Abstract
Understanding the actions performed by other people is a key aspect of social interaction, including in clinical settings where patients are learning from therapists and caregivers. While lesions of the left cerebral hemisphere induce praxic disorders, the hemispheric specialisation of intention understanding remains unclear. Do patients with a right hemispheric lesion understand the intentions of other people properly? The present study investigates how a split-brain patient understands the means (what) and intentions (why) of the actions of other people. Results show a significant left hemispheric dominance for understanding what is done, and a significant right hemispheric dominance for understanding why an action is carried out. This discovery might have important clinical implications in neurological patients, especially when those with right hemisphere lesions are faced with important decisions related to the interpretation of other’s intentions.
BACKGROUND
Understanding the actions and intentions of others is a key aspect of social interaction, notably in clinical settings where patients are trying to learn from therapists and caregivers. However, one cannot access other’s intentions directly; information is only available from observing their actions and surrounding contextual environment.1 The classical view on how individuals understand other’s intentions suggests we use automatic cognitive reasoning to evaluate other’s actions and compare them with similar representations stored from past self-related experiences.1–6 Along these lines, individuals can know what others are doing (eg, grasping a bottle of water) and also why (eg, to drink).1,2,7 While lesions of the left cerebral hemisphere induce praxic disorders, the hemispheric specialisation and neural substrates of intention understanding is unclear. Localisation might be within the human mirror neuron system (MNS), a bilateral parietal and inferior frontal network activated during action execution, observation and imitation.1,4,5,7 Many brain imaging studies of action understanding suggest that understanding actions in terms of objects or the details of a movement are mostly lateralised to the left MNS.1,4 This activation is mostly related to the what of motor acts.4,8,10 In contrast, a few neuroimaging studies suggest a right MNS involvement for understanding other’s intentions (the why of an action).1,2,4,7,9,11 If this right-lateralised specialisation is correct, one would expect hemispheric differences in neurological patients. To date, however, no case has been reported to reinforce this left-right hemispheric dissociation between the what and why of actions. Split-brain patients offer the unique opportunity to examine the specialisation of intention understanding within each hemisphere independently from each other.12
CASE PRESENTATION
Here, we describe a 55-year-old right-handed woman who underwent a complete resection of her corpus callosum for intractable epilepsy in 1979.13,14 A follow-up MRI in 1984 revealed spared fibres in the rostrum and splenium. The spared rostral fibres comprised ∼1.8% of the total cross-sectional area of the corpus callosum and the spared splenial fibres comprised ∼1% of the area.14 The spared fibres did not allow much inter-hemispheric communication in the higher-order cognitive domain.13,14 Post-operative neurological examination revealed no focal deficits.13,14 Wechsler IQ scores were in the normal range.13 Although the patient lacked any obvious impairment of intention understanding in daily life, a deficit could be elicited when she performed a specific neuropsychological task in a randomised repeated design.
INVESTIGATIONS
The patient’s ability to understand other’s intentions was investigated using a means inference task (MIT) and an intention inference task (IIT). During these two tasks, the same stimuli were used; only the instruction was different. In both tasks, the patient observed one 500 ms frame from video clips displaying a scene including two daily objects (eg, a glass and a bottle of water, a hammer and a nail, or a lighter and a candle) followed by a second 1000 ms frame showing a hand grasping one of these two objects (eg, the bottle of water, the hammer or the lighter) and then a third 1000 ms frame displaying either a correct (eg, to pour water into the glass, to hammer the nail, or to light up the wick of the candle) or incorrect (to pour water outside the glass, to hammer near the nail, or to light up the bottom of the candle) outcome. Then, the patient was either asked to guess as rapidly and as accurately as possible if the means (what) of the agent’s actions were correct (MIT) or if the intentions (why) of the agent were correct (IIT). More precisely, for MIT, the instruction was as follows: “During this task, you will have to press the YES or NO button to indicate if the grasp (useful hand–object interaction) of each agent’s action is correct or not”. For IIT, the instruction was as follows: “During this task, you will have to press the YES or NO button to indicate if the outcome of each agent’s action is correct or not”. Each trial began with a fixation cross that was presented for 150 ms. A 5000 ms maximum inter-trial interval separated the onset of each movie presentation. The tasks were run using JAVA on a MacBook Pro laptop computer with a 17-inch colour monitor located 70 cm from the patient. There were eight blocks in total. Thirty video clips were presented per block. The patient thus watched a total of 240 video clips (30×8). The order of the tasks was randomised and pre-determined according to an ABBA design. Responses were collected from two response keys that were randomly attributed across blocks. Prior to participation, the patient provided written informed consent. The study was approved by the Ethical Committee of the University of California, Santa Barbara.
