Abstract
Benign transient hyperphosphatasia (BTH) is a condition that occurs mainly in infants and children and is characterised by a transient increase of serum alkaline phosphatase (ALP) activity up to several fold the adult upper reference limit (URL). The present report concerns BTH in 2 patients, aged 59 and 52 years old, who showed no evidence of bone or liver disease but had an increase in ALP activity up to 20-fold and 13-fold the adult URL, respectively. The diagnosis of BTH in the first case was made retrospectively, and after excluding liver and bone disease. However, in the second case the diagnosis was made early in the course of the disease, by performing an ALP isoenzyme electrophoresis test. Lengthy and extensive investigations were avoided in the second case. These cases highlight the occurrence of this condition in adulthood as well as in infancy and childhood.
Background
Benign transient hyperphosphatasia (BTH) is a disorder characterised by transient marked increases in alkaline phosphatase (ALP) activity in the absence of bone or liver disease. It was first reported by Bach in 1954 as a condition occurring in infancy and early childhood only.
The aetiology of BTH remains unclear. However, a number of theories have been put forward to explain the transient rise in ALP activity. Firstly, increased hepatic ALP synthesis as part of the acute phase reaction1–3 was postulated, as infectious diseases have accompanied some cases of BTH.4–6 However, BTH has also been observed in healthy subjects and during the course of a wide variety of clinical disorders.2,4 Secondly, decreased clearance of ALP from circulation due to increased sialation of the enzyme is the most probable mechanism, although the cause of the altered sialation in BTH remains uncertain. This possibility is supported by the abnormal sialation of liver and bone isoenzyme fractions reported by Crofton.7
The prevalence of BTH in infants is unknown. In 1966, Asanti et al studied ALP in 260 healthy infants and 1.5% had an unexplained transient rise in ALP activity to more than 3 times the upper reference limit (URL) for the assay. In the acute medical setting, Parker studied a population of 204 adults and found that 32% of the cases of hyperphosphatasia were attributed to BTH. However in these cases the elevation in ALP was less than three times the upper reference limit.8 In 2001 this prevalence was supported by a similar finding in acutely ill children.9 This transient raised ALP is often found incidentally during routine biochemical investigations. The aim of this case report is to present two new cases of BTH in adults and to suggest criteria for the diagnosis of this condition at an early stage to avoid performing expensive and possibly invasive tests.
Case presentation
Case 1
A 59-year-old woman attended her general practitioner (GP) surgery for a routine check-up. She was found to have grossly elevated serum ALP activity at 5206 IU/litre (relative risk (RR): 70–300 IU/litre) but other liver function tests (bilirubin, alanine transaminase, γ-glutamyltransferase) were within reference limits. This result was confirmed 2 days later (6003 IU/litre). Subsequent isoenzyme analysis using a heat stability method showed an almost equal increase in liver and bone isoenzyme activities. Serum protein electrophoresis showed an acute phase response at the time of her raised ALP.
In view of her raised ALP, the patient was referred to a hepatologist and subsequently to the bone clinic. The patient had a history of osteoarthritis, hypothyroidism, hypertension and insulin dependent diabetes. She had not travelled abroad recently and there had been no known contact with anyone with an infectious liver disease. Her medication consisted of omeprazole, valsartan, atrovastatin, fluoxetine, indapamide, colacoxib, insulatard and thyroxine (75 μg/day), none of which are known to be associated with drug-induced cholestasis.
On the basis of physical examination and abdominal ultrasound there was no evidence of chronic liver disease. She had no bone deformity or tenderness. Chest x ray, a radionucleotide bone scan and skeletal survey showed no evidence of bone lesions. Initial biochemical investigations showed a normal adjusted calcium concentration at 2.29 mmol/litre (range 2.2–2.65), an appropriate parathyroid hormone concentration of 3.2 pmol/litre (range 1.5–7.6 in normocalcaemia) and a normal serum concentration of 25-hydroxy vitamin D of 77 nmol/litre. Her full blood count profile was also normal.
Anti-nuclear, anti-mitochondrial, gastric parietal, liver kidney microsomal antibodies and smooth muscle antibodies were negative and IgG, IgM and IgA were within reference limits. Microbiological investigations, including the hepatitis B antigen and hepatitis C antibodies, were negative.
The patient was reviewed at 5 and 11 weeks when ALP measurements were 365 IU/litre and 230 IU/litre, respectively.
Case 2
A 52-year-old woman with polyarticular psoriatic arthritis and large plaque skin psoriasis had a routine blood test for methotrexate (MTX) monitoring. It showed raised ALP activity (1624 IU/litre) with normal, bilirubin, alanine transaminase, γ-glutamyltransferase, calcium, phosphate and albumin. In view of the raised ALP, the GP referred the patient urgently to the rheumatology clinic.
