Abstract
Background:
Segmental bone defects pose reconstructive challenges. Composite tissue allotransplantation offers a potential solution but requires long-term immunosuppression with attendant health risks. This study demonstrates a novel method of composite-tissue allotransplantation, permitting long-term drug-free survival, with use of therapeutic angiogenesis of autogenous vessels to maintain circulation.
Methods:
Ninety-three rats underwent femoral allotransplantation, isotransplantation, or allografting. Group-1 femora were transplanted across a major histocompatibility complex barrier, with microsurgical pedicle anastomoses. The contralateral saphenous artery and vein (termed the AV bundle) of the recipient animal were implanted within the medullary canal to allow development of an autogenous circulation. In Group 2, allotransplantation was also performed, but with AV bundle ligation. Group 3 bones were frozen allografts rather than composite-tissue allotransplantation femora, and Group 4 bones were isotransplants. Paired comparison allowed evaluation of AV bundle effect, bone allogenicity (isogeneic or allogeneic), and initial circulation and viability (allotransplant versus allograft). Two weeks of immunosuppression therapy maintained blood flow initially, during development of a neoangiogenic autogenous blood supply from the AV bundle in patent groups. At eighteen weeks, skin grafts from donor, recipient, and third-party rats were tested for immunocompetence and donor-specific tolerance. At twenty-one weeks, bone circulation was quantified and new bone formation was measured.
Results:
Final circulatory status depended on both the initial viability of the graft and the successful development of neoangiogenic circulation. Median cortical blood flow was highest in Group 1 (4.6 mL/min/100 g), intermediate in Group 4 isotransplants (0.4 mL/min/100 g), and absent in others. Capillary proliferation and new bone formation were generally highest in allotransplants (15.0%, 6.4 μm3/μm2/yr) and isotransplants with patent AV bundles (16.6%, 50.3 μm3/μm2/yr) and less in allotransplants with ligated AV bundles (4.4%, 0.0 μm3/μm2/yr) or allografts (8.1%, 24.1 μm3/μm2/yr). Donor and third-party-type skin grafts were rejected, indicating immunocompetence without donor-specific tolerance.
Conclusions:
In the rat model, microvascular allogeneic bone transplantation in combination with short-term immunosuppression and AV bundle implantation creates an autogenous neoangiogenic circulation, permitting long-term allotransplant survival with measurable blood flow.
Clinical Relevance:
These methods may allow future composite-tissue allotransplantation of bone without the appreciable health risks that are associated with long-term immunosuppression or immune tolerance induction.
Large skeletal defects resulting from tumor resection, infection, or failed arthroplasty present a reconstructive challenge. Available reconstructive methods have limitations. Structural allografts (defined as allogeneic bone without blood supply or viable osteocytes) are incompletely revascularized1, as well as being prone to nonunion2 and late stress fracture3. A prosthetic replacement of diaphyseal bone may fail, loosen, or produce periprosthetic fractures4-7. Engineered bone replacements remain largely experimental.
Another alternative is the use of bone transplantation, in which the tissue, with its circulation and osteocyte viability intact, is placed in a new recipient site. Autogenous bone transplantation, commonly from the fibula as a free vascularized bone graft, is a common example. Bone may also be transplanted from a different donor of the same species. This is not a grafting procedure, but rather a form of composite-tissue allotransplantation with tissue limited to bone and its nutrient vessels. In this paper, for clarity, we will refer to such living tissue transfers as either allotransplants or isotransplants, depending on how closely the major histocompatibility complex (MHC) matched that of the recipient animal. For clarity, the term allograft will specifically refer to nonviable, previously frozen bone implanted without any native vascular supply.
Vascularized bone transplants are superior to bone grafts in both the ability to heal and the ability to remodel with applied stress, but expendable autogenous sources are frequently of insufficient size, shape, and strength. For larger defects, fibula and iliac crest are used, but these are generally poor structural replacements for segmental loss of the humerus, femur, and tibia. Humeral shaft and distal humeral reconstruction with autogenous fibula transplantation, for example, has a high rate of complications8.
