Abstract
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) belongs to a group of naturally-occurring psychoactive indolealkylamine drugs. It acts as a nonselective serotonin (5-HT) agonist and causes many physiological and behavioral changes. 5-MeO-DMT is O-demethylated by polymorphic cytochrome P450 2D6 (CYP2D6) to an active metabolite, bufotenine, while it is mainly inactivated through the deamination pathway mediated by monoamine oxidase A (MAO-A). 5-MeO-DMT is often used with MAO-A inhibitors such as harmaline. Concurrent use of harmaline reduces 5-MeO-DMT deamination metabolism and leads to a prolonged and increased exposure to the parent drug 5-MeO-DMT, as well as the active metabolite bufotenine. Harmaline, 5-MeO-DMT and bufotenine act agonistically on serotonergic systems and may result in hyperserotonergic effects or serotonin toxicity. Interestingly, CYP2D6 also has important contribution to harmaline metabolism, and CYP2D6 genetic polymorphism may cause considerable variability in the metabolism, pharmacokinetics and dynamics of harmaline and its interaction with 5-MeO-DMT. Therefore, this review summarizes recent findings on biotransformation, pharmacokinetics, and pharmacological actions of 5-MeO-DMT. In addition, the pharmacokinetic and pharmacodynamic drug-drug interactions between harmaline and 5-MeO-DMT, potential involvement of CYP2D6 pharmacogenetics, and risks of 5-MeO-DMT intoxication are discussed.
Keywords: 5-MeO-DMT, harmaline, metabolism, drug interaction, pharmacokinetics, pharmacogenetics, CYP2D6, serotonin toxicity
INTRODUCTION
Indolealkylamine drugs consist of many antimigraine triptans (e.g. sumatriptan, naratriptan and almotriptan) and psychedelic substances of abuse (e.g., 5-methoxy-N,N-dimethyltryptamine or 5-MeO-DMT) [1]. 5-MeO-DMT was initially isolated from the bark of Dictyoloma incanescens D.C. [2]. It is a major active ingredient of South American Virola snuffs and Ayahuasca beverage [3-5]. 5-MeO-DMT also represents the active constituent of the venom of Colorado River Bufo alvarius toads, and it accounts for 15% of the dry weight of parotoid and tibial glands [6]. In addition, 5-MeO-DMT may be synthesized in human pineal and retina, and has been identified in human body fluids including urine, blood, and cerebrospinal fluid [7-11]. 5-MeO-DMT is regarded as an endogenous psychotoxin, and elevated concentrations of 5-MeO-DMT and its analogs in body fluids might be associated with psychotic disorders such as schizophrenic psychosis [12-19].
5-MeO-DMT is a potent, fast-acting hallucinogen with short duration in humans. Following different administration routes, e.g., inhalation (~6-20 mg), intravenous injection (~0.7-3.1 mg), sublingual or intranasal insufflation (~10 mg), and oral administration (~30 mg; with MAO inhibitor), 5-MeO-DMT produces psychedelic effects in human subjects [20]. 5-MeO-DMT also induces various physiological and behavioral changes in animal models [21-24]. 5-MeO-DMT has high affinity for the serotonin 5-HT1A receptor, and it is 4- to 10-fold more potent than N,N-dimethyltryptamine (DMT) in human subjects [25, 26]. Unlike its chemically and pharmacologically related drugs, such as 5-hydroxy-N,N-dimethyltryptamine (bufotenine), DMT, 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT) and α-methyltryptamine (AMT) that all have been Schedule I controlled drugs for years, 5-MeO-DMT was not a federally controlled substance in United States until August 2009 when the Drug Enforcement Administration issued a notice intending to place 5-MeO-DMT into Schedule I of the Controlled Substances Act [20]. By contrast, 5-MeO-DMT had been a controlled substance in many European countries including the United Kingdom.
As a tryptamine derivative, 5-MeO-DMT is mainly inactivated through a deamination pathway mediated by monoamine oxidase A (MAO-A), and it is O-demethylated by cytochrome P450 2D6 (CYP2D6) enzyme to produce an active metabolite, bufotenine [27, 28], which binds to the 5-HT2A receptor with much higher affinity than 5-MeO-DMT itself [29-31]. Concurrent use of 5-MeO-DMT with an MAO inhibitor (MAOI), or plant preparations (e.g., ayahuasca) or Syrian rue (Peganum harmala) seeds containing an MAOI (e.g., harmaline) [4, 32, 33], often leads to an enhanced and prolonged drug effect or more severe toxicity. Mechanistically, both MAOI and 5-MeO-DMT act agonistically on serotonergic systems that readily causes hyperserotonergic effects or serotonin toxicity [34-36]. In addition, MAOI increases exposure to the parent drug 5-MeO-DMT and the active metabolite bufotenine through the inhibition of deamination metabolism [37]. Such complex pharmacokinetic and pharmacodynamic interactions may even cause fatal toxicity [38, 39]. This review, therefore, aims to introduce the current understanding of 5-MeO-DMT biotransformation, pharmacokinetics and pharmacological actions, as well as drug-drug interactions (DDI) between 5-MeO-DMT and the MAOI, harmaline, and potential impact of CYP2D6 genetics.
PHARMACO/TOXICOLOGICAL EFFECTS AND DRUG ACTIONS OF 5-MEO-DMT
5-MeO-DMT is psychoactive in humans following inhalation of the vapor [20]. Human self-experiments have revealed that 5-MeO-DMT causes visionary and auditory changes, and distorts the perception of time. The effects start at 3-4 min, peak about 35-40 min, and end around 60-70 min after insufflation [3]. However, oral ingestion of 30-35 mg of 5-MeO-DMT produces either no psychoactive effect at all [40] or only one-third of the potency as that generated from intranasal or sublingual ingestion, or oral administration of pharmahuasca that contains both 5-MeO-DMT and the MAOI, harmaline [3, 26].
The toxicity of 5-MeO-DMT was firstly reported as a lethal syndrome called “staggers” in sheep after grazing on Phalaris tuberose, a plant containing 5-MeO-DMT [41]. Studies with mouse, rat, sheep, and monkey models revealed remarkable ataxia, mydriasis, head nodding, tremor, convulsion and shivering after administration of 5-MeO-DMT. Sheep was found to be the most susceptible to 5-MeO-DMT, and exhibited stringy salivation, tachycardia, and respiratory failure when exposed to 1 mg/kg of 5-MeO-DMT. The LD50 values of 5-MeO-DMT in mice ranged from 48 to 278 mg/kg for different administration routes [12, 21].