In the rheumatology clinic, the patient showed no joint pain, no bone deformity or tenderness, no abdominal pain or other symptoms that indicate a liver pathology. In addition to MTX 20 mg weekly orally she took folic acid 5 mg six times a week and ciclosporin A 75 mg daily.
There was no other medical history of note. On direct questioning there was, however, a history of a flu vaccination (fluarix) given 24 h before the blood tests. The patient recalled being “fluish” before the flu jab and finding the injection very uncomfortable with pain during and after the procedure. On physical examination in the rheumatology clinic (8 days after injection) there was a red, indurated, mildly tender lesion on the upper left arm. Apart from a generalised flare of her cutaneous psoriasis, which was put down to her general state of anxiety after being told about the abnormal blood tests, there were no other positive findings of note.
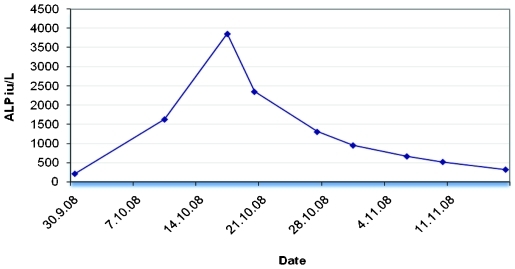
Repeat biochemical testing by the rheumatologist revealed a further increase in ALP activity to 3856 IU/litre with electrolytes, liver function test and calcium within reference ranges. ALP activity measured 1 week before the first rise was within normal limits (ALP 204 IU/litre). In view of this ALP isoenzyme determination by heat stability and electrophoresis were performed. Heat stability ALP isoenzymes for bone and liver were 1588 IU/litre and 2268 IU/litre, respectively. The electrophoretic pattern was typical of BTH, (fig 1). Further investigations were postponed and the patient had regular ALP determinations which showed ALP activity gradually return to the baseline 5 weeks after the peak rise in ALP activity (fig 2).
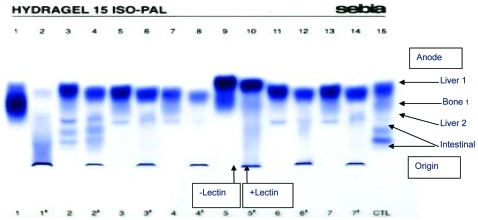
Figure 1.
Alkaline phosphatase (ALP) electrophoresis (EP). In normal serum, two or three distinct bands can be identified: a liver band which moves rapidly towards the anode; a diffuse slower running bone band; an intestinal band (when present) lying behind the bone band. Lectin is use to retard sialated forms (usually bone). Tracks 5 and 6 show EP without (track 5) and with (track 6) lectin for a normal serum. Tracks 9 (no lectin) and 10 (with lectin) show EP for patient 2. In benign transient hyperphosphatasia (BTH), the liver ALP isoenzyme EP mobility is increased (track 9) but with lectin the increased sialation slows mobility (track 10).
Figure 2.
The full duration of the rise and decrease of alkaline phosphatase (ALP) activity in the second case.
Discussion
ALP is a glycoprotein that consists of four isoenzymes. These isoenzymes are the products of four gene loci. These are: (1) a tissue non-specific isoenzyme found in the liver, bone and kidney; (2) placenta; (3) intestine; (4) placental-like germ cells. The serum activity of ALP varies among healthy individuals according to age, gender and pregnancy status. Serum activity increases modestly during the first year of life and up to twofold to threefold of the URL for adults at puberty. In the adult, bone and liver isoenzymes contribute equally to the total enzyme activity but in children the bone isoenzyme predominates.
The hyperphosphatasia in our first patient had the same features of transient hyperphosphatasia that are classically reported in children and more recently reported in adults. Namely, a transient marked rise of serum ALP with absence of any clinical, biochemical or radiological evidence of liver or bone diseases. In agreement with the reported cases of BTH, ALP activity in the first patient was about 20-fold the URL for adults at the time of presentation, and normalised at about 5–6 weeks. Increased ALP activity in BTH can vary from 3 to 53 URL of adults.1,2,10,11 This variability may be attributed to the timing of the ALP sample in relation to the peak ALP activity.
The full duration of the rise of ALP activity in BTH has only been documented in a few patients and it has been found that ALP peaks at about 6 weeks and normalises at about 12 weeks.5,12 In our first case, we coincidentally made the second ALP measurement near to the probable peak time; ALP was almost normal at 5–6 weeks (365 IU/litre) and was completely normal at 11 weeks (230 IU/litre). However, in the second case the whole duration from the first rise of ALP activity until it returned to baseline again was 7–8 weeks. ALP peaked at 2 weeks and returned to normal within 5 weeks from the peak time.