Analysis of accuracy did not reveal any significant differences (p>0.05). During MIT, similar accuracy rates were observed with the right hand/left hemisphere (mean (SD): 81% (11.55%)) and the left hand/right hemisphere (mean (SD): 73% (10.99%); p>0.05). During IIT, similar accuracy rates were observed with the right hand/left hemisphere (mean (SD): 83% (8.52%)) and the left hand/right hemisphere (mean (SD): 86% (5.31%); p>0.05). No difference was observed as a function of the type of means or the agent’s intentions (correct/incorrect; p>0.05).
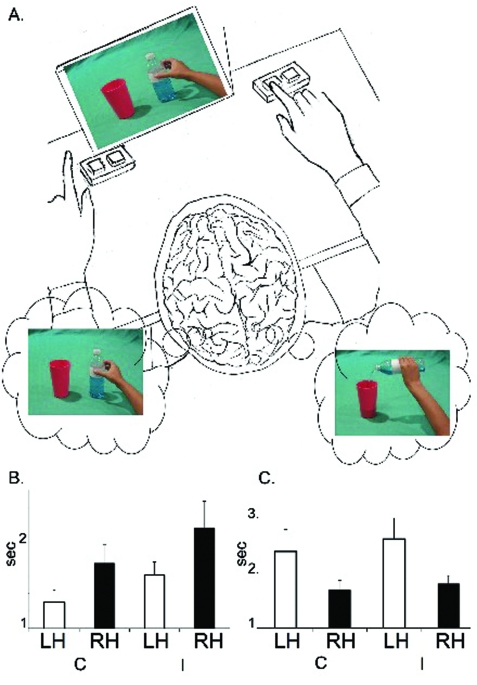
Interestingly, analysis of the patient’s reaction times revealed the following significant effects. During MIT, faster reaction times were observed with the right hand/left hemisphere (mean (SD): 1522 (161) ms) in comparison with the left hand/right hemisphere (mean (SD): 1963 (287) ms; F(1,17)=6.28; p=0.023). This left hemispheric advantage for the what was independent of the type of means (correct/incorrect). Faster reaction times were observed for right hand/left hemisphere for detecting both correct (mean (SD): 1300 (172) ms) and incorrect (mean (SD): 1623 (150) ms) action’s means in comparison with the left hand/right hemisphere (mean (SD): 1748 (268) ms for correct; mean (SD): 2178 (306) ms for incorrect; fig 1). Conversely, during IIT, slow reaction times (mean (SD): 2500 (390) ms) were collected from the right hand/left hemisphere, while faster reaction times were collected from the left hand/right hemisphere (mean (SD): 1750 (154) ms; F(1,22)=12.5; p=0.0019). This right hemispheric advantage for the why was independent of the type of the agent’s intentions (correct/incorrect). Faster reaction times (paired t test: p<0.05) were observed for left hand/right hemisphere when detecting both correct (mean (SD): 1695 (163) ms) and incorrect (mean (SD): 1805 (144) ms) intentions in comparison with the right hand/left hemisphere (mean (SD): 2386 (398) ms for correct; mean (SD): 2614 (381) ms for incorrect). Thus it is suggested that the reaction-time measurement is a good indicator of the level of patients’ decoding of the actions and intentions of other people. Notably, the right hemisphere shows a greater sensitivity for understanding the why of an observed action, while the left hemisphere is more sensitive to understanding the what of an observed action.
Figure 1.
(A) Graphic representation of the experimental design with stimuli. (B) Reaction times obtained during the means inference task (MIT) for both types of action’s means (C, correct; I, incorrect) and for both hemispheres (LH, left hemisphere; RH, right hemisphere). (C) Reaction times obtained during the intention inference task (IIT) for both types of agent’s intentions (C, correct; I, incorrect) and for both hemispheres (LH, left hemisphere; RH, right hemisphere).
OUTCOME AND FOLLOW-UP
By demonstrating the role of the right hemisphere in understanding the intentions of other people’s actions and the left hemisphere in evaluating how actions are performed, the present case report suggests there are hemispheric differences of action understanding. This might have important clinical implications in how rehabilitation therapy is designed for neurological patients with right or left brain lesions. The systematic screening for intention or means understanding using our task in patients with either right or left brain damage might be helpful to detect those who struggle with understanding the purpose of the actions performed on them by medical staff or therapists. The prediction would be that patients with right hemisphere lesions would benefit from instructions or explanations focused on action means and would have difficulty inferring why a person is performing a particular act. In contrast, patients with left hemisphere lesions would benefit from instructions related to what the therapist or caregiver is trying to do but would have difficulty understanding specific movements, gestures or actions.