The 5-HT2A receptor subtype has been shown to play a major role in the stimulus effects of indolealkylamine and phenethylamine hallucinogens, while the 5-HT2C receptor acts as a modulator [42-44]. However, the 5-HT2A receptor appears to be less important for the stimulus control of 5-MeO-DMT, and the potencies of drugs in substituting 5-MeO-DMT-induced stimulus effect are correlated well with their affinities with the 5-HT1A receptor [29]. In addition, the discriminative stimuli induced by 5-MeO-DMT are attenuated by 5-HT1A antagonists including TVX Q7821, WAY-100635 and pindolol [29, 45, 46], supporting the conclusion that 5-MeO-DMT induces stimulus control mainly via 5-HT1A receptor. Nevertheless, a partial generalization of 5-MeO-DMT-induced stimulus by (−)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane [(−)-DOM] suggests that, besides the 5-HT1A receptor, the 5-HT2A receptor may also contribute to 5-MeO-DMT-mediated stimulus complex [45]. Interestingly, other structurally-related tryptamines including DMT and 5-MeO-DiPT also exhibit considerable binding affinities toward 5-HT1A receptor, as well as partial or full agonistic activities against 5-HT2A receptor [46-49].
Besides drug-induced discriminative stimulus control, 5-MeO-DMT provokes a variety of other behavioral effects in animal models, such as head shaking, forepaw treading, flat-body posture, straub tail, and hindlimb abduction [50-55] that are shared with many other hallucinogens including lysergic acid diethylamide (LSD), DOM and 1-(2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI) [24, 56-58]. The similar effects of 5-MeO-DMT and 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT), a 5-HT1A receptor agonist, on forepaw treading behavior in rats support the action of 5-MeO-DMT on postsynaptic 5-HT1A receptor [51, 54]. In mice, 5-MeO-DMT induces head-twitch and head-weaving responses via the activation of 5-HT2A and 5-HT1A receptors, respectively [50, 59]. Among a series of methylated serotonin derivatives, 5-MeO-DMT has been identified as one of the most potent agents to induce “sham rage” responses in cats [12]. 5-MeO-DMT also decreases locomotor activity and investigatory behavior in rats that can be attenuated by WAY-100635, a selective 5-HT1A/7 antagonist but not by the 5-HT2A-selective antagonist, M100907, suggesting the involvement of the 5-HT1A receptor [55]. Pretreatment with MAOI significantly modifies the locomotor activity induced by 5-MeO-DMT to a biphasic effect in rats, which may involve the activation of 5-HT2A receptor [60]. In addition, 5-MeO-DMT induces body temperature change in rats, causing hypothermia at low doses (0.5-1.0 mg/kg) and hyperthermia at high dose (3-10 mg/kg). The hyperthermic effect may be completely attenuated or even converted into hypothermia by the 5-HT2A antagonist, ketanserin. Chronic treatment with MAOI nialamide not only diminishes the hypothermic effect but also attenuates the hyperthermic response [61, 62].
5-MeO-DMT indeed binds to the 5-HT1A receptor subtype with much higher affinity (Ki, < 10 nM) than 5-HT2 receptor (>1000 nM) [29]. Studies using rat brain synaptosomes [63] show that 5-MeO-DMT also inhibits 5-HT re-uptake with an IC50 value comparable to other psychostimulants such as cocaine and methamphetamine, whereas it has little effect on dopamine re-uptake or the release of monoamine neurotransmitters. Furthermore, 5-MeO-DMT is the most potent tryptamine in stimulating G protein binding, with an EC50 value around 100 nM, which is approximately 115% of the maximum activation of 5-HT itself [64]. In addition, 5-MeO-DMT and other endogenous hallucinogens (e.g., DMT and bufotenine) are thought to act as ligands for the recently discovered trace amine receptors that may be involved in sensory perception [65].
BIOTRANSFORMATION OF 5-MEO-DMT
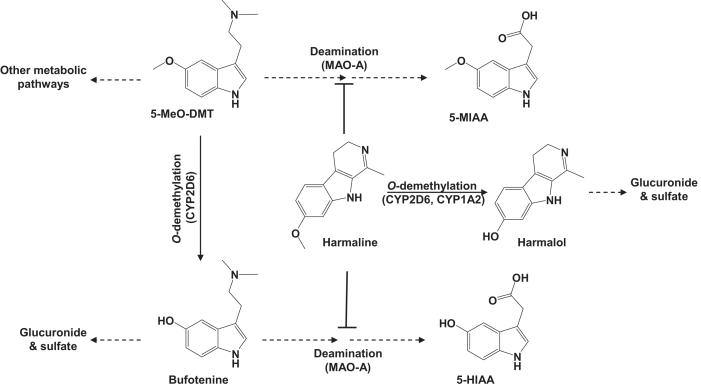
Similar to tryptamine, DMT and bufotenine [66-69], 5-MeO-DMT primarily undergoes MAO-A-mediated deamination (Fig. 1). Other metabolic pathways reported for 5-MeO-DMT include O-demethylation, N-demethylation and N-oxygenation. After intraperitoneal (i.p.) administration of 14C-labeled 5-MeO-DMT, 5-methoxyindoleacetic acid (5-MIAA) was identified as the major metabolite (54%) in rat urine, followed by 5-hydroxy-N,N-dimethyltryptamine glucuronide (23%), 5-hydroxyindoleacetic acid (5-HIAA, 14%), and bufotenine (9%) [70]. Because 5-HIAA cannot be transformed from 5-MIAA but from bufotenine, the 5-HIAA radioactivity may be attributed to consequential O-demethylation and deamination, suggesting that oxidative deamination and O-demethylation are the two major biotransformation pathways for 5-MeO-DMT in rats. 5-MeO-DMT was also found to be N-oxidized in rats [71-74]. In rat models, though the role of N-oxidation is uncertain, deaminated and O-demethylated metabolites were found in all studies. Our studies [27, 28, 37, 75] using human liver microsomes, hepatocytes and recombinant enzymes, as well as wild-type and CYP2D6-humanized mouse models, support the conclusion that 5-MeO-DMT is mainly inactivated through an MAO-A-catalyzed deamination pathway in humans although some 5-MeO-DMT is O-demethylated to bufotenine.
Figure 1.
5-MeO-DMT biotransformation and the metabolic interactions with harmaline. O-demethylation of 5-MeO-DMT by CYP2D6 produces an active metabolite bufotenine. Both 5-MeO-DMT and bufotenine are readily deaminated by MAO-A to indoleacetic acid derivatives. The MAOI harmaline, which is inactivated by CYP2D6, blocks the deamination metabolism of 5-MeO-DMT and bufotenine.
It is noteworthy that bufotenine, the O-demethylated product of 5-MeO-DMT, is also a hallucinogenic compound co-existing with 5-MeO-DMT in many plants and toad species [4-6, 76]. Bufotenine exhibits about 5- to 10-fold higher affinity to the 5-HT2A receptor than 5-MeO-DMT in vitro, and about 3-fold higher potency than 5-MeO-DMT when they are present at similar level in brain [31, 77, 78]. Although the psychoactivity of bufotenine had been questioned due to its lower ability to cross blood-brain barrier (BBB), bufotenine does produce psychoactive effects in humans after intravenous injection or intranasal and sublingual administration [76, 79, 80]. Thus, the production of bufotenine from 5-MeO-DMT may be viewed as an activation process similar to the production of serotonin from 5-methoxytryptamine [81]. Indeed, severe toxicity or even lethality associated with the use of bufotenine has been documented [82, 83], and bufotenine has been a Schedule I controlled substance in United State for decades.
