Abstract
Backgound
Adiposity is associated with cystatin C. Cystatin C-based glomerular filtration rate (GFR) equations may result in the over-estimation of chronic kidney disease (CKD) prevalence at higher body mass index (BMI) levels.
Study Design
Cross-sectional
Setting and Participants
6,709 US adult NHANES III participants.
Factor
Body mass index
Outcome
Absolute percent difference in the prevalence of stage 3 or 4 CKD between creatinine- and cystatin C-based estimating equations by level of BMI.
Measurements
Normal weight, overweight, and obesity were defined as BMI levels of 18.5 to <25.0, 25 to <30.0, and ≥30 kg/m2, respectively. Stage 3 or 4 CKD (eGFR of 15 to 59 ml/min/1.73m2) was defined using the abbreviated creatinine-based Modification of Diet in Renal Disease equation (eGFRMDRD); a cystatin C, age, sex, and race equation (eGFRCysC,age,sex,race); a cystatin C only equation (eGFRCysC); cystatin C≥1.12 mg/L (elevated cystatin C); and an equation incorporating serum creatinine, cystatin C, age, sex, and race (eGFRCr,CysC,age,sex,race).
Results
The differences in stage 3 or 4 CKD prevalence between eGFRCysC,age,sex,race, eGFRCysC, and elevated cystatin C, separately, and eGFRMDRD were larger at higher BMI levels. Specifically, compared to estimates derived using eGFRMDRD, for normal weight, overweight, and obese participants, the prevalence of stage 3 or 4 CKD was 2.1%, 3.0%, and 6.5% higher, respectively, when estimated by eGFRCysC,age,sex,race (p-trend=0.005); 0.1%, 0.6%, 2.2% higher, respectively, for eGFRCysC (p-trend=0.028); 2.9%, 5.2%, and 9.5% higher, respectively, for elevated cystatin C (p-trend<0.001); and −0.1%, −0.4%, and 0.0% higher, respectively, for eGFRCr,CysC,age,sex,race (p-trend=0.719).
Limitations
No gold standard measure of GFR was available.
Conclusions
BMI may influence the prevalence of stage 3 or 4 CKD when cystatin C-based equations are used.
In the identification, classification, and treatment of adults with chronic kidney disease (CKD), it is crucial to accurately estimate glomerular filtration rate (GFR). 1 The most widely used measure of kidney function is GFR estimated using the creatinine-based Modification in Diet and Renal Disease (MDRD) study formula.2 However, limitations associated with the use of serum creatinine have prompted interest in the use of other biomarkers for measuring kidney function.3, 4 Results from several studies suggest serum cystatin C may provide a more accurate estimate of GFR than serum creatinine alone, or GFR estimated by the MDRD study equation, especially among adults with estimated GFR ≥60 ml/min/1.73m2.5, 6 Stevens et al. recently derived three equations that incorporate cystatin C, all of which equally or more accurately, estimated measured GFR compared to the MDRD study formula.7
An ideal biomarker for estimating GFR should be freely filtered by the glomerular membrane without being reabsorbed, secreted or metabolized by the renal tubules.8 Since cystatin C has a low molecular weight and is freely filtered by the glomerular membrane,9 and because it is independent of muscle mass, it has been suggested as a better measure of GFR. However, several studies have demonstrated an association between cystatin C and BMI10-12, even among individuals without CKD, raising the concern that cystatin C may be associated with adiposity independently of kidney function.13, 14 The mechanism of the association between cystatin C and overweight and obesity is not clear; however, laboratory evidence has suggested that adipocytes secrete cystatin C.15
The purpose of the current analysis was to compare the estimation of GFR and prevalence of stage 3 or 4 CKD by cystatin C and creatinine-based equations, across BMI categories. We hypothesized that, due to the association of cystatin C with adiposity, the difference in the prevalence of CKD using cystatin C versus creatinine-based equations would be larger at progressively higher BMI categories.
METHODS
The Third National Health and Nutrition Examination Survey (NHANES III) was conducted between 1988 and 1994. A detailed description of the study participants and methods has been published elsewhere.16 In brief, a stratified multi-stage probability design was employed to obtain a representative sample of the civilian non-institutionalized US general population. The study design included over-sampling of those who were very young, elderly, non-Hispanic black and Mexican-American to improve the precision of estimates in these groups.