In healthy people, the half-life of ALP varies according to the type of isoenzyme. The half life of the liver ALP is 5–9 days and it is 1.5–2.3 days for bone ALP.13 In our patients, ALP half-lives estimated from the declining phase of ALP were 8.2 days for first and 9.4 days for the second patient. These half-lives are within the range of those reported previously which ranged from 5–25 days depending on coexisting morbidities.7,12 In the second case in this report the half-life was calculated from several ALP measurements in the declining phase. ALP half-life varied from 4.5 days at around the peak time and progressively increased to 14 days when ALP declines towards the baseline (fig 2).
Most patients presenting with BTH are healthy; however, BTH has coexisted with a variety of conditions, such as gastroenteritis, respiratory infection, viral infections, enteroviruses1,14 in children receiving chemotherapy for leukaemia and lymphoma7 and following transplant.15 BTH in this report was associated with a viral infection in the first case and flu vaccination in the second case.
ALP isoenzyme identification and quantification is performed using methods including electrophoresis, heat stability and high pressure liquid chromatography (HPLC). In our laboratory we use the heat stability method and in both cases this showed a rise in liver and bone isoform activities. This finding is consistent with the most probable postulated mechanism of the disease, which relates the increase in ALP activity to increased sialation of both isoenzymes, which in turn decreases their clearance rate.4 Increased sialation of ALP does not alter the heat stability of liver or bone isoenzymes;11 however, the electrophoretic mobility of the liver isoenzyme is affected. Therefore, the diagnostic test for BTH is isoenzyme analysis using serum electrophoresis to demonstrate an enhanced liver mobility compared to sera from patients with normal ALP activity. Unfortunately, in our first case, by the time investigations to exclude other causes for raised ALP activity were completed and the diagnosis of BTH became more likely, the patient’s ALP had returned to normal. We were therefore unable to perform serum ALP electrophoresis. However, when the diagnosis of BTH was suspected in the second patient, ALP isoenzyme by electrophoresis was performed early and unnecessary investigations avoided.
It has been suggested that the name for this condition should be changed from benign transient hyperphosphatasia of infancy to benign transient hyperphosphatasia, to include cases reported in children and adults. However, to date, only 10 adult cases have been reported. These are summarised in table 1.The two cases reported here highlight the possibility of this condition in adults as well as in children and perhaps the proposal to change the name of this condition to allow the inclusion of adult cases should be revisited.
Table 1.
Previously reported adult cases of benign transient hyperphosphatasia
| Age, years | Gender | Coexisting disease | Reference |
| 29 | Male | Immune deficiency | Onica et al10 |
| 34 | Male | Post renal transplant | Ilham et al15 |
| 47 | Female | Post renal transplant | Ilham et al15 |
| 45 | Female | Post renal transplant | Ilham et al15 |
| 21 | Male | Crohn disease | Rosalki et al16 |
| 55 | Female | Malignant lymphoma | Maekawa et al17 |
| 58 | Male | Rhabdomyolysis | Chisholm et al18 |
| 32 | Male | HIV/chronic hepatitis | Trower et al19 |
| 19 | Male | Upper respiratory infection | Rosalki et al20 |
| 27 | Female | Not available | Schambeck et al21 |
| 59 | Female | Viral infection | This report |
| 52 | Female | Viral infection/flu vaccination | This report |
Where electrophoretic ALP isoenzyme analysis is not available, BTH is a diagnosis of exclusion. However, the diagnosis of BTH can be suspected even in the absence of electrophoresis by the following findings: (1) a marked rise in ALP activity more than threefold URL in patients from all age groups, (2) absence of symptoms or biochemical evidence related to bone or liver diseases, (3) a steady decline in ALP activity that returns to baseline in about 12 weeks. When comorbidities exist, determination of ALP isoenzyme by electrophoresis becomes essential to make the diagnosis of BTH. The electrophoretic isoenzyme pattern characteristic of BTH consists of two bands of increased activity; one band of a homogenous compact appearance with biochemical characteristics of liver origin but with enhanced anodal mobility, and a second diffuse band with characteristics and mobility of bone ALP. The different mobility of the liver ALP band from that of a normal liver is due to increased sialation. The diagnosis is confirmed by the ALP activity and/or electrophoresis isoenzyme pattern returning to normal within 3–4 months.
Learning points
Alkaline phosphatase (ALP) level is a non-specific test that fails to identify the pathological origin of any increase in its activity and it is likely that a finding of an isolated raise in ALP activity will trigger several investigations.
