Abstract
PURPOSE
To develop a severity scale for diabetic macular edema (DME) and to assess relationships between severity and duration of DME and visual acuity (VA).
METHODS
From the Early Treatment Diabetic Retinopathy Study (ETDRS), mean baseline VA scores were tabulated for 7422 eyes cross-classified by (1) location of retinal thickening (RT) and its area within 1 disc diameter of the macular center, and (2) degree of RT at the center. Adjacent (row, column, and off-diagonal) cells with the greatest similarity in baseline VA (mean and SD) based on a Gaussian (normal) likelihood were merged. An initial eight-step scale was chosen using the Schwarz criterion (Bayesian information criterion; BIC) and was revised based on clinical judgment to nine steps. Relationships between baseline VA and other photographic and fluorescein angiographic characteristics were examined singly and in combination with the scale.
RESULTS
Modeling baseline VA as a function of the nine-step scale yielded an R2 of 38.0%, compared with 38.4% using the full cross-classification of these variables. Addition of each of the other baseline characteristics changed the adjusted R2 for the combination very little. Between scale levels 1A and 5B mean (SD) VA decreased from 86.8 (5.8) letters to 59.8 (13.6) letters. In a model of change in VA as a function of time spent at each DME severity level, VA loss increased progressively from 1 letter per year at level 2 to 17 letters per year at level 5B.
CONCLUSIONS
The scale facilitates documentation of the relationship of severity and duration of DME with VA.
In its initial report, the Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that focal/grid photocoagulation treatment of diabetic macular edema (DME) reduces the 3-year risk of moderate visual loss (MVL, a decrease in visual acuity [VA] score of 15 or more letters, corresponding to a doubling of the visual angle) by approximately 50%, from 24% in untreated eyes to 12% in treated eyes. 1
In previous ETDRS reports, the principal measure of the morphologic severity of DME has been the presence or absence of “clinically significant macular edema” (CSME), which may be characterized as retinal thickening (RT) or adjacent hard exudate that involves or threatens the center of the macula.1 CSME may be expanded to a three-step scale by subdividing the CSME-present category by the absence or presence of involvement of the macular center. On this scale, in untreated eyes with DME the 3-year risks of MVL were 17%, 22%, and 33%, respectively, in eyes with non-CSME, CSME without center involvement, and CSME with center involvement. 2
The ETDRS also reported several factors associated with baseline VA, the most notable of which were area of RT, degree of RT at the macular center, and severity of fluorescein leakage within 1 disc diameter (DD) of the macular center. In a subset of 741 eyes with mild to moderate nonproliferative retinopathy and macular edema questionably or definitely involving the macular center, VA score at baseline was ≥70 letters (corresponding to 20/40 or better) in 92.5% of eyes in which the area of RT within 1 DD of center was <0.5 disc area (DA) versus 43.4% of eyes in which this area was ≥2.0 DA. Corresponding proportions for eyes in the lowest and highest categories of fluorescein leakage were 85.2% and 50.0% and for degree of RT at the macular center, they were 91.3% and 63.4%.3
The objectives of this report are to describe the development of a more detailed scale to categorize the severity of DME and to use the scale to assess the relationship between the duration of severe DME and VA. The scale is based on associations between baseline gradings of features characteristic of DME in stereoscopic color fundus photographs and best corrected baseline VA.4,5
METHODS
Baseline VA scores (mean and SD) were tabulated for eyes cross-classified by degree of RT at the macular center and by a combination of location of RT within a 30° photographic field centered on the macula (Field 2) and area of RT within 1 DD of the center.5 The resultant table had 26 cells. Both eyes of the 3711 patients enrolled in the ETDRS were included. Patient’s ETDRS records were managed according to the provisions of Declaration of Helsinki to protect privacy. An agglomerative clustering algorithm was used to generate candidate scales with 1 to 26 distinct steps. Starting with all cells as distinct steps, two adjacent (row, column or off-diagonal) steps with the greatest similarity in baseline VA scores (mean and SD) based on the smallest decrease in the Gaussian (normal) likelihood were merged. From these 26 candidate scales, an initial eight-step scale was chosen according to the Schwarz criterion (Bayesian information criterion; BIC).6 The initial scale was revised slightly to nine steps based on clinical judgment (see the Results section).
Relationships between baseline VA and other ocular characteristics included in the baseline gradings of fundus photographs and fluorescein angiograms were also examined singly and in combination with the revised scale, by using linear models with an exchangeable variance structure. R2 was calculated as 1 minus the ratio of the estimated residual variance for a given model to the estimated residual variance for the null model (no covariates). These characteristics were retinopathy severity on the ETDRS scale, severity of hard exudates (HEs), severity of hemorrhages and microaneurysms (H/Mas) in field 2, and severity of fluorescein leakage, cystoid spaces, and capillary loss in fluorescein angiograms.5,7,8
Relationships between baseline VA and nonocular characteristics included in the baseline assessment were examined in a similar fashion. These characteristics included age, sex, body mass index (BMI), systolic and diastolic blood pressure, type and duration of diabetes and total, LDL, and HDL cholesterol and triglycerides.