Using stored serum specimens, cystatin C was measured on all NHANES III participants 60 years and older, as well as a random sample of those aged 12 to 59 years. Additionally, cystatin C was measured in all adult men and women with a serum creatinine ≥1.2 and 1.0 mg/dL, respectively. The current analysis was limited to adult NHANES III participants, aged 20 years or older, with serum cystatin C measurements. Participants were excluded from the current analysis if they were pregnant (n=71), missing a valid height and/or weight measurement (n=21), were underweight (BMI<18.5 kg/m2; n=133), or had an estimated GFR < 15 ml/min/1.73m2 (based on the MDRD study equation; n=17). After these exclusions, the current analyses were based on the experience of 6709 participants.
NHANES III data were collected by administration of a standardized questionnaire during a home interview, followed by the completion of a detailed physical examination with collection of blood specimens at a mobile examination center or the participant’s home. Of relevance to the current analysis, variables collected using questionnaires included age, race-ethnicity, sex, cigarette smoking, and leisure-time physical activity. Participants who reported having smoked more than 100 cigarettes during their lifetime and responded affirmatively to “Do you now smoke cigarettes?” were classified as current smokers. Being physically active was defined as participating in any leisure time physical activity or strength training in the month preceding the NHANES visit.
Body weight and height were measured according to a standard protocol. Height was measured with participants standing on the floor using a fixed stadiometer with a vertical backboard and movable headboard. Weight was taken by asking each participant to stand on the center of the platform of a Toledo digital scale while wearing underwear, a disposable gown, and foam slippers. BMI was calculated as weight in kilograms divided by height in meters squared and categorized into three categories (18.5 to <25 kg/m2 - normal weight; 25 to <30 kg/m2 – overweight; and ≥30 kg/m2 – obese). Waist circumference was measured using a steel measuring tape to the nearest 0.1 cm at the high point of the iliac crest at minimal respiration when the participant was in a standing position. The examiner stood behind the participant, palpated the hip area for the right iliac crest, marked a horizontal line at the high point of the iliac crest, and crossed the line to indicate the midaxillary line of the body. Abdominal obesity was defined as waist circumference > 88 centimeters in women and > 102 centimeters in men17.
Serum samples were obtained during the clinical examination. Serum creatinine was measured by the modified kinetic Jaffe reaction using a Roche Hitachi 737 analyzer (Roche Diagnostics Corporation, Indianapolis, Indiana) and re-calibrated in order to calculate estimated GFR (eGFR) using the 4-variable, IDMS-traceable, Modification of Diet in Renal Disease study equation: eGFRMDRD = 175 * serum creatinine [mg/dL]−1.154 * age [years]−0.203 * 0.742 [if female] * 1.210 [if black]18, 19. Cystatin C was measured using an automated particle-enhanced nephelometric assay on the Dade Behring Nephelometer II. This assay maintains a range from 0.23 to 7.25 mg/L and intra- and inter-assay coefficients of variation of 2.0% to 3.0% and 3.2% to 4.4%, respectively. For the main analysis, cystatin C was used to calculate eGFR using the following formula: eGFRCysC,age,sex,race = 127.7 * cystatin C [mg/L]−1.17 * age [years]−0.13 * 0.91 [if female] * 1.06 [if black]. Individuals with an eGFR of 15 to 59 ml/min/1.73 m2 were considered to have stage 3 or 4 CKD. For secondary analyses, the prevalence of stage 3 or 4 CKD was calculated using the cystatin C only equation (eGFRCysC = 76.7 * cystatin C [mg/L] −1.19), serum cystatin C 1.12 mg/L (elevated cystatin C, the 99th percentile for adults 20 to 39 years of age without hypertension, diabetes mellitus or CKD), and an equation incorporating creatinine, cystatin C, age, sex and race (eGFRCr,CysC,age,sex,race = 177.6 * serum creatinine [mg/dL]−0.65 * cystatin C [mg/L]−0.57 * age [years]−0.20 * 0.82 [if female] * 1.11 [if black]).7 Both the MDRD study formula and the cystatin C formulas were developed to predict measured GFR standardized to 1.73m2 body surface area. eGFR levels > 200 ml/min/1.73m2 were truncated at 200 ml/min/1.73m2.
The protocol for NHANES III was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. Informed consent was obtained from each NHANES III participant.