Transplantation of living allogeneic bone could provide a replacement closely matched to the dimensions and mechanical properties of the resected bone, combined with the desirable healing properties of autotransplants. Few such procedures have been performed clinically because they require the use of long-term immunosuppression to maintain circulation and viability9,10. The results in human trials have been disappointing. A long-term review of femoral and knee allotransplants found failure in five of six cases11. In one transplant, viability was being maintained with long-term use of tacrolimus (FK-506) and mycophenolate mofetil immunosuppression. Methods permitting vascularized bone allotransplantation without the health risks that accompany the use of immunosuppressive drugs or immune tolerance induction would be an important advance.
The present study investigates a novel alternative to long-term immune modulation in which principles of therapeutic angiogenesis are used to create a new autogenous circulation within the allogeneic bone transplant. At the time of surgery, after repair of the allogeneic nutrient vessels, vascularized tissue from the recipient animal is implanted within the bone. Short-term immunosuppression therapy is used to maintain nutrient blood flow until the new autogenous circulation develops from the implanted vessels. Following withdrawal of drug therapy, long-term viability of the transplanted bone is maintained despite nutrient vessel thrombosis.
In this experiment, we have quantified blood flow and extent of angiogenesis, demonstrated biologic activity through new bone formation, and assessed whether a state of immune tolerance could otherwise account for maintained tissue viability. Similar methods applied clinically may eventually provide the ability to replace missing bone with living tissue of a similar size and shape, while maintaining the superior healing and remodeling abilities of living bone that have been noted in vascularized autogenous bone grafts.
Materials and Methods
Experimental Design
The experimental groups are shown in Table I. Inbred female Dark Agouti (DA) rats, weighing 150 to 175 g, were used as femoral allotransplant donors in Groups 1 and 2 and as allograft donors in Group 3. For the isotransplant controls (Group 4), we used female Piebald-Virol-Glaxo (PVG) rats weighing 200 to 250 g. Male PVG rats, weighing 200 to 250 g, were used as the recipient animals. The haplotypes of the rat groups used were DA (RT1.AaBa) and PVG (RT1.AcBc), comprising a full MHC mismatch. All animals were obtained from the same supplier (Harlan Sprague Dawley, Madison, Wisconsin). Groups 1 and 2 differed only in the patency of the implanted AV bundle (the contralateral saphenous artery and vein). Paired comparison between groups allowed evaluation of AV bundle effect in allotransplanted femora (ligated in Group 2 versus patent in Group 1), bone allogenicity (isogeneic in Group 4 rather than allogeneic in all other groups), and initial tissue circulation and viability (Group 1 allotransplant versus Group 3 allograft). All experiments were performed according to established National Institutes of Health guidelines and under the direction of our Institutional Animal Care and Use Committee.
TABLE I.
Experimental Groups and Independent Variables
| Group | Number of PVG Recipients | Group Descriptions* | Independent Variables |
||
| Length of Immunosuppression† | AV Bundle | Survival (wk) | |||
| 1 | 35 | Allotransplant: DA female to PVG male | 2 wk | Patent | 21 |
| 2 | 28 | Allotransplant: DA female to PVG male | 2 wk | Ligated | 21 |
| 3 | 11 | Allograft: DA female to PVG male | 2 wk | Patent | 21 |
| 4 | 19 | Isotransplant: PVG female to PVG male | 2 wk | Patent | 21 |
DA = Dark Agouti, and PVG = Piebald-Virol-Glaxo.
Immunosuppression was accomplished with use of FK-506 (1 mg/kg/day).
Sterile technique was maintained throughout the procedures. Donor animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (35 mg/kg) for the nonsurvival harvest surgery. The animals were killed with an intracardiac injection (Sleepaway, 200 mg/kg; Fort Dodge Animal Health, Fort Dodge, Iowa). Recipient animals were anesthetized with an intramuscular injection of a combination of ketamine (90 mg/kg) and xylazine (10 mg/kg). If necessary, during the surgical procedure, the animal received an additional dose of ketamine intraperitoneally (20 mg/kg). Fragmin (dalteparin; 10 IU per day) and butorphanol (0.05 mg/kg per day) were given subcutaneously for five days postoperatively. Staples and sutures were removed seven to ten days postoperatively. Animals were allowed to move freely in their cages and were fed standard rodent feed and water ad libitum.
Animal Model
Microvascular transplantation of the rat femur was first described in 1984 on the basis of prior anatomical dissections12. The vascular distribution to the bone is provided by proximal and distal nutrient branches arising from the lateral femoral circumflex artery and femoral artery, respectively.