For analyses of the effect of duration of severe DME on VA over the first three years of follow-up, analyses were restricted to eyes with baseline VA ≥ 70 letters, thus excluding eyes that may have had severe DME for long periods before enrollment. The number of these eyes at baseline was 3280 and 3294, respectively, in the early-treatment and deferral groups. The number of eyes with VA at the 3-year follow-up visit were 3042 and 3058, respectively. Calculation of duration at DME severity levels required gradable photographs at all scheduled follow-up visits (4 months, 1 year, 2 years, and 3 years). In these groups, respectively, 687 and 737 eyes had one or more missed visits or missing or ungradable photographs at a completed visit, leaving 2355 and 2321 eyes for analysis.
Linear regression models were used to assess the relationship between duration in steps of the DME scale over the first 3 years of follow-up and change in VA from baseline to 3 years. To define duration at each DME step, DME status was determined at each scheduled follow-up visit (4 months, 1 year, 2 years, and 3 years) and the eye was assumed to be in the current step for the entire interval since the prior scheduled visit (4 months for the 4-month visit, 8 months for the 1-year visit, and 12 months for the 2- and 3-year visits). Total duration at each DME step was obtained by summation over all four intervals. Duration at each DME step was included in the linear regression models, using step 1A as a reference category. These models included baseline DME status.
RESULTS
Table 1 provides mean (SD) baseline VA within each of 24 of the original 26 cells of the cross-classification of location and area of RT and degree of RT at center. The highest step in the grading scale for degree of RT at center, ≥0.5 DD, in which there were only 17 eyes, all in the last 2 rows of the table, has been merged with the next highest step, now more than or equal to two times reference thickness.5 From the original 26-step scale an initial 8-step scale was selected based on the agglomerative clustering algorithm. It was revised slightly based on clinical judgment, resulting in a nine-step scale. The nine steps were grouped into five major steps, numbered one to five, some of which have alphabetic subdivisions. The changes between the initial and revised scales were (1) division of the first step in the initial scale (no RT within 1 DD of center) into step 1A (no RT in Field 2) and step 1B (RT in Field 2 but none within 1 DD of center); (2) division of the highest step in the initial scale into steps 5A and 5B; and (3) the merger of eyes with questionable central thickening and RT area ≥2 DA within 1 DD of center, which was a separate step in the initial scale, into step 3A (see bold outlines of these cells in Table 1). Modeling baseline VA as a function of the initial eight-step scale, the revised nine-step scale and its reduced five-step version yielded R2 of 37.9%, 38.0%, and 37.3%, respectively, compared with an R2 of 38.4% using the full cross-classification of these variables. In a quality-control exercise the weighted κ for interobserver agreement on the nine-step scale was 0.64 (SE = 0.05) and agreement within 1-step was 88%.
TABLE 1.
Baseline VA (Letters) in Eyes Cross-Classified by Location/Area of Retinal Thickening and Thickness at the Macular Center
| Retinal Thickness at Macular Center | Total | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Questionable | Definite, <1X Ref* | ≥1X, <2X Ref | ≥2X Ref | ||||||||||||||||
| Location of RT† and area in CZ‡ | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Level 1A | |
| None in Field 2§ | 86.8 | 5.8 | 2463 | 86.8 | 5.8 | 2463 | Level 1B | |||||||||||||
| Field 2, not CZ | 86.2 | 6.2 | 761 | 86.2 | 6.2 | 761 | Level 1C | |||||||||||||
| Quest‖ in CZ | 85.0 | 7.1 | 401 | 82.2 | 8.5 | 216 | 84.0 | 7.7 | 617 | Level 2 | ||||||||||
| <1/2 DA# in CZ | 85.2 | 6.7 | 522 | 82.5 | 8.9 | 315 | 81.2 | 8.5 | 175 | 81.8 | 8.2 | 102 | 75.0 | NA | 1 | 83.5 | 8.0 | 1115 | Level 3A | |
| ≥1/2, <1 DA in CZ | 83.2 | 8.2 | 145 | 81.1 | 10.2 | 216 | 78.9 | 9.6 | 235 | 77.2 | 10.9 | 223 | 72.5 | 14.1 | 6 | 79.8 | 10.1 | 825 | Level 3B | |
| ≥1, ≤ 2 DA in CZ | 82.2 | 9.0 | 30 | 79.6 | 11.9 | 90 | 75.1 | 12.3 | 282 | 72.8 | 12.4 | 571 | 66.7 | 15.0 | 34 | 74.1 | 12.7 | 1007 | Level 4 | |
| ≥ 2 DA in CZ | 60.7 | 13.9 | 3 | 87.0 | 8.5 | 7 | 72.1 | 13.3 | 58 | 64.3 | 14.6 | 467 | 59.8 | 13.6 | 99 | 64.5 | 14.7 | 634 | Level 5A | |
| Total | 86.2 | 6.3 | 4325 | 81.8 | 9.5 | 844 | 77.5 | 11.1 | 750 | 71.3 | 13.9 | 1363 | 62.2 | 14.4 | 140 | 81.6 | 11.2 | 7422 | Level 5B | |
Cells with similar VA combined as indicated by color code to form a nine-step scale. Cells outlined in black show changes from an initial eight-step scale: (1) separation of level 1A from 1B, (2) inclusion of bottom cell in second column (initially a separate step) in level 3A, and (3) separation of level 5B from 5A.