The production of bufotenine from 5-MeO-DMT is predominately mediated by human CYP2D6 (Fig. 1), which is well known for its genetic polymorphism and clinical importance [28, 84-87]. This biotransformation was initially discovered using recombinant CYP2D6 enzyme [27]. Our recent studies [37] indicate that 5-MeO-DMT O-demethylation is significantly correlated with bufuralol 1′-hydroxylation and CYP2D6 content in human liver microsomes when the deamination pathway is blocked by an MAO inhibitor. In addition, the CYP2D6.10 allelic isoform has much lower enzymatic activity in catalyzing 5-MeO-DMT O-demethylation than wild-type CYP2D6.1 enzyme, suggesting that subjects carrying the CYP2D6*10 allelic variant may produce less bufotenine from 5-MeO-DMT. Given the important role for CYP2D6 in 5-MeO-DMT O-demethylation activation, elucidation of the formation of bufotenine would be necessary to understand 5-MeO-DMT pharmacokinetics and risk of intoxication.
PHARMACOKINETICS OF 5-MEO-DMT
After i.p. administration, 5-MeO-DMT reaches the maximum drug concentration (Cmax) at around 5-7 min and is then eliminated with a terminal half-life (t1/2) of 12-19 min in mice [75]. The fast absorption and short t1/2 are also true for 5-MeO-DMT in rats [72]. 5-MeO-DMT is predominantly eliminated through MAO-A-mediated metabolism, as supported by the low urinary recovery and biliary excretion of the parent compound [70, 71] and an over 4-fold increase in systemic exposure to 5-MeO-DMT when co-administered with an MAOI [37]. 5-MeO-DMT also shows a relatively high oil/water partition coefficient (3.30) [88], suggesting that 5-MeO-DMT may easily penetrate various lipoprotein barriers including the BBB. Indeed, 5-MeO-DMT significantly accumulates in many organs (e.g., liver, kidney and brain) in different animal models (e.g., mouse, rat and rabbit) [72, 73, 89]. The brain concentration of 5-MeO-DMT is about 1.7-fold higher than that in blood at 45 min after i.p. administration [72], and the drug is widely distributed in different rat brain regions including cortex, thalamus, hippocampus, basal ganglia, medulla, pons and cerebellum [90]. Our unpublished results also support the idea that 5-MeO-DMT is readily distributed and accumulated in mouse cortex, hippocampus, hypothalamus, and striatum after i.p administration.
As mentioned above, monitoring bufotenine formation may provide improved understanding of the complex pharmacological and toxicological effects of 5-MeO-DMT because bufotenine is the active metabolite of 5-MeO-DMT, and has a higher binding affinity for the 5-HT2A receptor than 5-MeO-DMT does. Bufotenine is also rapidly eliminated from the body. In healthy volunteers receiving an intravenous infusion of 14C-labeled bufotenine, nearly all the radioactivity is recovered in the first 12 hr urine sample [91]. Furthermore, only 1-6% of total recovered radioactivity is identified as unchanged bufotenine, while the deaminated metabolite 5-HIAA accounts for 68-74% of radioactivity. Compared to 5-MeO-DMT, bufotenine has a poor partition coefficient and shows a low penetration across the BBB but a high accumulation in rat lungs after subcutaneous injection [68, 88].
Bufotenine is readily detected in mouse blood samples, following the administration of a lower dose of 5-MeO-DMT (2 mg/kg, i.p.) [37, 75]. Bufotenine reaches the Cmax at around 13 min, and is eliminated with an apparent half-life of about 25 min. The systemic exposure (AUC) to bufotenine appears to be less than 10% of that of the parent 5-MeO-DMT in mice [37, 75]. Considering the low systemic exposure to bufotenine produced from 5-MeO-DMT and the low BBB penetration for bufotenine, the peripheral bufotenine produced from 5-MeO-DMT may not be able to cross the BBB and thus maintain sufficient concentration in brain to exert neurotoxicity. In addition, our unpublished findings indicate that bufotenine may not be extensively produced from 5-MeO-DMT within mouse brain. Therefore, it is likely that only peripherally-generated bufotenine contributes to the apparent activity of 5-MeO-DMT.
DRUG INTERACTIONS BETWEEN 5-MEO-DMT AND MAOI HARMALINE
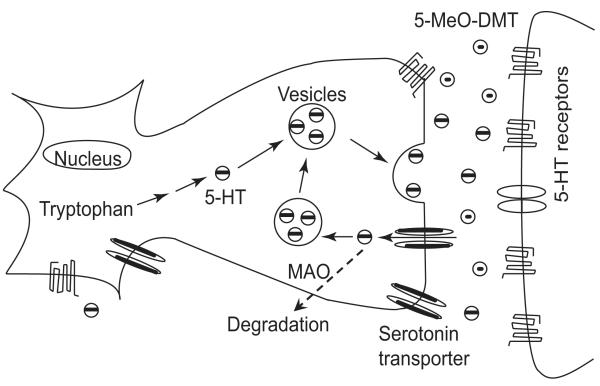
DDI may be arisen when subjects are exposed to polypharmacy [36, 92-95]. At the pharmacokinetic level, a perpetrator drug alters the absorption, disposition, metabolism, or excretion of the victim drug, which may be translated into a significant change in drug efficacy and/or toxicity. The metabolic DDI is often observed because metabolism represents the major route of drug elimination. At the pharmacodynamic level, concurrent drugs both act on the common targets, leading to synergistic or antagonistic responses. It should be noted that some drugs may interact at both pharmacokinetic and pharmacodynamic levels, and lead to severe or even fatal toxicity. This may be true for the concomitant use of 5-MeO-DMT and harmaline or another MAOI. By inhibiting MAO-A-mediated 5-HT degradation, MAOI itself promotes serotonergic transmission (Fig. 2). Furthermore, harmaline is also a 5-HT agonist [96, 97] that could potentiate serotonergic actions of 5-MeO-DMT. In addition, harmaline reduces 5-MeO-DMT deamination metabolism, leading to an increased and prolonged exposure to 5-MeO-DMT, as well as the psychoactive metabolite bufotenine that depends upon CYP2D6 status. Indeed, cases of severe and lethal intoxication due to the combined use of a tryptamine (e.g., 5-MeO-DMT or 5-MeO-DiPT) and an MAOI drug (e.g., harmaline) have been reported [38, 39, 98, 99].
Figure 2.