Statistical Analysis
Demographic characteristics, mean height, weight, and the prevalence of cigarette smoking and physical activity were calculated by BMI category (normal weight, overweight, and obese) for participants with and without stage 3 or 4 CKD defined using eGFRMDRD and eGFRCysC,age,sex,race, separately. Using each equation, mean eGFR and the prevalence of stage 3 or 4 CKD were calculated by BMI category. Differences in mean eGFR, (eGFR CysC,age,sex,race minus eGFRMDRD), within BMI category were calculated, with the statistical significance of the trend across BMI categories determined using linear regression. For all regression models assessing trends, the mean BMI (22.3, 27.2, and 34.7 kg/m2 for normal weight, overweight, and obese, respectively) was included for each BMI category. Because the eGFRCysC,age,sex,race and eGFRMDRD prevalence estimates of stage 3 or 4 CKD were calculated on the same individuals, the prevalence estimates will not be independent. Furthermore, individuals with prevalent stage 3 or 4 CKD by both equations, and lack of prevalence by both equations, contributed no information to the analysis. To address these issues, we calculated the absolute percent difference in the prevalence of stage 3 or 4 CKD as the percentage of adults with stage 3 or 4 CKD by eGFRCysC,age,sex,race but not eGFRMDRD minus the percentage with stage 3 or 4 CKD by eGFRMDRD but not eGFRCysC,age,sex,race. Since these are two independent groups, the standard errors for the difference in these prevalence estimates could be calculated directly. An example of this calculation is provided in the Appendix. The statistical significance of trends in the difference in the prevalence of CKD estimates across BMI categories was determined by modeling adults with stage 3 or 4 CKD by eGFRCysC,age,sex,race but not eGFRMDRD versus their counterparts with stage 3 or 4 CKD by eGFRMDRD but not eGFRCysC,age,sex,race by BMI category using logistic regression. Differences in the prevalence of stage 3 or 4 CKD between the eGFRMDRD and eGFRCysC,age,sex,race equations by BMI category were calculated for sub-groups defined by age (<70 and ≥70 years, the mean age of participants with stage 3 or 4 CKD by eGFRMDRD), race-ethnicity (non-Hispanic whites, non-Hispanic blacks, and Mexican-Americans), and sex. In secondary analyses, differences in the prevalence of stage 3 or 4 CKD were compared between eGFRMDRD and eGFR derived using eGFRCysC, elevated cystatin C, and eGFRCr,CysC,age,sex,race. Also, the differences in the prevalence of stage 3 or 4 CKD were compared between eGFRMDRD and eGFR derived using eGFRCysC,age,sex,race, eGFRCysC, elevated cystatin C, and eGFRCr,CysC,age,sex,race by abdominal obesity.
Data were analyzed using SUDAAN (version 9.0; Research Triangle Institute, Research Triangle Park, NC) to account for the complex NHANES III sampling design including unequal probabilities of selection, over-sampling, non-response, and measurement of cystatin C in a sub-sample of NHANES III participants.
RESULTS
The prevalence of stage 3 or 4 CKD was 5.7% and 9.2% by eGFRMDRD and eGFRCysC,age,sex,race, respectively. Defined using the eGFRMDRD or eGFRCysC,age,sex,race, participants with stage 3 or 4 CKD were older, more likely to be women, non-Hispanic white, have a higher mean BMI, and were less likely to be current smokers and physically active than their counterparts without stage 3 or 4 CKD (Table 1).
Table 1.
Characteristics of adults with and without stage 3 or 4 chronic kidney disease (CKD) based on the Modification of Diet in Renal Disease (MDRD) study equation and the cystatin C, age, sex, and race equation
| eGFRMDRD | eGFRCysC,age,sex,race | |||
|---|---|---|---|---|
| Without Stage 3 or 4 CKD (n=5791) |
Stage 3 or 4 CKD (n=918) |
Without Stage 3 or 4 CKD (n=5134) |
Stage 3 or 4 CKD (n=1575) |
|
| Age, years | 43.5 (0.7) | 69.7 (1.0) | 42.3 (0.7) | 71.5 (0.9) |
| Women, % | 50.5 | 62.7 | 50.0 | 63.2 |
| Non-Hispanic white, % | 82.4 | 90.9 | 82.1 | 90.8 |
| Non-Hispanic black, % | 11.9 | 7.6 | 12.2 | 7.1 |
| Mexican American, % | 5.7 | 1.5 | 5.8 | 2.1 |
| Body mass index, kg/m2 | 26.8 (0.2) | 27.8 (0.2) | 26.7 (0.2) | 28.4 (0.3) |
| Current cigarette smokers, % | 29.3 | 13.4 | 29.5 | 17.8 |
| Physically active, % | 79.1 | 68.3 | 79.8 | 65.3 |
eGFRMDRD was derived from an equation including creatinine, age, sex, race
eGFRCysC,age,sex,race was derived from an equation including cystatin C, age, sex, race
Numbers in table are mean (standard error) or percentage
All comparisons between the stage 3 or 4 CKD and no stage 3 or 4 CKD groups are significant (p<0.001)
Primary Analysis
Mean eGFR was lower, and the prevalence of stage 3 or 4 CKD was higher, at higher BMI categories using eGFRMDRD and eGFRCysC,age,sex,race (Table 2). The difference in mean eGFR and prevalence of stage 3 or 4 CKD between the two estimating equations was greater at progressively higher BMI categories. Specifically, the difference in eGFR (eGFRCysC,age,sex,race minus eGFRMDRD) was +1.4, −1.9, and −5.4 ml/min/1.73m2 for normal weight, overweight, and obese participants, respectively (p-trend<0.001). Compared to estimates derived using eGFRMDRD, the prevalence of stage 3 or 4 CKD was 2.3%, 3.0%, and 6.4% higher for normal weight, overweight, and obese participants, respectively, when estimated by eGFRCysC,age,sex,race (p-trend=0.005).