Reference thickness, the maximum thickness of normal retina 0.5 to 1.0 disc diameter from the macular center.
Retinal thickening.
The area within 1 disc diameter of the macular center.
Field 2, a 30° photograph focused on the macular center.
Questionable (50%–90% likely in the opinion of the grader).
Disk area.
Table 2 gives R2 values for models with other baseline characteristics, either alone or in combination with the nine-step scale. When considered alone, severity of fluorescein leakage, cystoid spaces, and capillary loss in fluorescein angiograms (a model combining these variables), severity of HE (a model combining HE in Field 2, HE at macular center and prominence of HE rings) and H/Ma in field 2 produced an R2 of 24.3%, 21.6%, and 10.1%, respectively, but when included in models with the nine-step scale they raised the R2 of 38.0% to 42.2%, 39.3%, and 38.6%, respectively. The revised scale was therefore adopted as the ETDRS DME severity scale.
TABLE 2.
R2 Values for Association with VA in Models with Other Baseline Characteristics
| Characteristic | Alone (%) | With DME Level (%) |
|---|---|---|
| DME Level (9-step scale) | 38.0 | |
| Model combining fluorescein leakage, cystoid spaces and capillary loss | 24.3 | 42.2 |
| Model combining HE in Field 2, at center, and prominence of HE rings | 21.6 | 39.3 |
| Severity of HMA in Field 2 | 10.1 | 38.6 |
| Retinopathy severity level | 0.9 | 38.1 |
| Age | 15.1 | 41.9 |
| Diabetes type | 9.4 | 39.5 |
| Systolic BP | 2.4 | 38.3 |
| Duration of diabetes | 2.0 | 38.5 |
| BMI | 1.4 | 38.2 |
| Total cholesterol | 1.3 | 38.1 |
| Triglycerides | 0.6 | 38.2 |
| LDL | 0.6 | 38.1 |
| Diastolic BP | 0.1 | 38.0 |
| HbA1c | 0.1 | 38.1 |
| HDL | 0.0 | 38.0 |
Among the nonocular variables in Table 2, only age and diabetes type were notable for producing an R2 above 5%: 15.1% for age (2.9 fewer letters per decade), and 9.4% for diabetes type (8.8 fewer letters for type II versus type I diabetes). They added, respectively, 3.9% and 1.5% to the R2 for the nine-step scale alone.
Table 3 provides, for each step in the ETDRS DME severity scale, the mean and SD for VA at the baseline and 3-year visits and for change between these visits in eyes assigned to deferral of photocoagulation (by the 3-year visit, ~40% of these eyes had been treated with focal/grid photocoagulation, which was allowed after the protocol was changed in conjunction with the first ETDRS report). 1 Between the least and most severe steps of the scale, mean VA ranged from 86.8 to 59.8 letters at baseline and from 82.3 to 50.7 letters at 3 years. Mean change in VA between baseline and 3 years was also strongly associated with increasing DME severity (from −4.8 letters at level 1A to −13.3 letters at level 5A).
TABLE 3.