Along these lines, we propose future clinical studies should systematically investigate intention understanding skills in neurological patients with a focal brain damage and/or inter-hemispheric disorder in order to better understand the patients’ ability to understand the actions and intentions of other people during (and also after) hospitalisation.
DISCUSSION
By demonstrating the first clinical evidence of a dissociate neural network between the understanding of the what and why of other’s actions and intentions, our case report opens a new avenue to the understanding of the social active brain in clinical settings. Up until now, the lack of assessment of this mechanism in clinical settings led to the absence of neurological reports regarding the hemispheric representation of the what and why of actions and intentions. Thus, the present double dissociation between left and right brain highlights the dynamics of hemispheric specialisation and integration in the context of goal-directed behaviors.15 This reinforces the idea that the functional participation of both hemispheres in understanding goal-directed behaviour is not fixed but based on versatile dynamics between the hemispheres.15,16 Clinically, our results may have important implications in neurological patients, especially when those with right hemisphere lesions are faced with important decisions related to the interpretation of other’s intentions.
LEARNING POINTS
The way people understand what others are doing and why they are acting calls for different neural networks in the human brain.
The left hemisphere is dominant for understanding the what of others’ actions, while the right hemisphere is dominant for understanding the why of others’ actions.
A focal cerebral lesion limited to the right or left hemisphere might have important consequences for the patient’s social life, and also for their ability to fully understand medical staff or therapists during (and/or after) their hospitalisation.
For clinicians, it may be important to evaluate the severity of action understanding deficits in patients with a focal right or left hemispheric lesions.
The rapid detection of action understanding deficits is a challenge for medical personal and may be important for tailoring dialogues and therapy to a patient level of understanding and for establishing if a patient has the ability to understand medical acts and their possible outcomes.
Acknowledgments
The authors thank Professor Giacomo Rizzolatti for helpful discussions. We also thank Catherine Chang for her drawing, and Nisa Patel for technical assistance.
This study was supported by NIH #R01NS031443-10A2 to MBM and MG, and grant DAAD19-03-D-0004 to STG.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Rizzolatti G, Cragheiro L. The mirror neuron system. Annu Rev Neurosci 2004; 27: 169–92 [DOI] [PubMed] [Google Scholar]
- 2.Grafton ST, Hamilton A. Evidence for a distributed hierarchy of action representation in the brain. Hum Mov Sci 2007; 26: 590–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci 2007; 11: 194–6 [DOI] [PubMed] [Google Scholar]
- 4.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 2006; 7: 942–51 [DOI] [PubMed] [Google Scholar]
- 5.Rizzolatti R, Sinigaglia C. Mirror neurons and motor intentionality. Funct Neurol 2007; 22: 205–10 [PubMed] [Google Scholar]
- 6.Cattaneo L, Fabbri-Destro M, Boria S, et al. Impairment of actions chains in autism and its possible role in intention understanding. Proc Natl Acad Sci U S A 2007; 104: 17825–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iacoboni M, Molnar-Szakacs I, Gallese V, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol 2005; 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz-Zadeh L, Koski L, Zaidel E, et al. Lateralization of the human mirror neuron system. J Neurosci 2006; 26: 2964–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lange FP, Spronk M, Willems RM, et al. Complementary systems for understanding action intentions. Curr Biol 2008; 18: 454–7 [DOI] [PubMed] [Google Scholar]
- 10.Aziz-Zadeh L, Maeda F, Zaidel E, et al. Lateralization in motor facilitation during action observation: a TMS study. Exp Brain Res 2002; 144: 127–31 [DOI] [PubMed] [Google Scholar]
- 11.Aziz-Zadeh L, Iacoboni M, Zaidel E, et al. Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci 2004; 19: 2609–12 [DOI] [PubMed] [Google Scholar]
- 12.Gazzaniga M. Forty-five years of split-brain research and still going strong. Nat Rev 2005; 6: 653–9 [DOI] [PubMed] [Google Scholar]
- 13.Gazzaniga M, Nass R, Reeves A, et al. Neurologic perspectives on right hemisphere language following surgical section of the corpus callosum. Sem Neurol 1984; 4: 126–35 [Google Scholar]
- 14.Funnell MG, Corballis PM, Gazzaniga MS. Insights into the functional specificity of the human corpus callosum. Brain 2000; 123: 920–6 [DOI] [PubMed] [Google Scholar]
- 15.Serrie DJ, Ivry R, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev 2006; 7: 160–7 [DOI] [PubMed] [Google Scholar]
- 16.Ivry R, Robertson L. The two sides of perception. Cambridge, MA: MIT Press, 1997 [Google Scholar]