Table 2.
Mean estimated glomerular filtration rate (eGFR) and prevalence of stage 3 or 4 chronic kidney disease (CKD) calculated using the Modification of Diet in Renal Disease (MDRD) study equation and the cystatin C age, sex, race equation by body mass index
| Body mass index category (range, kg/m2) | ||||
|---|---|---|---|---|
| Normal weight (18.5 - 24.9) |
Overweight (25.0 – 29.9) |
Obesity (≥30.0) |
P-Trend | |
| Mean (SE) eGFR, ml/min/1.73m2 | ||||
| eGFRCysC,age,sex,race * | 96.5 (1.0) | 88.8 (0.9) | 84.1 (1.3) | |
| eGFRMDRD** | 95.2 (0.9) | 90.7 (0.9) | 89.5 (1.1) | |
| Difference | ||||
| eGFRCysC,age,sex,race estimate minus eGFRMDRD estimate |
1.4 (1.0) | −1.9 (0.9) | −5.4 (1.0) | 0.0009 |
| Stage 3 or 4 CKD prevalence † , % (SE) | ||||
| eGFRCysC,age,sex,race | 6.5 (0.7) | 9.5 (0.8) | 13.8 (1.5) | |
| eGFRMDRD | 4.2 (0.3) | 6.5 (0.5) | 7.4 (0.7) | |
| Difference | ||||
| eGFRCysC,age,sex,race estimate minus eGFRMDRD estimate |
2.3 (0.4) | 3.0 (0.7) | 6.4 (1.2) | 0.005 |
SE – standard error
eGFRCysC,age,sex,race was derived from an equation including cystatin C, age, sex, race
eGFRMDRD was derived from an equation including creatinine, age, sex, race
Percent prevalence of stage 3 or 4 CKD was defined by either eGFRCysC,age,sex,race or eGFRMDRD
Sub-group Analysis
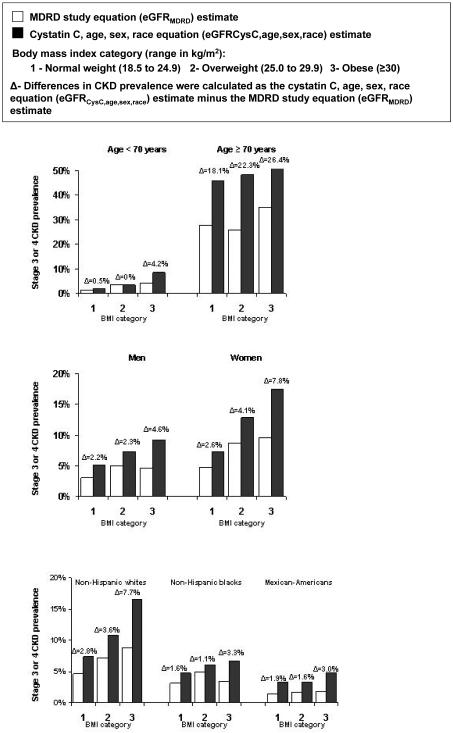
A graded increase in the difference (i.e., eGFRCysC,age,sex,race minus eGFRMDRD) in the estimates of stage 3 or 4 CKD prevalence across BMI categories was present among participants ≥ 70 years of age, men and women, and non-Hispanic Whites (Figure 1). Also, for participants <70 years of age and non-Hispanic blacks and Mexican-Americans, the difference in stage 3 or 4 CKD prevalence estimates was higher for obese compared with normal weight participants. Testing for interaction indicated that results were consistent by age group, sex, and race-ethnicity. The p-values for interaction were p=0.889 across age group, p=0.267 across gender, and p=0.61 and p=0.33 for non-Hispanic blacks and Mexican-Americans, respectively, compared to non-Hispanic whites.