VA (Letters) at Baseline and 3-Year Visits and Change between These Visits for Eyes Assigned to Deferral of Photocoagulations, by ETDRS DME Scale Level
| 9- step scale | 5- step scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | |||||||||
| Level 1A | Baseline | 86.8 | 5.9 | 1256 | |||||||
| 3 year | 82.3 | 15.2 | 1195 | ||||||||
| Change | −4.8 | 14.3 | 1195 | ||||||||
| Level 1B | Baseline | 86.1 | 6.3 | 370 | |||||||
| 3 year | 79.8 | 17.3 | 343 | ||||||||
| Change | −6.4 | 16.0 | 343 | Mean | SD | N | |||||
| Level 1C | Baseline | 85.0 | 6.7 | 512 | Level 1 | Baseline | 86.3 | 6.2 | 2138 | Level 1A | |
| 3 year | 78.3 | 17.1 | 477 | 3 year | 80.9 | 16.1 | 2015 | Level 1B | |||
| Change | −6.9 | 14.8 | 477 | Change | −5.6 | 14.7 | 2015 | Level 1C | |||
| Level 2 | Baseline | 81.6 | 9.2 | 525 | Level 2 | Baseline | 81.6 | 9.2 | 525 | Level 2 | |
| 3 year | 72.1 | 21.1 | 473 | 3 year | 72.1 | 21.1 | 473 | Level 3A | |||
| Change | −10.0 | 18.9 | 473 | Change | −10.0 | 18.9 | 473 | Level 3B | |||
| Level 3A | Baseline | 79.5 | 10.7 | 170 | Level 4 | ||||||
| 3 year | 68.7 | 22.8 | 147 | Level 5A | |||||||
| Change | −11.1 | 20.1 | 147 | Level 5B | |||||||
| Level 3B | Baseline | 77.8 | 10.2 | 232 | Level 3 | Baseline | 78.5 | 10.5 | 402 | ||
| 3 year | 68.5 | 21.5 | 201 | 3 year | 68.6 | 22.0 | 348 | ||||
| Change | −9.7 | 19.1 | 201 | Change | −10.3 | 19.5 | 348 | ||||
| Level 4 | Baseline | 72.7 | 12.4 | 341 | Level 4 | Baseline | 72.7 | 12.4 | 341 | ||
| 3 year | 62.4 | 21.2 | 302 | 3 year | 62.4 | 21.2 | 302 | ||||
| Change | −10.6 | 17.8 | 302 | Change | −10.6 | 17.8 | 302 | ||||
| Level 5A | Baseline | 64.6 | 14.9 | 256 | |||||||
| 3 year | 52.6 | 21.8 | 221 | ||||||||
| Change | −13.3 | 19.4 | 221 | ||||||||
| Level 5B | Baseline | 59.8 | 13.6 | 49 | Level 5 | Baseline | 63.8 | 14.7 | 305 | ||
| 3 year | 50.7 | 19.6 | 45 | 3 year | 52.3 | 21.4 | 266 | ||||
| Change | −9.2 | 20.5 | 45 | Change | −12.6 | 19.6 | 266 | ||||
| Total | Baseline | 81.7 | 11.2 | 3711 | |||||||
| 3 year | 74.5 | 20.5 | 3404 | ||||||||
| Change | −7.7 | 16.8 | 3404 | ||||||||
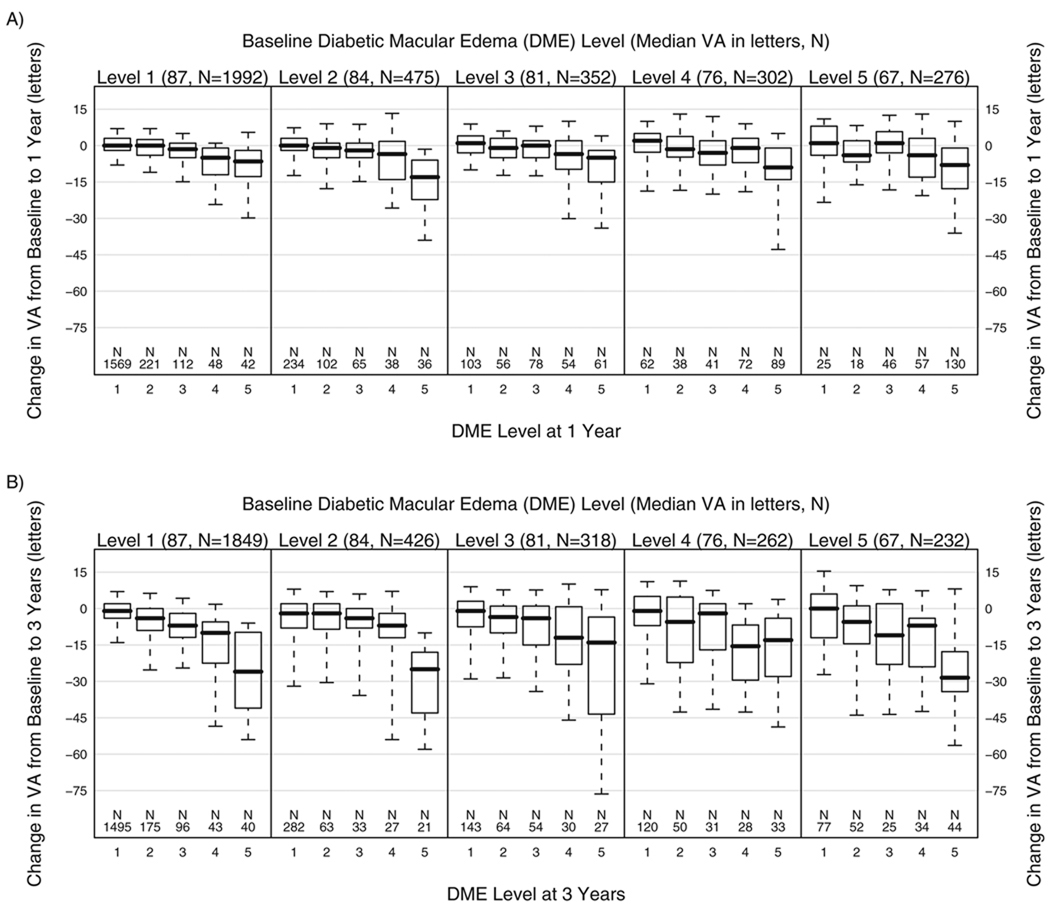