Hyperserotonergic effects may be induced when harmaline blocks 5-HT degradation, and the concurrent 5-MeO-DMT activates 5-HT receptors within the synaptic cleft, beside their pharmacokinetic interactions.
Pharmacokinetic and Pharmacodynamic Interactions
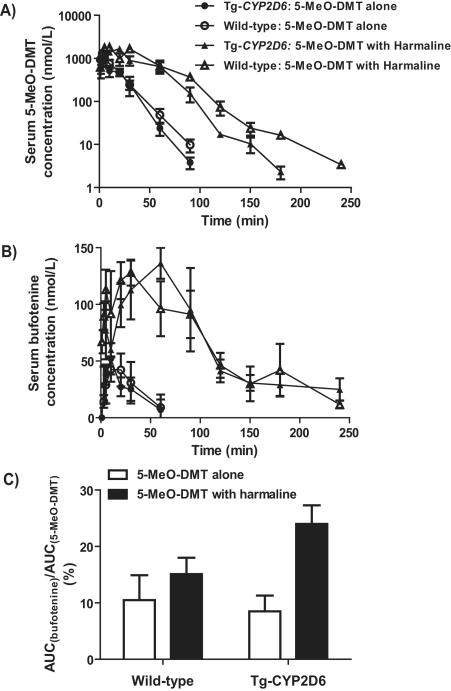
The pharmacokinetics of 5-MeO-DMT can be significantly altered by a co-administered MAOI. Co-incubation of 5-MeO-DMT with harmaline in human hepatocytes completely blocks the depletion of 5-MeO-DMT, and reduces the in vitro intrinsic clearance (CLint) by over 24-fold [37]. In rats, pretreatment with iproniazid significantly increases 5-MeO-DMT levels in urine, blood, and other tissues [71, 72]. In mice, pretreatment with harmaline decreases the clearance of 5-MeO-DMT by 4.4-fold, leading to a 4.4-fold higher systemic exposure to 5-MeO-DMT (Fig. 3) [37]. The concomitant administration of harmaline not only affects the pharmacokinetics of 5-MeO-DMT, but also influences formation of the active metabolite bufotenine (Fig. 3). When deamination metabolism is inhibited, more 5-MeO-DMT is diverted to other metabolic pathways including O-demethylation with increased production of bufotenine. In human CYP2D6 extensive metabolizer hepatocytes, bufotenine formation is significantly increased when 5-MeO-DMT is co-incubated with harmaline [37]. Since bufotenine is also mainly inactivated through the MAO-A-mediated deamination pathway [91], MAOI may not only increase bufotenine production but also reduce its elimination. As a result, the Cmax and AUC of bufotenine are increased to 2.6- and 6-fold, respectively, in mice after harmaline pretreatment [37].
Figure 3.
Harmaline (5 mg/kg, i.p.) co-administered with 5-MeO-DMT (2 mg/kg, i.p.) markedly alters blood concentrations of the parent drug 5-MeO-DMT (A), the active metabolite bufotenine (B), and the ratio of their systemic exposures (C) in wild-type and/or Tg-CYP2D6 mouse models [37].
Besides the impact on 5-MeO-DMT pharmacokinetics, harmaline acts on serotonergic systems and exhibits a variety of neuropharmacological activities. Harmaline is one of the most potent inhibitors of MAO-A [100], and it may interact with 5-HT, dopamine, gamma-amino-butyric acid (GABA), and N-methyl-d-aspartate (NMDA) receptors [101-105]. The pharmacokinetic and dynamic interactions of harmaline and 5-MeO-DMT are manifested by a remarkable change in drug responses. For instance, pretreatment with harmaline and clorgyline in rats significantly modifies 5-MeO-DMT-induced locomotor activity to a biphasic effect, of which the late hyperactivity is attenuated by MDL 11939, a selective 5-HT2A antagonist, suggesting the important contribution of the 5-HT2A receptor to the hyperactivity induced by combined use of 5-MeO-DMT and MAOI [60]. Studies in our laboratory indicate that harmaline enhances the hyperthermic effect of 5-MeO-DMT and potentiates stimulus control in mice (unpublished data). Anecdotal reports by human self-experimenters suggest an enhancement of the psychedelic effects of 5-MeO-DMT when combined with harmaline or harmine [3, 40]. For instance, 10 mg of 5-MeO-DMT administrated intranasally or sublingually causes significant visionary response, whereas the same dose of oral 5-MeO-DMT does not show any effects in humans, which is probably due to the extensive first-pass metabolism by MAO-A. However, when used with harmaline, the same oral dose 5-MeO-DMT (10 mg) shows nearly equal intensity of psychedelic effects as 10 mg of 5-MeO-DMT itself dosed intranasally or sublingually [3]. These findings indicate that 5-MeO-DMT pharmacological and toxicological effects are generally potentiated by a concurrent MAOI because of pharmacokinetic and dynamic interactions.
The Impact of CYP2D6 Genetic Polymorphism
CYP2D6 is an important P450 enzyme that has more than 90 allelic variants, leading to considerable interindividual variability in metabolism of some CYP2D6 substrate drugs including therapeutic agents and drugs of abuse [1, 106-108]. Because 5-MeO-DMT O-demethylation is dependent upon CYP2D6 [27], the impact of CYP2D6 phenotype/genotype on the production of bufotenine from 5-MeO-DMT has been examined [37]. The blockage of MAO activity results in a strong correlation between 5-MeO-DMT O-demethylation and CYP2D6 activity in human liver microsomes. As expected, the CYP2D6.1, CYP2D6.2 and CYP2D6.10 allelic isoforms shows variable catalytic activities in producing bufotenine from 5-MeO-DMT, and human CYP2D6 poor hepatocytes lacking CYP2D6 activity do not produce any bufotenine. In addition, CYP2D6-humanized (Tg-CYP2D6) mice [109] show a significantly higher systemic exposure (AUC) to the active metabolite bufotenine than wild-type mice treated i.p. with 20 mg/kg of 5-MeO-DMT. It should be noted that the Tg-CYP2D6 and wild-type control mice are useful animal models in delineating the impact of CYP2D6 on drug metabolism and pharmacokinetics [28, 110, 111]. Therefore, it is expected that subjects with regular or increased CYP2D6 activity would be exposed to both the substrate drug 5-MeO-DMT and the active metabolite bufotenine, and thus might have more complex drug effects [37].