Figure 1.
Difference in the prevalence of stage 3 or 4 chronic kidney disease using the age, sex, race and cystatin C equation (eGFRCysC,age,sex,race) and the Modification of Diet in Renal Disease (MDRD) study equation (eGFRMDRD) by body mass index for demographic subgroups.
Secondary analyses
Compared to stage 3 or 4 CKD defined by eGFRMDRD, the prevalence of stage 3 or 4 CKD defined by eGFRCysC was 0.2%, 0.6%, 2.2% higher for normal weight, overweight, and obese participants, respectively (Table 3; p-trend=0.028). The differences (elevated cystatin C minus eGFRMDRD) in stage 3 or 4 CKD prevalence estimates were 2.9%, 5.2%, 9.5% for normal weight, overweight, and obese participants, respectively (p-trend<0.001). In contrast, there was no difference in the estimates of stage 3 or 4 CKD prevalence by BMI categories between eGFRMDRD and eGFRCr,CysC,age,sex,race (eGFRCr,CysC,age,sex,race minus eGFRMDRD; −0.1%, −0.4%, 0.0% for normal weight, overweight, and obese participants, respectively; p-trend=0.719). Similar to the trend observed with BMI, cystatin C-based equations demonstrated higher stage 3 or 4 CKD prevalence estimates in participants with, compared to those without, abdominal obesity. For example, the differences for eGFRCr,CysC,age,sex,race minus eGFRMDRD in stage 3 or 4 CKD prevalence estimates were 6.3% and 1.4% for participants with and without abdominal obesity, respectively (p<0.001).
Table 3.
Prevalence of stage 3 or 4 chronic kidney disease calculated using the Modification of Diet in Renal Disease (MDRD) study equation and other cystatin C-based estimates, by body mass index
| Body mass index category (range, kg/m2) | ||||
|---|---|---|---|---|
| Normal weight (18.5 - 24.9) |
Overweight (25.0 − 29.9) |
Obesity (≥30.0) |
P- Trend |
|
| Stage 3 or 4 CKD prevalence, % (SE) | ||||
| eGFRcysC | 4.3 (0.5) | 7.1 (0.7) | 9.6 (1.1) | |
| eGFRMDRD | 4.2 (0.3) | 6.5 (0.5) | 7.4 (0.7) | |
| Difference | ||||
| eGFRcysC estimate minus eGFRMDRD estimate |
0.2 (0.3) | 0.6 (0.7) | 2.2 (0.8) | 0.03 |
| Stage 3 or 4 CKD prevalence, % (SE) | ||||
| Elevated cystatin C | 7.1 (0.7) | 11.7 (1.0) | 16.9 (1.8) | |
| eGFRMDRD | 4.2 (0.3) | 6.5 (0.5) | 7.4 (0.7) | |
| Difference | ||||
| Elevated cystatin C estimate minus eGFRMDRD estimate |
2.9 (0.5) | 5.2 (0.9) | 9.5 (1.4) | 0.0009 |
| Stage 3 or 4 CKD prevalence, % (SE) | ||||
| eGFRcreat, cysC, age, sex, race | 4.1 (0.4) | 6.1 (0.6) | 7.4 (0.8) | |
| eGFRMDRD | 4.2 (0.4) | 6.5 (0.6) | 7.4 (0.7) | |
| Difference | ||||
| eGFRcreat, cysC, age, sex, race estimate minus eGFRMDRD |
−0.1 (0.3) | −0.4 (0.4) | 0.0 (0.4) | 0.7 |
SE − Standard error
Prevalence of stage 3 or 4 CKD was defined by estimated glomerular filtration rate (eGFR):
eGFRcysC was derived from an equation including only cystatin C.
eGFRMDRD was derived from an equation including creatinine, age, sex, and race.
Elevated cystatin C was defined as cystatin C ≥ 1.12 mg/L (the 99th percentile for adults 20 to 39 years of age without hypertension, diabetes mellitus or CKD).
eGFRcreat, cysC, age, sex, race was derived from an equation including creatinine, cystatin C, age, sex, and race.