It is important to recognise that benign transient hyperphosphatasia (BTH) of infancy may also occur in adults, to avoid carrying out unnecessary testing that can be expensive and may promote patient anxiety and unnecessary exposure to radiation.
Once BTH is suspected, it is important to request ALP isoenzyme analysis by electrophoresis as this is the only method that can identify the electrophoretic pattern of ALP isoenzymes that is diagnostic of BTH.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Rosalki SB, Foo AY, Went J, et al. Transient hypophosphatasemia of infancy and childhood in an adult. Clin Chem 1991; 37: 1137–8 [PubMed] [Google Scholar]
- 2.Brensilver HL, Kaplan MM. Significance of elevated liver alkaline phosphatase in serum. Gastroenterology 1975; 68: 1556–62 [PubMed] [Google Scholar]
- 3.Asanti R, Hutin H, Visakorpi JK. Serum alkaline phosphates in healthy infants, occurrence of abnormally high values without known cause. Ann Paediatr Fenn 1966; 12: 139. [PubMed] [Google Scholar]
- 4.Stein P, Rosalki A, Foo Y, et al. Transient hyperphosphatasemia and early childhood: clinical and biochemical features of 21 cases and a literature review. Clin Chem 1987; 33: 313–18 [PubMed] [Google Scholar]
- 5.Holt PA, Steel AE, Armstrong AM. Transient hyperphosphatasaemia of infancy following rotavirus infection. J Infect 1984; 9: 283–5 [DOI] [PubMed] [Google Scholar]
- 6.Fennoy I, Laraque D. Benign transient hyperphosphatasia and HIV infection. Clin Pediatr 1989; 28: 180–4 [DOI] [PubMed] [Google Scholar]
- 7.Crofton PM. What is the cause of benign transient hyperphosphatasemia? A study of 35 cases. Clin Chem 1988; 34: 335–40 [PubMed] [Google Scholar]
- 8.Parker SG. Transient hyperphosphatasaemia in association with acute infection in adults. Postgrad Med J 1991; 67: 638–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caroll AJ, Coakley JC. Transient hyperphosphatasaemia: an important condition to recognize. J paediatr. Child Health 2001; 37: 359–62 [DOI] [PubMed] [Google Scholar]
- 10.Onica D, Trassander J, Waldenlind L. Recurrent transient hyperphosphatasia of infancy in an adult. Clin Chem 1992; 38: 1913–15 [PubMed] [Google Scholar]
- 11.Rosalki SB, Foo AY. More on transient hyperphosphatasemia of infancy. Clin Chem 1983; 29: 723. [PubMed] [Google Scholar]
- 12.Nathan E. Transient hyperphosphatasemia of infancy. Acta Paediatr Scand 1980; 69: 235–8 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg DM, Moss DW, Schmidt E, et al. Enzymes - tools and targets (advances in clinical enzymology). 6th edn Basel, Switzerland: Karger, 1988 [Google Scholar]
- 14.Rosalki SB, Foo AY. Transient hyperphosphatasemia of infancy: four new cases, and a suggested etiology. Clin Chem 1980; 26: 1109–10 [PubMed] [Google Scholar]
- 15.Ilham MA, Cookson A, Dheerendra A, et al. Idiopathic severe elevation of serum alkaline phosphatase following adult renal transplantation: case reports. Transplant Proc 2008; 40: 2059–61 [DOI] [PubMed] [Google Scholar]
- 16.Rosalki SB, Hurst NP. Transient presence in serum of an atypical alkaline phosphatases. Clin Chem Acta 1976; 73: 149–55 [DOI] [PubMed] [Google Scholar]
- 17.Maekawa M, Sugiura K, Azuma Y, et al. Benign transient hyperphosphatasemia in an adult with malignant lymphoma. Clin Chem 1989; 35: 897. [PubMed] [Google Scholar]
- 18.Chisholm JC. Transient benign, serum alkaline hyperphosphatasia in an adult. J Natl Med Assoc 1986; 78: 338–41 [PMC free article] [PubMed] [Google Scholar]
- 19.Trower K, Wilmot R, Barlow G, et al. A case of transient hyperphosphatasemia of infancy and early childhood in an HIV-positive adult possibly related to atazanavir. AIDS 2006; 20: 135–6 [DOI] [PubMed] [Google Scholar]
- 20.Rosalki SB, Foo AY, Went J, et al. Transient hyperphosphatasemia of infancy and childhood in an adult. Clin Chem 1991; 37: 1137–8 [PubMed] [Google Scholar]
- 21.Schambeck CM, Koppa A, Mora-Maza G, et al. Transient alkaline hyperphosphatasaemia in an adult: biochemical peculiarities. Eur J Clin Chem Clin Biochem 1997; 35: 441–4 [DOI] [PubMed] [Google Scholar]