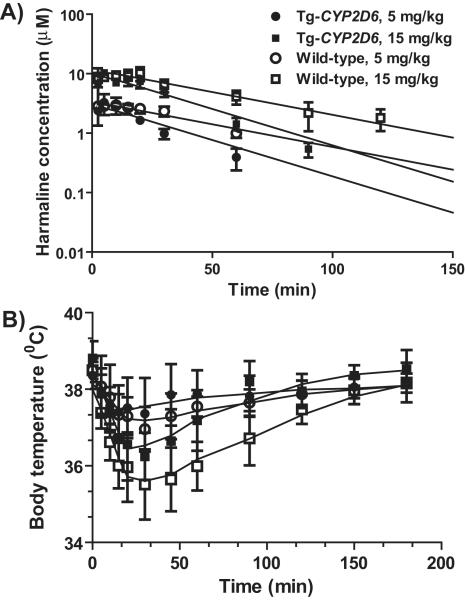
Harmaline co-administered with 5-MeO-DMT is also metabolized by CYP2D6 [112], and the effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics have been defined separately [113]. Depletion of harmaline is 2.5 times slower in human CYP2D6 poor metabolizer hepatocytes than that in CYP2D6 extensive metabolizer hepatocytes. Further studies using Tg-CYP2D6 and wild-type mouse models reveal that wild-type mice have a longer and higher exposure to harmaline, as well as a more severe hypothermia (Fig. 4). In addition, wild-type mice lacking CYP2D6 activity are more sensitive to harmaline in marble-burying test [113]. Therefore, a lower exposure to harmaline in Tg-CYP2D6 mice might counteract the effect of CYP2D6 on 5-MeO-DMT O-demethylation. Indeed, levels of bufotenine formed from 5-MeO-DMT do not differ significantly between wild-type and Tg-CYP2D6 mice after co-administration of harmaline [37], which may be due to the low dose (2 mg/kg, i.p.) of 5-MeO-DMT used in the study and the significant contribution of mouse P450s to 5-MeO-DMT O-demethylation [27, 28]. Nevertheless, systemic exposure to bufotenine is about 24% of that to 5-MeO-DMT in Tg-CYP2D6 mice, whereas it is 15% in wild-type mice, indicating the impact of CYP2D6 status on harmaline-5-MeO-DMT pharmacokinetic drug interaction.
Figure 4.
Studies using wild-type and Tg-CYP2D6 mouse model demonstrate that CYP2D6 status has significant impact on harmaline pharmacokinetics (A) and harmaline-induced hypothermia (B) [113].
CONCLUSIONS
5-MeO-DMT represents a natural psychoactive tryptamine indolealkylamine drug of abuse. As a fast-acting drug, 5-MeO-DMT induces many physiological and behavioral changes in humans and animal models, such as visionary and auditory distortion, hyperthermia, head-twitch, and stimulus control, which involve the actions of 5-HT receptors. It is readily inactivated through the MAO-A-mediated deamination pathway, while a small portion is transformed to the active metabolite bufotenine by polymorphic CYP2D6. Receptor binding studies have revealed that 5-MeO-DMT is more selective for the 5-HT1A receptor, while bufotenine mainly acts as a 5-HT2A agonist. The apparent non-selectivity of 5-MeO-DMT may be attributed, at least in part, to bufotenine produced from 5-MeO-DMT, which is dependent on CYP2D6 enzymatic activity.
5-MeO-DMT is often co-abused with an MAOI such as harmaline to enhance hallucinations. There are two levels of interactions between harmaline and 5-MeO-DMT, pharmacokinetic and pharmacodynamic. When deamination metabolism is inhibited by harmaline, the systemic and cerebral exposure to 5-MeO-DMT, as well as to the metabolite bufotenine, is sharply elevated and prolonged. Meanwhile, harmaline and 5-MeO-DMT both act agonistically on the serotonergic systems. As a result, coadministration of harmaline potentiates 5-MeO-DMT drug responses and sometimes leads to severe or fatal serotonin toxicity in animal models. Several cases of intoxication or even death have also been reported in humans associated with the abuse of 5-MeO-DMT and harmaline. In addition, depending upon the dose combination, an influence of CYP2D6 genotype/phenotype on harmaline-5-MeO-DMT DDI may be seen, despite the fact that the CYP2D6 enzyme inactivates harmaline whereas it activates 5-MeO-DMT. Therefore, CYP2D6 may serve as a marker for bufotenine production during pharmacokinetic interaction between 5-MeO-DMT and harmaline.
ACKNOWLEDGEMENT
This project was supported by Award Number R01DA021172 from the National Institute On Drug Abuse, National Institutes of Health (NIH).
ABBREVIATIONS
- 5-MeO-DMT
5-methoxy-N,N-dimethyltryptamine
- Bufotenine
5-hydroxy-N,N-dimethyltryptamine
- CYP2D6
cytochrome P450 2D6
- MAO-A
monoamine oxidase A
- MAOI
monoamine oxidase inhibitor
- DDI
drug-drug interaction
- Serotonin
5-HT
- DMT
N,N-dimethyltryptamine
- 5-MeO-DiPT
5-methoxy-N,N-diisopropyltryptamine
- BBB
blood-brain barrier
- Tg-CYP2D6
CYP2D6-humanized
REFERENCES
- [1].Yu AM. Indolealkylamines: biotransformations and potential drug-drug interactions. Aaps J. 2008;10:242–253. doi: 10.1208/s12248-008-9028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pachter IJ, Zacharias DE, Ribeiro O. Indole alkaloids of acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J Org Chem. 1959;24:1285–1287. [Google Scholar]
- [3].Ott J. Pharmepena-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–407. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- [4].McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther. 2004;102:111–129. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [5].Gambelunghe C, Aroni K, Rossi R, Moretti L, Bacci M. Identification of N,N-dimethyltryptamine and beta-carbolines in psychotropic ayahuasca beverage. Biomed Chromatogr. 2008;22:1056–1059. doi: 10.1002/bmc.1023. [DOI] [PubMed] [Google Scholar]
- [6].Weil AT, Davis W. Bufo alvarius: a potent hallucinogen of animal origin. J Ethnopharmacol. 1994;41:1–8. doi: 10.1016/0378-8741(94)90051-5. [DOI] [PubMed] [Google Scholar]
- [7].Narasimhachari N, Heller B, Spaide J, Haskovec L, Meltzer H, Strahilevitz M, Himwich HE. N,N-dimethylated indoleamines in blood. Biol Psychiatry. 1971;3:21–23. [PubMed] [Google Scholar]
- [8].Oon MC, Murray RM, Rodnight R, Murphy MP, Birley JL. Factors affecting the urinary excretion of endogenously formed dimethyltryptamine in normal human subjects. Psychopharmacology (Berl) 1977;54:171–175. doi: 10.1007/BF00426775. [DOI] [PubMed] [Google Scholar]
- [9].Smythies JR, Morin RD, Brown GB. Identification of dimethyltryptamine and O-methylbufotenin in human cerebrospinal fluid by combined gas chromatography/mass spectrometry. Biol Psychiatry. 1979;14:549–556. [PubMed] [Google Scholar]
- [10].Sitaram BR, Blackman GL, McLeod WR, Vaughan GN. The ion-pair extraction, purification, and liquid chromatographic analysis of indolealkylamines in human urine. Anal Biochem. 1983;128:11–20. doi: 10.1016/0003-2697(83)90337-8. [DOI] [PubMed] [Google Scholar]
- [11].Forsstrom T, Tuominen J, Karkkainen J. Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scand J Clin Lab Invest. 2001;61:547–556. doi: 10.1080/003655101753218319. [DOI] [PubMed] [Google Scholar]