DISCUSSION
In the current analysis, the differences in the prevalence of stage 3 or 4 CKD, estimated using several cystatin C equations and the MDRD study equation were progressively larger at higher BMI categories. Among obese adults, the prevalence of stage 3 or 4 CKD was almost two-fold greater when estimated by eGFRCysC,age,sex,race versus eGFRMDRD. These findings suggest cystatin C-based equations may result in the over-estimation of CKD prevalence, in overweight and obese adults.
Because it is not practical to measure GFR directly in clinical practice, the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend that creatinine-based estimating equations (such as the MDRD study equation or Cockcroft-Gault) be used to estimate GFR1. The accuracy of estimating GFR using various cystatin and creatine-based estimating equations was recently examined by Stevens et al.7 In a pooled analysis of adults with CKD, cystatin C alone and cystatin C with age, race and sex provided an estimation of GFR that was as accurate as a creatinine-based estimating equation. The authors suggested that cystatin C is a better measure of GFR because it is independent of muscle mass. That report did not present sub-group analyses by BMI category. However, the impact of BMI on cystatin C-based prediction equations, in the context of CKD, may be limited since the prevalence of obesity is likely to be lower in a disease-based sample.20
Cystatin C is produced at a steady rate by all types of nucleated cells in the body and is, thus, found in detectable amounts in most body fluids. Its low molecular mass allows it to be freely filtered by the glomerular membrane in the kidney.21 Several researchers have hypothesized that serum cystatin C may be a better marker of GFR as it is independent of body composition;21-24 however, this generally refer to muscle mass and not to body fat. Our study suggests that cystatin C may not be independent of adiposity.
Cystatin C has been associated with overweight and obesity in previous population-based research studies. In a cross-sectional analysis of the NHANES III population without CKD,13 a graded association between BMI categories and elevated cystatin C was reported. Specifically, compared to normal weight, overweight, class I obesity, and class II-III obesity, were associated with a 1.46, 2.36, and 2.82 greater odds of cystatin C ≥ 1.09 mg/L (p for trend <0.001). In an analysis of the NHANES III population older than 60 years that included persons with CKD,12 higher BMI was associated with a statistically significant increased odds ratio of elevated cystatin C (≥1.12 mg/L). The reported multivariable adjusted odds ratios between BMI and elevated cystatin C in these two NHANES III studies were not adjusted for eGFR. In a population-based sample of 8,058 adults in Groningen, The Netherlands, a significant correlation (r=0.22; p<0.001) was present between body weight and serum cystatin C independent of creatinine clearance.11 Also, Fried and colleagues10 demonstrated that mean BMI was significantly associated with increasing levels of cystatin C in a study of 2,135 elderly men and women. However, a multivariable adjusted association was not reported. It is also possible that obesity leads to CKD25, a reduction in GFR, and elevations in serum creatinine and cystatin C. Hsu et al conducted a study among 320,252 adult members of Kaiser Permanente who volunteered for screening health checkups between 1964 and 1985 and linked them to the US Renal Data registry through 2000. A strong, independent association of increasing BMI with end-stage renal disease was present in this study. Because the current study did not have a gold standard measure of GFR or longitudinal measures of BMI, we were not able to explore whether the differences in estimation of GFR by creatinine versus cystatin C-based equations could be a result of decreased kidney function due to increasing BMI .
The mechanism underlying an association between BMI and cystatin C remains unclear. Adipose tissue secretes endocrine and paracrine hormones and thus plays a vital role in metabolism and homeostasis.26, 27 The growth of adipose tissue is the consequence of increased accumulation of lipids in the adipocytes as well as new adipocytes formed from precursor cells.28 These “preadipocytes” become mature adipocytes when exposed to a multitude of inhibitory and stimulatory factors. Laboratory studies have examined the expression of cathepsin S as a new biomarker of adiposity and have demonstrated that human adipose tissue secretes and expresses cathepsin S which is upregulated in obesity.29 Cystatin C regulates cathepsin S activity by acting as an endogenous inhibitor. In a recent laboratory study, researchers examined the effect of cathepsin S on adipogenesis by studying the effects of inhibition of cathepsin S using cystatin C in human preadipocytes. This study showed that cystatin C secretion increased and cathepsin S decreased during pre-adipocyte differentiation suggesting a possible role of cystatin C in adipogenesis.28
The evidence of an association between cystatin C and overweight and obesity raises the concern that the estimation of kidney function using cystatin C-based equations may result in biased estimation of GFR and CKD prevalence, in overweight and obese adults. Without a gold standard measurement of GFR, our study could not address this directly. However, we did show a significant trend in increasing differences in CKD prevalence estimated by cystatin C and creatinine-based equations increased with increasing BMI. It is unlikely that this trend is due to bias in the MDRD formula at higher BMI levels since studies have shown that adiposity is associated with CKD estimation by cystatin C-based equations but not creatinine-based equations30. Given the high prevalence of overweight and obesity in US adults, this finding has important implications. Data from the NHANES 2005-2006 indicate that over a third of US adults (72 million Americans) are obese.31 While cystatin C has been advocated as a measure of GFR that is independent of muscle mass, the current data suggest that cystatin C may not be independent of body fat and may lead to the overestimation of CKD among overweight and obese persons.