Operative Procedure
Donor Femur Harvest
In Groups 1, 2, and 4, vascularized femoral allotransplants or isotransplants were harvested as follows. Under anesthesia, the right femoral and lateral femoral circumflex vessels were exposed. We used both the proximal and distal nutrient vessels to the femur, which branch from these vessels and pass between the pectineus muscle and the vastus medialis muscle into the femoral shaft. To facilitate microvascular anastomoses, the vessel dissection was extended proximally to include the common iliac artery and vein. The femur was then further dissected from surrounding tissues and disarticulated at the hip and knee joints. The femoral head and neck and distal femoral condyles were resected, and the medullary canal was reamed by hand with a 2-mm drill. The femoral transplant, once removed, was irrigated with heparinized saline solution and cooled during the preparation of the recipient site.
In Group 3, allografts were harvested without vascular pedicles. All soft tissue was removed from the allograft, which, after irrigation, was frozen in liquid nitrogen without cryoprotectants for thirty minutes, followed by storage at –70°C for a minimum of twenty-four hours.
Transplantation Procedure
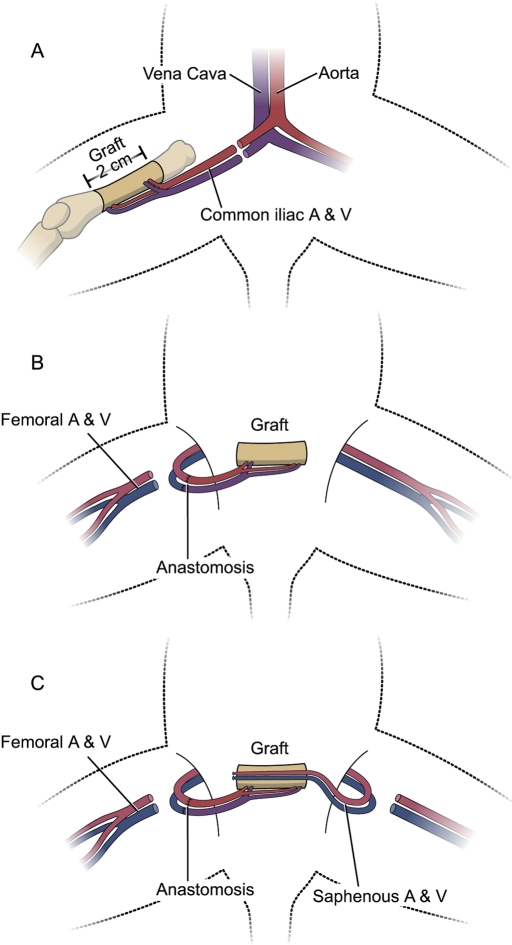
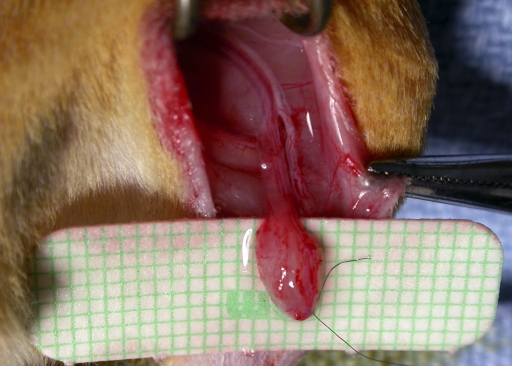
In the recipient animals for Groups 1, 2, and 4, the femur was transplanted heterotopically to a subcutaneous abdominal pocket and then revascularized by repair of the nutrient circulation. In the Group 3 allograft-recipient animals, the previously frozen femoral allograft was similarly positioned but without microvascular repairs. In all groups, a saphenous AV bundle was raised from the left hind limb of the recipient animal (Fig. 1) and placed within the medullary canal. The implanted AV bundle was a small pedicled fascial flap raised from the left limb, including the saphenous artery and venae comitantes (Fig. 2). Circulation was restored to the transplanted femur by end-to-end repair of its pedicle (common iliac artery and vein) to the femoral vessels, just proximal to the origin of the saphenous vessels, with use of 11-0 and 10-0 nylon sutures for the artery and vein, respectively. No vascular compromise to the hind limb was seen postoperatively, as sufficient nutrition is provided through pudendal branches and vessels traveling with the ischial nerve.
Fig. 1.