- [12].Benington F, Morin RD, Clark LC., Jr. 5-methoxy-N, N-dimethyltryptamine, a possible endogenous psychotoxin. Ala J Med Sci. 1965;2:397–403. [PubMed] [Google Scholar]
- [13].Nishimura T, Gjessing LR. Failure to detect 3,4-dimethoxyphenylethylamine and bufotenine in the urine from a case of periodic catatonia. Nature. 1965;206:963–964. doi: 10.1038/206963b0. [DOI] [PubMed] [Google Scholar]
- [14].Narasimhachari N, Heller B, Spaide J, Haskovec L, Fujimori M, Tabushi K, Himwich HE. Urinary studies of schizophrenics and controls. Biol Psychiatry. 1971;3:9–20. [PubMed] [Google Scholar]
- [15].Gillin JC, Wyatt RJ. Evidence for and against the involvement of N,N-dimethyl-tryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in schizophrenia. Psychopharmacol Bull. 1976;12:12–13. [PubMed] [Google Scholar]
- [16].Angrist B, Gershon S, Sathananthan G, Walker RW, Lopez-Ramos B, Mandel LR, Vandenheuvel WJ. Dimethyltryptamine levels in blood of schizophrenic patients and control subjects. Psychopharmacology (Berl) 1976;47:29–32. doi: 10.1007/BF00428697. [DOI] [PubMed] [Google Scholar]
- [17].Takeda N, Ikeda R, Ohba K, Kondo M. Bufotenine reconsidered as a diagnostic indicator of psychiatric disorders. Neuroreport. 1995;6:2378–2380. doi: 10.1097/00001756-199511270-00024. [DOI] [PubMed] [Google Scholar]
- [18].Cichon S, Nothen MM, Rietschel M, Propping P. Pharmacogenetics of schizophrenia. Am J Med Genet. 2000;97:98–106. doi: 10.1002/(sici)1096-8628(200021)97:1<98::aid-ajmg12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [19].Emanuele E, Colombo R, Martinelli V, Brondino N, Marini M, Boso M, Barale F, Politi P. Elevated urine levels of bufotenine in patients with autistic spectrum disorders and schizophrenia. Neuro Endocrinol Lett. 2010;31:117–121. [PubMed] [Google Scholar]
- [20].DEA-2009-0008 [Accessed Sep 23, 2010];Placement of 5-Methoxy-N,N-Dimethyltryptamine Into Schedule I of the Controlled Substances Act. http://edocket.access.gpo.gov/2009/E9-20204.htm. [PubMed]
- [21].Gillin JC, Tinklenberg J, Stoff DM, Stillman R, Shortlidge JS, Wyatt RJ. 5-Methoxy-N,N-dimethyltryptamine: behavioral and toxicological effects in animals. Biol Psychiatry. 1976;11:355–358. [PubMed] [Google Scholar]
- [22].Glennon RA. Do classical hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacology. 1990;3:509–517. [PubMed] [Google Scholar]
- [23].Duvvuri V, Risbrough VB, Kaye WH, Geyer MA. 5-HT1A receptor activation is necessary for 5-MeODMT-dependent potentiation of feeding inhibition. Pharmacol Biochem Behav. 2009;93:349–353. doi: 10.1016/j.pbb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McKenna DJ, Towers GH. Biochemistry and pharmacology of tryptamines and beta-carbolines. A minireview. J Psychoactive Drugs. 1984;16:347–358. doi: 10.1080/02791072.1984.10472305. [DOI] [PubMed] [Google Scholar]
- [26].Ott J. Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs. 1999;31:171–177. doi: 10.1080/02791072.1999.10471741. [DOI] [PubMed] [Google Scholar]
- [27].Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- [28].Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36:243–277. doi: 10.1081/dmr-120034000. [DOI] [PubMed] [Google Scholar]
- [29].Spencer DG, Jr., Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology (Berl) 1987;93:158–166. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- [30].Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K. Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem. 1994;37:1929–1935. doi: 10.1021/jm00039a004. [DOI] [PubMed] [Google Scholar]
- [31].Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther. 1997;280:576–583. [PubMed] [Google Scholar]
- [32].Lamchouri F, Settaf A, Cherrah Y, El Hamidi M, Tligui N, Lyoussi B, Hassar M. Experimental toxicity of Peganum harmala seeds. Ann Pharm Fr. 2002;60:123–129. [PubMed] [Google Scholar]
- [33].Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem Toxicol. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- [34].Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- [35].Sun-Edelstein C, Tepper SJ, Shapiro RE. Drug-induced serotonin syndrome: a review. Expert Opin Drug Saf. 2008;7:587–596. doi: 10.1517/14740338.7.5.587. [DOI] [PubMed] [Google Scholar]
- [36].Gillman PK. A review of serotonin toxicity data: implications for the mechanisms of antidepressant drug action. Biol Psychiatry. 2006;59:1046–1051. doi: 10.1016/j.biopsych.2005.11.016. [DOI] [PubMed] [Google Scholar]
- [37].Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol. 2010;80:122–128. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42:191–195. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- [39].Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J Anal Toxicol. 2005;29:838–841. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- [40].Shulgin AT, Shulgin A. TIKAL The Continuation. Transform Press; Berkeley, CA: 1997. [Google Scholar]
- [41].Gallagher CH, Koch JH, Moore RM, Steel JD. Toxicity of Phalaris Tuberosa for Sheep. Nature. 1964;204:542–545. doi: 10.1038/204542a0. [DOI] [PubMed] [Google Scholar]
- [42].Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- [43].Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- [44].Winter JC, Fiorella DJ, Timineri DM, Filipink RA, Helsley SE, Rabin RA. Serotonergic receptor subtypes and hallucinogen-induced stimulus control. Pharmacol Biochem Behav. 1999;64:283–293. doi: 10.1016/s0091-3057(99)00063-5. [DOI] [PubMed] [Google Scholar]
- [45].Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- [46].Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology (Berl) 2009;203:251–263. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- [47].Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology (Berl) 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- [48].Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- [49].Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- [50].Matsumoto K, Mizowaki M, Takayama H, Sakai S, Aimi N, Watanabe H. Suppressive effect of mitragynine on the 5-methoxy-N,N-dimethyltryptamine-induced head-twitch response in mice. Pharmacol Biochem Behav. 1997;57:319–323. doi: 10.1016/s0091-3057(96)00314-0. [DOI] [PubMed] [Google Scholar]