In contrast to the other estimating equations, there was no difference in stage 3 or 4 CKD prevalence estimates across BMI categories between eGFRCr,CysC,age,sex,race and eGFRMDRD. Thus, eGFRCr,CysC,age,sex,race appears to not have a substantial bias across BMI categories while benefiting in precision from addition of cystatin C to serum creatinine, age, sex and race. In a previous report, the eGFRCr,CysC,age,sex,race equation successfully estimated GFR within 30% of measured GFR for 89% of patients screened for CKD, compared to 81% for eGFRCysC, 83% for eGFRCysC,age,sex,race and 85% for eGFRMDRD. Equations using both serum creatinine and cystatin C may provide more accurate estimate of the overall burden of CKD than other published cystatin C-based estimating equations, especially among populations with a high percentage of overweight and obese adults.
There are limitations to the current analysis that warrant mention. The lack of a gold standard measurement of GFR means we are unable to determine whether there is a bias, or the extent of any bias, in the estimation of GFR using these equations. For example, it is possible that equations using serum creatinine overestimate GFR, equations using cystatin C underestimate GFR or, more likely, the answer is somewhere in between but cannot be assessed due to the lack of a gold-standard. However, this bias is unlikely to affect BMI groups differentially. Secondly, only a single measurement of serum creatinine and cystatin C was obtained. Ideally, the presence of CKD would be defined based on at least two serum measurements over a period of several months due to intra-individual variation.32
These limitations are balanced by the strengths of NHANES III, which included a large multi-ethnic sample of US adults. NHANES III also incorporated an over-sampling of older individuals to ensure valid estimates could be obtained for this population sub-group. This is important because of the higher prevalence of CKD at older age and the growing number of older adults in the US population. As previously reported, NHANES III response rates were high, missing data were rare and data were collected by trained staff, following a standardized protocol.16
In conclusion, data from the current study demonstrated a greater difference in CKD prevalence, estimated by cystatin C versus creatinine-based equations, at higher BMI categories. However, no such trend across BMI categories was present when an equation that incorporated serum creatinine and cystatin C was compared to a creatinine-based equation. The impact of overweight and obesity on cystatin C-based equations requires further research.
Table 4.
Prevalence of stage 3 or 4 chronic kidney disease calculated using the Modification of Diet in Renal Disease (MDRD) study equation and other cystatin C-based estimates, by abdominal obesity
| Abdominal obesity* | |||
|---|---|---|---|
| No | Yes | P- Value |
|
| Stage 3 or 4 CKD prevalence, % (SE) | |||
| eGFRCysC,age,sex,race | 4.5 (0.5) | 15.6 (1.2) | |
| eGFRMDRD | 3.1 (0.3) | 9.4 (0.7) | |
| Difference | |||
| eGFRCysC,age,sex,race estimate minus eGFRMDRD estimate |
1.4 (0.3) | 6.2 (0.9) | 0.002 |
| Stage 3 or 4 CKD prevalence, % (SE) | |||
| eGFRcysC | 3.4 (0.3) | 11.6 (0.9) | |
| eGFRMDRD | 3.1 (0.3) | 9.4 (0.7) | |
| Difference | |||
| eGFRcysC estimate minus eGFRMDRD estimate |
0.3 (0.3) | 2.2 (0.7) | 0.012 |
| Stage 3 or 4 CKD prevalence, % (SE) | |||
| Elevated cystatin C | 5.7 (0.6) | 18.0 (1.5) | |
| eGFRMDRD | 3.1 (0.3) | 9.4 (0.7) | |
| Difference | |||
| Elevated cystatin C estimate minus eGFRMDRD estimate |
2.6 (0.5) | 8.6 (1.1) | <0.001 |
| Stage 3 or 4 CKD prevalence, % (SE) | |||
| eGFRcreat, cysC, age, sex, race | 2.9 (0.3) | 9.1 (0.8) | |
| eGFRMDRD | 3.1 (0.3) | 9.4 (0.7) | |
| Difference | |||
| eGFRcreat, cysC, age, sex, race estimate minus eGFRMDRD |
−0.2 (0.2) | −0.3 (0.5) | 0.93 |
Abdominal obesity (waist circumference > 88 cm for women; > 102 cm for men)
SE – Standard error
Prevalence of stage 3 or 4 CKD was defined by estimated glomerular filtration rate (eGFR):
eGFRcysC was derived from an equation including only cystatin C.
eGFRMDRD was derived from an equation including creatinine, age, sex, and race.