Change in VA between the baseline and follow-up visits, by DME severity (five-step scale) at these visits, is presented in Figure 1 for eyes assigned to deferral of photocoagulation. Among the 1992 eyes with follow-up at 1 year that were at level 1 at baseline, median change in VA between baseline and 1 year (Fig. 1A, leftmost panel) ranged from 0 to −6.5 letters in eyes that were at levels 1 and 5, respectively, at 1 year. Baseline VA was strongly associated with baseline DME severity level. Change in VA was strongly associated with DME severity at 1 year in all baseline DME severity groups, although there was no consistent pattern across the baseline groups. Among the 1849 eyes with follow-up at 3 years that were at level 1 at baseline, median change in VA between baseline and 3 years (Fig. 1B, leftmost panel) ranged from −1 to −26 letters in eyes that were at levels 1 and 5, respectively, at 3 years. As was the case at 1 year, changes from baseline depended mainly on the level at 3 years and were similar across baseline DME severity levels.
FIGURE 1.
Change in median VA (number of letters) between baseline and 1 year (1A) and between baseline and 3 years (1B) in eyes assigned to deferral of photocoagulation, by DME severity level on the five-step scale (see Table 1) at baseline and at follow-up. Boxes show the 25th and 75th percentiles, whiskers the 10th and 90th percentiles, and the line within box the median.
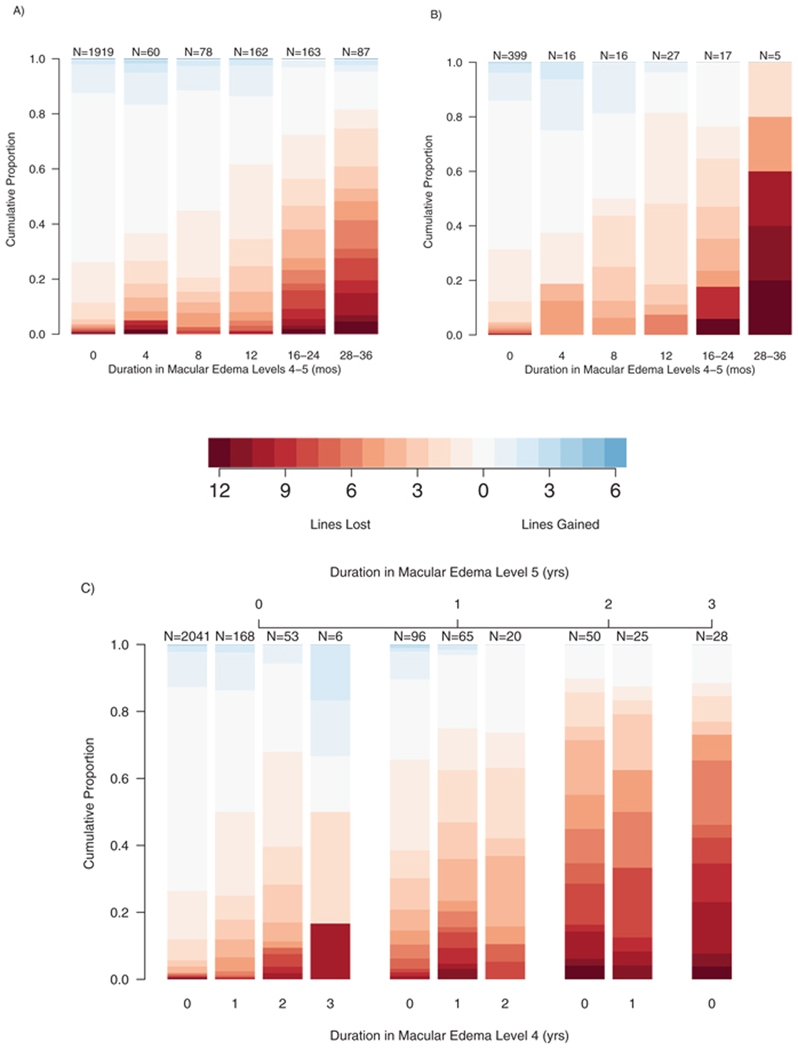
Change in VA between the baseline and 3-year visits in eyes that had baseline VA of ≥70 letters (corresponding to 20/40 or better) is shown in Figure 2, by duration of severe DME (defined as ≥ level 4) during that interval, for all eyes assigned to deferral of photocoagulation (Fig. 2A) and for eyes with mild to moderate nonproliferative diabetic retinopathy (NPDR) and DME at baseline assigned to immediate focal/grid photocoagulation and delay of scatter photocoagulation unless retinopathy progressed to severe NPDR or to proliferative retinopathy (PDR) of any severity (Fig. 2B). These were the only eyes assigned to early treatment in which the initial treatment did not include scatter photocoagulation. The number of patients in this group at baseline was 727, approximately 20% of the 3711 patients enrolled, in all of whom one eye was assigned to early photocoagulation and the other to deferral of photocoagulation unless high-risk PDR developed. Among eyes assigned to deferral that had 28 to 36 months of severe DME, median VA loss was 4 lines, and 41% of eyes lost at least 6 lines. In contrast, only 5% of eyes without severe DME lost three or more lines of VA, and 1% lost at least 6 lines. Results were similar in treated eyes, but the proportions with severe DME for long periods were smaller (16–36 months in 10.1% of deferral eyes vs. 4.6% of treated eyes).
