Abstract
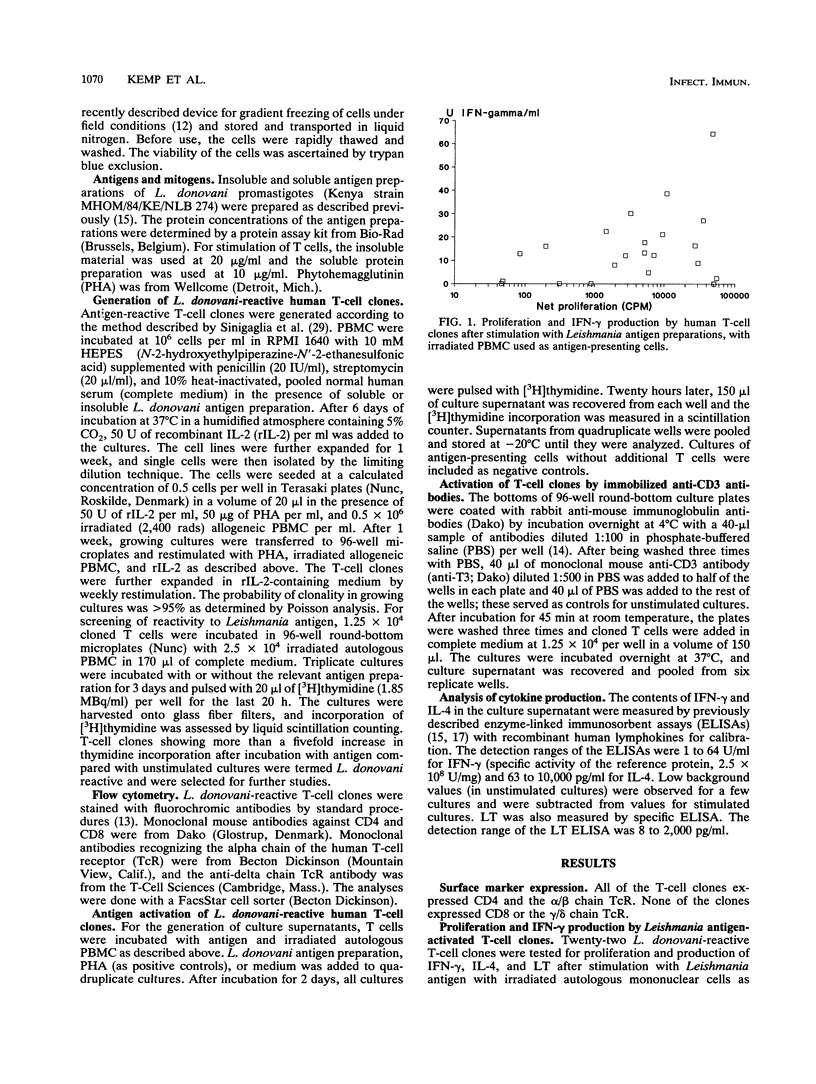
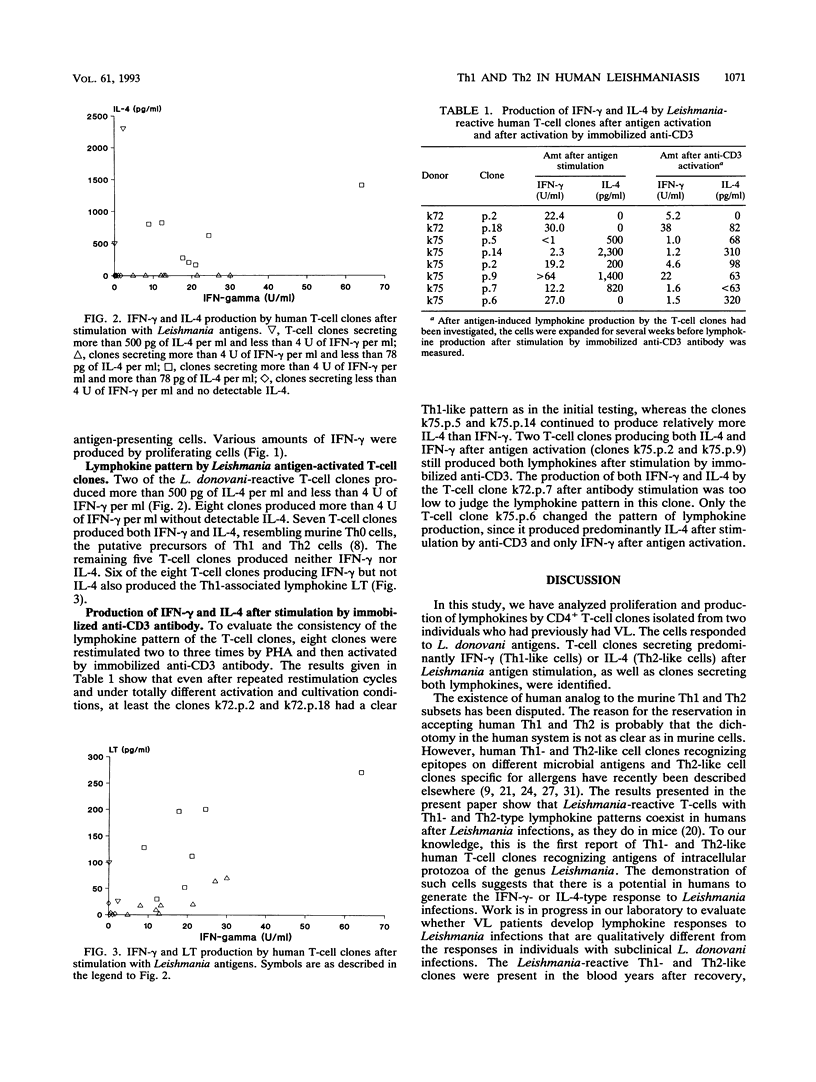
Infections in humans by Leishmania donovani parasites can result in a fatal disease, visceral leishmaniasis (VL), or in a self-limiting asymptomatic infection. In murine models of the infection employing Leishmania major, the course of the disease can be directed into a VL-like syndrome by interleukin-4 (IL-4)-producing Th2 cells, or cure may result by Th1 cells secreting gamma interferon (IFN-gamma). The present study examined the potential of human T cells to generate Th1 or Th2 responses to L. donovani. The profiles of IFN-gamma, IL-4, and lymphotoxin secretion after antigen stimulation were analyzed in a panel of L. donovani-reactive CD4+ human T-cell clones generated from individuals who had recovered from VL after antimonial treatment. Two of the T-cell clones produced large amounts of IL-4 without production of IFN-gamma, seven clones produced both IFN-gamma and IL-4, and eight produced only IFN-gamma. This is the first report of a Th1- and Th2-type response in human leishmaniasis. These results suggest that in analogy with murine models, there is a dichotomy in the human T-cell response to L. donovani infections. Preferential activation of IL-4-producing Th2-like cells may be involved in the exacerbation of human VL, whereas activation of IFN-gamma-producing Th1 cells may protect the host from severe disease. Identification of leishmanial antigens activating one or the other type of T cells will be important in the development of vaccines against leishmaniasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akuffo H. O., Britton S. F. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992 Jan;87(1):58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaro R., Falcoff E., Badaro F. S., Carvalho E. M., Pedral-Sampaio D., Barral A., Carvalho J. S., Barral-Netto M., Brandely M., Silva L. Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med. 1990 Jan 4;322(1):16–21. doi: 10.1056/NEJM199001043220104. [DOI] [PubMed] [Google Scholar]
- Berenguer J., Moreno S., Cercenado E., Bernaldo de Quirós J. C., García de la Fuente A., Bouza E. Visceral leishmaniasis in patients infected with human immunodeficiency virus (HIV). Ann Intern Med. 1989 Jul 15;111(2):129–132. doi: 10.7326/0003-4819-111-2-129. [DOI] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Guerrero M. L., Aguado J. M., Buzón L., Barros C., Montalbán C., Martín T., Bouza E. Visceral leishmaniasis in immunocompromised hosts. Am J Med. 1987 Dec;83(6):1098–1102. doi: 10.1016/0002-9343(87)90948-x. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday B. J., Sadick M. D., Wang Z. E., Reiner S. L., Heinzel F. P., Parslow T. G., Locksley R. M. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991 Sep 1;147(5):1653–1658. [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Hviid L., Sørensen A. L., Kharazmi A., Theander T. G. Functional and phenotypic changes in human lymphocytes after coincubation with Leishmania donovani in vitro. Infect Immun. 1990 Oct;58(10):3163–3167. doi: 10.1128/iai.58.10.3163-3167.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M., Hansen M. B., Theander T. G. Recognition of Leishmania antigens by T lymphocytes from nonexposed individuals. Infect Immun. 1992 Jun;60(6):2246–2251. doi: 10.1128/iai.60.6.2246-2251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzhals J. A., Hey A. S., Theander T. G., Odera E., Christensen C. B., Githure J. I., Koech D. K., Schaefer K. U., Handman E., Kharazmi A. Cellular and humoral immune responses in a population from the Baringo District, Kenya to Leishmania promastigote lipophosphoglycan. Am J Trop Med Hyg. 1992 Apr;46(4):480–488. doi: 10.4269/ajtmh.1992.46.480. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Lohoff M., Sommer F., Solbach W., Röllinghoff M. Coexistence of antigen-specific TH1 and TH2 cells in genetically susceptible BALB/c mice infected with Leishmania major. Immunobiology. 1989 Oct;179(4-5):412–421. doi: 10.1016/S0171-2985(89)80045-2. [DOI] [PubMed] [Google Scholar]
- Maggi E., Biswas P., Del Prete G., Parronchi P., Macchia D., Simonelli C., Emmi L., De Carli M., Tiri A., Ricci M. Accumulation of Th-2-like helper T cells in the conjunctiva of patients with vernal conjunctivitis. J Immunol. 1991 Feb 15;146(4):1169–1174. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. S., Fish D., Golden R., Evans D. A., Bryceson A. D., Pinching A. J. Visceral leishmaniasis in HIV infection and AIDS: clinical features and response to therapy. Q J Med. 1990 Nov;77(283):1101–1111. doi: 10.1093/qjmed/77.2.1101. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Sinigaglia F., Matile H., Pink J. R. Plasmodium falciparum-specific human T cell clones: evidence for helper and cytotoxic activities. Eur J Immunol. 1987 Feb;17(2):187–192. doi: 10.1002/eji.1830170206. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Weinbaum F. I., Herrod H. R. Characterization of in vitro proliferative responses of human lymphocytes to leishmanial antigens. J Infect Dis. 1979 Aug;140(2):215–221. doi: 10.1093/infdis/140.2.215. [DOI] [PubMed] [Google Scholar]
- Yssel H., Shanafelt M. C., Soderberg C., Schneider P. V., Anzola J., Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991 Sep 1;174(3):593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwingenberger K., Harms G., Pedrosa C., Omena S., Sandkamp B., Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin Immunol Immunopathol. 1990 Nov;57(2):242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]