Elevated cystatin C was defined as cystatin C ≥ 1.12 mg/L (the 99th percentile for adults 20 to 39 years of age without hypertension, diabetes mellitus or CKD).
eGFRcreat, cysC, age, sex, race was derived from an equation including creatinine, cystatin C, age, sex, and race.
ACKNOWLEDGEMENTS
Cystatin C assays and Dr. Coresh were supported by the CKD-EPI Collaboration funded by grants UO1 DK 053869, UO1 DK 067651 and UO1 DK 35073.
Appendix
Example of the process used to calculate the difference in the prevalence of stage 3 or 4 chronic kidney disease using two estimating equations (eGFRcysC,age,sex,race minus eGFRMDRD).
| eGFRMDRD | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| eGFRcysC,age,sex,race | Yes | 3.1% (0.3) |
 |
6.5% (0.7) |
| No | 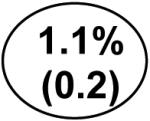 |
92.4% (0.7) |
93.5% (0.7) |
|
| Total | 4.2% (0.4) |
95.8% (0.4) |
100% | |
Numbers in table represent percent (standard error)
The difference in stage 3 or 4 chronic kidney disease was calculated as the percent of individuals with stage 3 or 4 CKD by eGFRcysC,age,sex,race but not eGFRMDRD (shaded circle: 3.4%) minus the percent with stage 3 or 4 CKD by the eGFRMDRD equation but not the eGFRcysC,age,sex,race equation (unshaded circle:1.1%). The difference in this example is 2.3%.
The standard error of the difference between these two percentages was calculated as the square root of the sum of the variance for each percentage (square root of 0.4*0.4 + 0.2*0.2). The standard error of the difference in this example is 0.4.
Data presented in this example are for normal weight individuals (see table 2).
Footnotes
Disclosure of any potential conflicts of interest: There are no conflicts of interest to disclose.
REFERENCES
- 1.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004 Dec 21;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006 Jan;69(2):399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 5.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002 May;48(5):699–707. [PubMed] [Google Scholar]
- 6.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002 Aug;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008 Mar;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin A, Stevens LA. Executing change in the management of chronic kidney disease: perspectives on guidelines and practice. Med Clin North Am. 2005 May;89(3):701–709. doi: 10.1016/j.mcna.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006 Nov;15(6):610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 10.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006 May;54(5):750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 11.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004 Apr;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 12.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008 Mar;51(3):385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, Winston J, Uribarri J, Mann D, Fox CS. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008 Apr;121(4):341–348. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Greene T, Li C, et al. Factors Other Thank GFR Influencing Serum Cystatin. J Am Soc Nephrol. 2007;18:547A–548A. abstract. [Google Scholar]
- 15.Kratchmarova I, Kalume DE, Blagoev B, et al. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002 Mar;1(3):213–222. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics . Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-1994. 1994:1. US Dept of Health and Human Services publication; Washington, D.C.: pp. 94–1308. [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002 May;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003 Mar;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005 Jan;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Jones CY, Jones CA, Wilson IB, et al. Cystatin C and Creatinine in an HIV Cohort: The Nutrition for Healthy Living Study. Am J Kidney Dis. 2008 Jun;51(6):914–24. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998 May;101(5):875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005 May 19;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006 Jan 3;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000 Aug;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol Metab. 2002 Jan-Feb;13(1):5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- 28.Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006 Oct;147(10):4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 29.Taleb S, Lacasa D, Bastard JP, et al. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. Faseb J. 2005 Sep;19(11):1540–1542. doi: 10.1096/fj.05-3673fje. [DOI] [PubMed] [Google Scholar]
- 30.Young JA, Hwang SJ, Sarnak MJ, et al. Association of visceral and subcutaneous adiposity with kidney function. Clin J Am Soc Nephrol. 2008 Nov;3(6):1786–1791. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity Among Adults in the United States -- No Statistically Significant Change Since 2003-2004. 2007 NCHS Data Brief. [PubMed] [Google Scholar]
- 32.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006 Jan;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]