- [51].Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- [52].Eison AS, Wright RN. 5-HT1A and 5-HT2 receptors mediate discrete behaviors in the Mongolian gerbil. Pharmacol Biochem Behav. 1992;43:131–137. doi: 10.1016/0091-3057(92)90649-z. [DOI] [PubMed] [Google Scholar]
- [53].Cancela LM, Volosin M, Molina VA. Gangliosides attenuate stress-induced changes on body weight, motor activity and on the behavioral response to 5-methoxy-N,N-dimethyltryptamine. Brain Res Bull. 1996;40:105–110. doi: 10.1016/0361-9230(96)00040-8. [DOI] [PubMed] [Google Scholar]
- [54].Sanchez C, Arnt J, Moltzen E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- [55].Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- [56].Knapp DJ, Sim-Selley LJ, Breese GR, Overstreet DH. Selective breeding of 5-HT(1A) receptor-mediated responses: application to emotion and receptor action. Pharmacol Biochem Behav. 2000;67:701–708. doi: 10.1016/s0091-3057(00)00415-9. [DOI] [PubMed] [Google Scholar]
- [57].Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- [58].Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- [59].Matsumoto K, Morishige R, Murakami Y, Tohda M, Takayama H, Sakakibara I, Watanabe H. Suppressive effects of isorhynchophylline on 5-HT2A receptor function in the brain: behavioural and electrophysiological studies. Eur J Pharmacol. 2005;517:191–199. doi: 10.1016/j.ejphar.2005.05.015. [DOI] [PubMed] [Google Scholar]
- [60].Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gudelsky GA, Koenig JI, Jackman H, Meltzer HY. Suppression of the hypo- and hyperthermic responses to 5-HT agonists following the repeated administration of monoamine oxidase inhibitors. Psychopharmacology (Berl) 1986;90:403–407. doi: 10.1007/BF00179199. [DOI] [PubMed] [Google Scholar]
- [62].Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- [63].Nagai F, Nonaka R, Kamimura K. Satoh Hisashi. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- [64].Nonaka R, Nagai F, Ogata A, Satoh K. In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes. Biol Pharm Bull. 2007;30:2328–2333. doi: 10.1248/bpb.30.2328. [DOI] [PubMed] [Google Scholar]
- [65].Wallach JV. Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception. Med Hypotheses. 2009;72:91–94. doi: 10.1016/j.mehy.2008.07.052. [DOI] [PubMed] [Google Scholar]
- [66].Suzuki O, Katsumata Y, Oya M. Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochem Pharmacol. 1981;30:1353–1358. [PubMed] [Google Scholar]
- [67].Raynaud F, Pevet P. 5-Methoxytryptamine is metabolized by monoamine oxidase A in the pineal gland and plasma of golden hamsters. Neurosci Lett. 1991;123:172–174. doi: 10.1016/0304-3940(91)90923-h. [DOI] [PubMed] [Google Scholar]
- [68].Fuller RW, Snoddy HD, Perry KW. Tissue distribution, metabolism and effects of bufotenine administered to rats. Neuropharmacology. 1995;34:799–804. doi: 10.1016/0028-3908(95)00049-c. [DOI] [PubMed] [Google Scholar]
- [69].Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Kupfer A, Idle JR, Gonzalez FJ. The relative contribution of monoamine oxidase and cytochrome p450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther. 2003;304:539–546. doi: 10.1124/jpet.102.043786. [DOI] [PubMed] [Google Scholar]
- [70].Agurell S, Holmstedt B, Lindgren JE. Metabolism of 5-methoxy-N,-N dimethyltryptamine- 14 C in the rat. Biochem Pharmacol. 1969;18:2771–2781. doi: 10.1016/0006-2952(69)90185-3. [DOI] [PubMed] [Google Scholar]
- [71].Sitaram BR, Lockett L, Blackman GL, McLeod WR. Urinary excretion of 5-methoxy-N,N-dimethyltryptamine, N,N-dimethyltryptamine and their N-oxides in the rat. Biochem Pharmacol. 1987;36:2235–2237. doi: 10.1016/0006-2952(87)90159-6. [DOI] [PubMed] [Google Scholar]
- [72].Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem Pharmacol. 1987;36:1509–1512. doi: 10.1016/0006-2952(87)90118-3. [DOI] [PubMed] [Google Scholar]
- [73].Sitaram BR, McLeod WR. Observations on the metabolism of the psychotomimetic indolealkylamines: implications for future clinical studies. Biol Psychiatry. 1990;28:841–848. doi: 10.1016/0006-3223(90)90566-k. [DOI] [PubMed] [Google Scholar]
- [74].Sitaram BR, Talomsin R, Blackman GL, McLeod WR. Study of metabolism of psychotomimetic indolealkylamines by rat tissue extracts using liquid chromatography. Biochem Pharmacol. 1987;36:1503–1508. doi: 10.1016/0006-2952(87)90117-1. [DOI] [PubMed] [Google Scholar]
- [75].Shen HW, Jiang XL, Yu AM. Development of a LC–MS/MS method to analyze 5-methoxy-N,N-dimethyltryptamine and bufotenine: application to pharmacokinetic study. Bioanalysis. 2009;1:87–95. doi: 10.4155/bio.09.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ott J. Pharmanopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine. J Psychoactive Drugs. 2001;33:273–281. doi: 10.1080/02791072.2001.10400574. [DOI] [PubMed] [Google Scholar]
- [77].McBride MC. Bufotenine: toward an understanding of possible psychoactive mechanisms. J Psychoactive Drugs. 2000;32:321–331. doi: 10.1080/02791072.2000.10400456. [DOI] [PubMed] [Google Scholar]
- [78].Vogel WH, Evans BD. Structure-activity-relationships of certain hallucinogenic substances based on brain levels. Life Sci. 1977;20:1629–1635. doi: 10.1016/0024-3205(77)90335-6. [DOI] [PubMed] [Google Scholar]
- [79].Fabing HD, Hawkins JR. Intravenous bufotenine injection in the human being. Science. 1956;123:886–887. doi: 10.1126/science.123.3203.886. [DOI] [PubMed] [Google Scholar]
- [80].McLeod WR, Sitaram BR. Bufotenine reconsidered. Acta Psychiatr Scand. 1985;72:447–450. doi: 10.1111/j.1600-0447.1985.tb02638.x. [DOI] [PubMed] [Google Scholar]
- [81].Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003;13:173–181. doi: 10.1097/01.fpc.0000054066.98065.7b. [DOI] [PubMed] [Google Scholar]
- [82].Chilton WS, Bigwood J, Jensen RE. Psilocin, bufotenine and serotonin: historical and biosynthetic observations. J Psychedelic Drugs. 1979;11:61–69. doi: 10.1080/02791072.1979.10472093. [DOI] [PubMed] [Google Scholar]
- [83].Kostakis C, Byard RW. Sudden death associated with intravenous injection of toad extract. Forensic Sci Int. 2009;188:e1–5. doi: 10.1016/j.forsciint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [84].Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- [85].Loovers HM, van der Weide J. Implementation of CYP2D6 genotyping in psychiatry. Expert Opin Drug Metab Toxicol. 2009;5:1065–1077. doi: 10.1517/17425250903081738. [DOI] [PubMed] [Google Scholar]