Diagram showing the surgical technique. A: Donor procedure: resection of the femoral diaphysis on the basis of the nutrient vessels to the bone. B: Microvascular anastomosis in the recipient; the slight size mismatch in donor and recipient animals permitted matching of the donor common iliac vessels to the recipient femoral vessels. C: AV bundle implantation; with the transplanted bone in the abdominal pocket, the recipient saphenous vessels were carefully pulled through the medullary canal and fixed to the abdominal wall.
Fig. 2.
Photograph of the AV bundle dissection in the left hind limb of a recipient animal. A 3 × 3-mm distal fascial flap, including the saphenous artery and venae comitantes, is elevated to the bifurcation in the groin. This permitted enough vascularity and length to ensure proper placement and survival inside the bone.
The abdominal location permitted isolation of the transplant from surrounding tissue by envelopment in a 0.18-mm-thick reinforced silicone sleeve (Bentec Medical, Woodland, California), enabling a more accurate assessment of blood flow differences between groups. The AV bundle was pulled through the medullary canal to fill its entire length and was then fixed with an 8-0 nylon suture. The AV bundle was ligated at this point in the Group 2 recipient rats. Short-term immunosuppression was provided with the administration of tacrolimus (Fujisawa Pharmaceutical, Osaka, Japan), at a dosage of 1 mg/kg per day for two weeks. Dosing parameters were based on our previous research, in which we found that a dosing period of two weeks was optimal to allow sufficient AV bundle angiogenesis to maintain blood flow and osteocyte viability after subsequent nutrient pedicle thrombosis13.
In a second survival procedure eighteen weeks after the initial surgery, three 15 × 15-mm full-thickness skin defects were made on the back of each recipient. Full-thickness skin grafts from donor-party, recipient-party, and third-party-type (Lewis [LEW]) rats were transplanted to each of the defects and fixed with a bolster dressing for five days.
Preparation for Transplant Harvest
The experiment concluded after a total survival period of twenty-one weeks. We used two methods to evaluate the circulatory status of the bone. Local blood flow to the cortical bone was measured quantitatively by the hydrogen washout method, and the extent of neoangiogenesis within the bone from the implanted autogenous AV bundle was quantified by measuring capillary density. Viability of osteocytes was assessed by a count of empty versus occupied lacunae, and the immune status and presence of tolerance to donor-specific antigens by skin grafting.
In a nonsurvival operation, anesthesia was induced. Arterial blood pressure was continuously monitored via a carotid artery catheter connected to a transducer (transducer pressure-monitoring kit, Edwards Lifesciences, Irvine, California; and LabVIEW software, National Instruments, Austin, Texas). A 14-gauge catheter connected to a T tube was inserted through a tracheotomy. One end of the T tube was connected via a Y-connector to an oxygen source and a hydrogen generator (Whatman Hydrogen Generator 75-32; Whatman, Haverhill, Massachusetts).
Bone Blood-Flow Measurement
The femoral transplant or graft was exposed through a transverse lower abdominal incision and by careful trimming of the silicone membrane. The patency of the microvascular anastomosis and AV bundle was evaluated. A patent vessel was observed to have both visible gross pulsation and a positive standard microsurgical patency test. Cortical blood flow was determined with use of the modified hydrogen washout method that we have previously described and validated14,15. Briefly, a shallow hole was drilled perpendicularly into the cortical bone. A hydrogen sensor (H2-50; Unisense, Aarhus, Denmark) connected through a picoammeter (PA2000; Unisense) to a data acquisition interface (DAQpad-6020E; National Instruments) was placed in the hole with a micromanipulator. Hydrogen was added to the breathing mixture (30% oxygen and 70% hydrogen), and tissue hydrogen saturation was achieved as determined by a steady-state sensor output. Blood flow was then calculated, by the rate of hydrogen washout from bone after the hydrogen generator was turned off, with use of the method and equation of Whiteside et al.16,17: C = C0e−Kt, where C is the hydrogen concentration at time t, and C0 the initial concentration. The flow constant is given by K = F/λ, where F = blood flow, expressed in mL/min/100 g tissue and λ is the partition coefficient of hydrogen in bone relative to that in blood16. We calculated the value of λ to be 0.72 for rat cortical diaphyseal bone in a previous experiment (authors’ unpublished data), as it had not been determined for this species previously.