FIGURE 2.
Distribution of change in VA (number of lines) by duration of severe DME. (A) Eyes assigned to deferral of photocoagulation and (B) eyes assigned to immediate focal/grid photocoagulation for DME. Severe DME is defined as DME levels 4 and 5 combined. (C) Time at levels 5 (top) and 4 (bottom) are shown separately for eyes assigned to deferral of photocoagulation.
Figure 2C provides additional information on the effect of duration of DME at levels 4 and 5 on change in VA between baseline and 3 years in eyes assigned to deferral. The single, rightmost bar shows the distribution of VA change between baseline and 3 years in eyes in level 5 at the 1-, 2-, and 3-year visits; 77% of these 28 eyes lost at least 3 lines and 65% lost at least 6 lines. The next pair of bars to the left shows this distribution for eyes in level 5 at two of the three visits, with the remaining visit in level 4 (the right member of the pair) or a lower level (the left member of the pair). The VA decreases in these two groups were similar to each other and little less than those in eyes in level 5 at all three visits (76%–79% lost at least 3 lines and 45%–50% lost at least 6 lines). Eyes at level 5 at only one of the first three annual visits fared substantially better, and the presence of level 4 at two, one, or none of the other two annual visits appeared to make little difference (30%–47% of eyes lost at least 3 lines and 10%–20% lost at least 6 lines). Eyes in which the worst level was 4 for 1, 2, or 3 years fared better still (17%–28% lost at least 3 lines and 2%–17% lost at least 6 lines), and those with all visits at a level less than 4 even better (6% lost at least 3 lines and 1% at least 6 lines).
Table 4 presents the results of regression analyses that estimate the rate of change in VA (number of letters lost per year) by time spent at each DME severity level. Each group has an estimated slope (rate of change of visual acuity) based on the observed letters lost over the length of time spent at that DME severity level. The analysis was performed separately for the early-treatment and deferral groups and compared the average number of letters lost per year. Compared with change over time at level 1A, there was little difference at levels 1B and 1C, but there was a progressive increase in average yearly VA loss at each succeeding level, reaching 12 letters per year at level 5A and 17 letters per year at level 5B. Comparison of treatment groups showed that treatment decreased the amount of time that eyes spent at higher DME severity levels, but did not modify the effect on VA of time spent at a level.
TABLE 4.
Number of Letters Lost per Year in Each DME Severity Level and Effect of Time in Each Level, Relative to Time in Level 1A
| Level | Eye-Years | Change | Effect | 95% CI | P |
|---|---|---|---|---|---|
| Deferral | |||||
| 1A | 2929 | −0.3 | 0 | ||
| 1B | 762 | −1.0 | −0.7 | −1.4 to 0.1 | 0.08 |
| 1C | 1413 | −0.7 | −0.4 | −1.0 to 0.2 | 0.16 |
| 2 | 774 | −1.5 | −1.2 | −1.9 to −0.4 | 0.003 |
| 3A | 244 | −2.3 | −1.9 | −3.3 to −0.6 | 0.004 |
| 3B | 282 | −3.9 | −3.6 | −4.8 to −2.3 | <0.0001 |
| 4 | 236 | −4.7 | −4.4 | −5.7 to −3.1 | <0.0001 |
| 5A | 297 | −11.7 | −11.4 | −12.4 to −10.3 | <0.0001 |
| 5B | 25 | −17.1 | −16.8 | −20.5 to −13.1 | <0.0001 |
| Early treatment | |||||
| 1A | 3768 | −0.9 | 0 | ||
| 1B | 657 | −0.7 | 0.2 | −0.5 to 1.0 | 0.54 |
| 1C | 1169 | −1.2 | −0.3 | −0.9 to 0.3 | 0.37 |
| 2 | 777 | −1.8 | −0.9 | −1.6 to −0.1 | 0.02 |
| 3A | 185 | −2.2 | −1.3 | −2.9 to 0.2 | 0.09 |
| 3B | 236 | −4.2 | −3.3 | −4.6 to −2.0 | <0.0001 |
| 4 | 128 | −9.2 | −8.3 | −10.1 to −6.6 | <0.0001 |
| 5A | 140 | −12.8 | −11.9 | −13.5 to −10.3 | <0.0001 |
| 5B | 6 | −17.1 | −16.2 | −26.2 to −6.3 | 0.001 |
Eye-years, sum of the number of years that each eye spent in the DME level for the study population.