- [86].Dorado P, Penas-Lledo EM, Llerena A. CYP2D6 polymorphism: implications for antipsychotic drug response, schizophrenia and personality traits. Pharmacogenomics. 2007;8:1597–1608. doi: 10.2217/14622416.8.11.1597. [DOI] [PubMed] [Google Scholar]
- [87].De Gregori M, Allegri M, De Gregori S, Garbin G, Tinelli C, Regazzi M, Govoni S, Ranzani GN. How and Why to Screen for CYP2D6 Interindividual Variability in Patients Under Pharmacological Treatments. Curr Drug Metab. 2010;11:276–282. doi: 10.2174/138920010791196274. [DOI] [PubMed] [Google Scholar]
- [88].Migliaccio GP, Shieh TL, Byrn SR, Hathaway BA, Nichols DE. Comparison of solution conformational preferences for the hallucinogens bufotenin and psilocin using 360-MHz proton NMR spectroscopy. J Med Chem. 1981;24:206–209. doi: 10.1021/jm00134a016. [DOI] [PubMed] [Google Scholar]
- [89].Berger G, Maziere M, Marazano C, Comar D. Carbon 11 labeling of the psychoactive drug o-methyl-bufotenine and its distribution in the animal organism. Eur J Nucl Med. 1978;3:101–104. doi: 10.1007/BF00251632. [DOI] [PubMed] [Google Scholar]
- [90].Barker SA, Littlefield-Chabaud MA, David C. Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC-APcI-MS-MS-isotope dilution method. J Chromatogr B Biomed Sci Appl. 2001;751:37–47. doi: 10.1016/s0378-4347(00)00442-4. [DOI] [PubMed] [Google Scholar]
- [91].Sanders-Bush E, Oates JA, Bush MT. Metabolism of bufotenine-2′-14C in human volunteers. Life Sci. 1976;19:1407–1411. doi: 10.1016/0024-3205(76)90441-0. [DOI] [PubMed] [Google Scholar]
- [92].Boobis A, Watelet JB, Whomsley R, Benedetti MS, Demoly P, Tipton K. Drug interactions. Drug Metab Rev. 2009;41:486–527. doi: 10.1080/10837450902891550. [DOI] [PubMed] [Google Scholar]
- [93].Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert Opin Drug Metab Toxicol. 2009;5:1513–1528. doi: 10.1517/17425250903307448. [DOI] [PubMed] [Google Scholar]
- [94].He SM, Yang AK, Li XT, Du YM, Zhou SF. Effects of herbal products on the metabolism and transport of anticancer agents. Expert Opin Drug Metab Toxicol. 2010 doi: 10.1517/17425255.2010.510132. [DOI] [PubMed] [Google Scholar]
- [95].Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, Woods J. Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab. 2008;9:1063–1120. doi: 10.2174/138920008786927785. [DOI] [PubMed] [Google Scholar]
- [96].Abdel-Fattah AF, Matsumoto K, Gammaz HA, Watanabe H. Hypothermic effect of harmala alkaloid in rats: involvement of serotonergic mechanism. Pharmacol Biochem Behav. 1995;52:421–426. doi: 10.1016/0091-3057(95)00131-f. [DOI] [PubMed] [Google Scholar]
- [97].Glennon RA, Dukat M, Grella B, Hong S, Costantino L, Teitler M, Smith C, Egan C, Davis K, Mattson MV. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend. 2000;60:121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- [98].Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K. A fatal poisoning with 5-methoxy-N,N-diisopropyltryptamine, Foxy. Forensic Sci Int. 2006;163:152–154. doi: 10.1016/j.forsciint.2005.11.026. [DOI] [PubMed] [Google Scholar]
- [99].Bjornstad K, Hulten P, Beck O, Helander A. Bioanalytical and clinical evaluation of 103 suspected cases of intoxications with psychoactive plant materials. Clin Toxicol (Phila) 2009;47:566–572. doi: 10.1080/15563650903037181. [DOI] [PubMed] [Google Scholar]
- [100].Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by beta-carboline derivatives. Arch Biochem Biophys. 1997;337:137–142. doi: 10.1006/abbi.1996.9771. [DOI] [PubMed] [Google Scholar]
- [101].Monsef HR, Ghobadi A, Iranshahi M, Abdollahi M. Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J Pharm Pharm Sci. 2004;7:65–69. [PubMed] [Google Scholar]
- [102].Hilber P, Chapillon P. Effects of harmaline on anxiety-related behavior in mice. Physiol Behav. 2005;86:164–167. doi: 10.1016/j.physbeh.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [103].Moura DJ, Rorig C, Vieira DL, Henriques JA, Roesler R, Saffi J, Boeira JM. Effects of beta-carboline alkaloids on the object recognition task in mice. Life Sci. 2006;79:2099–2104. doi: 10.1016/j.lfs.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [104].Hutchinson D, Ho V, Dodd M, Dawson HN, Zumwalt AC, Schmitt D, Colton CA. Quantitative measurement of postural sway in mouse models of human neurodegenerative disease. Neuroscience. 2007;148:825–832. doi: 10.1016/j.neuroscience.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80. doi: 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- [106].Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48:761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [107].Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48:689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [108].Narimatsu S, Yonemoto R, Saito K, Takaya K, Kumamoto T, Ishikawa T, Asanuma M, Funada M, Kiryu K, Naito S, Yoshida Y, Yamamoto S, Hanioka N. Oxidative metabolism of 5-methoxy-N,N-diisopropyltryptamine (Foxy) by human liver microsomes and recombinant cytochrome P450 enzymes. Biochem Pharmacol. 2006;71:1377–1385. doi: 10.1016/j.bcp.2006.01.015. [DOI] [PubMed] [Google Scholar]
- [109].Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60:1260–1267. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- [110].Gonzalez FJ, Yu AM. Cytochrome P450 and Xenobiotic Receptor Humanized Mice. Annu Rev Pharmacol Toxicol. 2006;46:41–64. doi: 10.1146/annurev.pharmtox.45.120403.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lin JH. Applications and limitations of genetically modified mouse models in drug discovery and development. Curr Drug Metab. 2008;9:419–438. doi: 10.2174/138920008784746355. [DOI] [PubMed] [Google Scholar]
- [112].Yu AM, Idle JR, Krausz KW, Kupfer A, Gonzalez FJ. Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. J Pharmacol Exp Ther. 2003;305:315–322. doi: 10.1124/jpet.102.047050. [DOI] [PubMed] [Google Scholar]
- [113].Wu C, Jiang XL, Shen HW, Yu AM. Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochem Pharmacol. 2009;78:617–624. doi: 10.1016/j.bcp.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]