Modeling VA at the 3-year visit as a function of duration of time spent at DME severity levels during the 3-year observation period yielded an R2 of 32.9%, compared with an R2 of 20.7%, using the concurrent DME severity level and 27.6% using baseline VA. The combination of baseline VA and duration at DME severity levels yielded an R2 of 47.5%.
Table 5 presents the change in VA between baseline and 3 years by baseline DME level in all eyes assigned to deferral and in the eyes assigned to immediate focal/grid photocoagulation. Also shown in the table is the treatment effect (treated minus deferral), unadjusted and adjusted for duration at DME levels during the 3-year period. In the deferral group, VA loss increased from 4.8 letters at level 1A to 13.3 letters at level 5A. The beneficial effect of treatment was demonstrated by smaller VA losses at all baseline levels except 1A. After we accounted for duration at DME severity levels over the 3-year observation period, we saw no differences between treatments. Based on these models, it appears that the mechanism underlying the benefit of focal/grid treatment is the reduction of the severity and duration of retinal thickening.
TABLE 5.
VA Change in Letters between Baseline and 3 Years by Baseline DME Level in All Eyes
| Deferral of PC | Immediate Focal/Grid PC |
Unadjusted | Adjusted for Duration in DME Levels over 3 Years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | N | Mean | (95% CI) | N | Mean | (95% CI) | Effect (I – D) |
(95% CI) | P | Effect (I – D) |
(95% CI) | P |
| 1A | 1195 | −4.8 | −5.6 to −4.0 | 100 | −4.9 | −6.9 to −2.8 | −0.1 | −2.3 to 2.2 | 0.95 | −1.6 | −3.4 to 0.2 | 0.08 |
| 1B | 343 | −6.4 | −8.1 to −4.7 | 63 | −3.1 | −5.6 to −0.5 | 3.4 | 0.3 to 6.4 | 0.03 | 0.8 | −1.1 to 2.7 | 0.42 |
| 1C | 477 | −6.9 | −8.2 to −5.6 | 141 | −4.1 | −6.0 to −2.1 | 2.9 | 0.6 to 5.1 | 0.01 | −0.5 | −2.4 to 1.5 | 0.64 |
| 2 | 473 | −10.0 | −11.7 to −8.3 | 116 | −7.0 | −9.7 to −4.3 | 3.0 | −0.1 to 6.1 | 0.05 | −1.4 | −4.0 to 1.1 | 0.26 |
| 3A | 147 | −11.1 | −14.3 to −7.8 | 46 | −5.7 | −9.2 to −2.3 | 5.3 | 0.6 to 10.1 | 0.03 | −2.9 | −6.5 to 0.8 | 0.12 |
| 3B | 201 | −9.7 | −12.4 to −7.1 | 58 | −3.9 | −6.9 to −0.9 | 5.8 | 2.0 to 9.6 | 0.003 | 0.4 | −2.8 to 3.7 | 0.79 |
| 4 | 302 | −10.6 | −12.6 to −8.6 | 73 | −4.2 | −7.5 to −0.9 | 6.5 | 2.6 to 10.3 | 0.0009 | 0.8 | −3.0 to 4.7 | 0.68 |
| 5A | 221 | −13.3 | −15.9 to −10.8 | 61 | −0.5 | −4.0 to 3.0 | 12.8 | 8.6 to 17.1 | <0.0001 | 1.1 | −4.0 to 6.2 | 0.67 |
| 5B | 45 | −9.2 | −15.2 to −3.3 | 9 | 0.2 | −8.2 to 8.6 | 9.5 | 1.0 to 17.9 | 0.03 | 0.9 | −6.6 to 8.4 | 0.82 |
DISCUSSION
Herein we describe a scale for DME severity developed by using an approach similar to that used in developing the ETDRS Diabetic Retinopathy Severity Scale.7 Although the characteristics used in the scale—location and area of RT and degree of RT at the macular center—correlate, their cross-classification produces a more useful scale with more even distribution of information across its steps than either of these variables alone. This enhanced result is particularly apparent for the large number of eyes with RT at center at least 1 but less than two times the reference thickness (1363 eyes with mean VA 71.3 letters), which when divided by area of RT within 1 DD of center into four moderately large groups resulted in differences in mean VA ranging from 81.8 letters (~20/25) in the group with smallest area of thickening to 64.3 letters (~20/50) in the group with the largest area of thickening (Table 1). As might be expected from cross-classification of two risk factors, the off-diagonal cells in the table (those cells higher in one parameter but lower in the other) often have similar visual acuity results and are combined to form the risk groups.
The association between DME severity and concurrent VA is well known, and has been quantified with the advent of optical coherence tomography (OCT).9–12 The strength of the correlation between a slightly modified version of our scale and concurrent VA approached that for RT measured with time-domain OCT and VA (R = 0.47 and 0.57, respectively).13
Clinical experience suggests that decreases of several lines in VA are likely to occur after several years of severe DME, but this impression has not, to our knowledge, been well documented. Among eyes with VA 20/40 or better at baseline, we found that the proportion losing three or more lines over the 3-year period of observation increased from 5% in those in which severe DME (levels 4–5) never developed, to 15%–25% in those that had severe DME for 4 to 12 months and to 61% in those that had severe DME for 28 to 36 months (Fig. 2A). Initially, we pooled levels 4 and 5 for analysis on the basis of our clinical impression that they were similar, but Figure 2C suggests that long periods at level 5 are substantially more damaging. Our estimates of letters lost per year in a specified DME severity level increased from 0.3 at level 1A to 17.1 at level 5B (Table 4).
In previous ETDRS reports, the principal outcome measure has been the proportion of eyes with moderate visual loss (MVL), defined as a decrease in VA of ≥15 letters (3 lines), equivalent to a doubling of the visual angle (e.g., from 20/20–20/40), between baseline and specified follow-up visits.1–3 Focal/grid treatment reduced the risk of MVL by ~50% overall and in most subgroups. There was suggestive evidence of a larger treatment effect in eyes with greater area of RT within 1 DD of center and in those with greater degree of thickening at the center.3 The comparison of mean letters lost over the 3-year observation period between the deferral and focal/grid photocoagulation groups showed a similar treatment effect at all levels above 1A, an effect that appeared to become larger at the more severe levels and that disappeared when results were adjusted for duration at the levels observed over the 3-year period (Table 5). This result supports the presumption that the benefit of macular photocoagulation for DME results from the reduction of both the severity and duration of RT. The similarity between Figures 2A and 2B is also consistent with this conclusion.
Estimating RT with fundus photography is more difficult, less sensitive, and less reproducible than doing so with OCT.13 Although these factors seem likely to limit the future usefulness of the scale in clinical trials of treatments for DME, we believe it may continue to be of value for further analyses of ETDRS data and of other data sets collected before the widespread availability of OCT.
In summary, we describe a severity scale for DME based on the association between photographic estimates of the extent and location of RT and concurrent best-corrected VA. We found no additional photographic or angiographic factors that added substantially to development of the scale. According to the scale, the association between duration of severe DME and VA loss was estimated to range from 1 ETDRS letter lost per year of very mild DME to 17 letters per year of very severe DME.
Acknowledgments
Supported by Grant R03 EY1029-01 from the National Eye Institute.
Footnotes
Disclosure: R.E. Gangnon, None; M.D. Davis, None; L.D. Hubbard, None; L.M. Aiello, None; E.Y. Chew, None; F.L. Ferris III, None; M.R. Fisher, None
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 4. Int Ophthalmol Clin. 1987;27:265–272. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema: relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline. ETDRS report no. 19. Arch Ophthalmol. 1995;113(9):1144–1155. [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS Report Number 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 6.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report no. 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS Report Number 11. Ophthalmology. 1991;98:807–822. [PubMed] [Google Scholar]
- 9.Ferris FL, III, Patz A. Macular edema: a complication of diabetic retinopathy. Surv of Ophthalmol. 1984;28:452–461. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 10.Ferris FL, Podgor MJ, Davis MD, et al. Macular edema in diabetic retinopathy study patients. Diabetic Retinopathy Study Report Number 12. Ophthalmology. 1987;94:754–760. doi: 10.1016/s0161-6420(87)33526-2. [DOI] [PubMed] [Google Scholar]
- 11.Hee MJ, Puliafito CA, Duker, et al. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998;105:360–370. doi: 10.1016/s0161-6420(98)93601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MD, Bressler SB, Aiello LP, et al. Diabetic Retinopathy Clinical Research Network. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:1745–1752. doi: 10.1167/iovs.07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]