Capillary Density
Before each animal was killed, the aorta distal to the renal arteries was cannulated and flushed with 60 mL of heparinized saline solution under physiologic pressure. After the animal was killed, Microfil (Flow Tech, Carver, Massachusetts), a colored synthetic angiographic polymer, was infused through the aorta under physiologic pressure. The AV bundle patency was again assessed. The compound was allowed to polymerize for twenty-four hours at 4°C. The bone was then fixed in 10% formalin for forty-eight hours and decalcified in 14% ethylenediaminetetraacetic acid (EDTA) for seven hours in a calibrated laboratory microwave at 750 W (Pelco Biowave 3450 Laboratory Microwave; Ted Pella, Redding, California). The intraosseous vasculature was visualized by modified Spalteholz methyl salicylate optical bone clearing18,19, and the capillary density (D, measured by dividing the vessel pixels by the total pixels) of each specimen was measured with use of image analysis software (Scion Image; Scion Corporation, Frederick, Maryland).
Grading of Skin-Graft Acceptance
Skin-graft acceptance was graded on the extent to which DA, PVG, and LEW grafts were viable at the time of experiment termination: a viability score of 0% to 69% was regarded as poor acceptance; 70% to 85%, fair acceptance; and 86% to 100%, good acceptance. Our grading system corresponds with clinical assessments.
Histologic Grading of Rejection
With use of specimens from a section of transplant pedicle and bone medullary canal, rejection was graded on a scale of 0 to 3 (with 0 indicating no rejection, 1 indicating mild rejection, 2 indicating moderate rejection, and 3 indicating severe rejection) on the basis of the amount of inflammation that could be seen in each hematoxylin-eosin-stained specimen at ×400 magnification20. To measure bone viability, the number of osteocyte-occupied lacunae versus empty lacunae was counted in three randomly selected fields (×400 magnification). The results were graded on a scale of 0 to 3, in which grade 0 indicated normal histology, grade 1 indicated less than half empty lacunae and decreased peritrabecular osteoblast lining, grade 2 indicated more than half empty lacunae and no peritrabecular osteoblast lining, and grade 3 indicated severe necrosis with empty lacunae and fragmentation.
Quantitative Histomorphometry
Calcein and tetracycline fluorescent markers were injected at the base of the tail vein at a dose of 20 mg/kg at two weeks and two days, respectively, prior to killing the animal. Glycol methylmethacrylate-embedded 5-μm-thick transverse sections were assessed with the use of quantitative bone histomorphometry software (OsteoMeasure; OsteoMetrics, Decatur, Georgia). Ten randomly selected fields at ×200 magnification were studied. Perimeters of trabecular bone surface, osteoblast-covered surface, eroded surface, and osteoclast-covered surface were measured. In three random samples from each group, adjacent transverse sections were stained with modified Goldner trichrome stain for mineral deposition and analyzed at ×20 and ×400 magnification.
Statistical Methods
Experimental characteristics are reported as medians (minimum, maximum) for continuous variables. Since the data were not normally distributed, nonparametric tests were used whenever possible, and all descriptive information was reported as the number and percentage of the total or the median and the range. Histology grading and skin-graft acceptance scores were compared overall and for each combination of two groups with use of a Cochran-Mantel-Haenszel extension of the Fisher exact test for ordered contingency tables21. Capillary density and blood flow values were evaluated overall with use of a Kruskall-Wallis test, and each combination of groups was evaluated with the Wilcoxon rank-sum test. The femoral medullary canal and cortex (ten random areas from ten different sections of ten specimens) were analyzed histologically by two independent reviewers. All statistical tests were two-sided, and p values of <0.05 were considered significant. Analyses were performed with use of SAS software (version 9.1; SAS Institute, Cary, North Carolina).
Source of Funding
This project was funded by a National Institutes of Health grant (AR49718).
Results
Significant differences between groups were found for blood flow, capillary density, and bone formation rates (p ≤ 0.003, Kruskal-Wallis test), as well as in bone viability grade and medullary canal inflammation (p < 0.001, Cochran-Mantel-Haenszel test). An overview of results is provided in Table II.
TABLE II.
Overview of Results*
| Group | Blood Flow (mL/min/100 g) | Capillary Density (%) | Bone Formation Rate (μm3/μm2/yr) | Medullary Canal Inflammation† | Bone Rejection Grade‡ |
| 1 | 4.58 | 15.0 | 6.4 | 1.0 | 1.0 |
| 2 | 0.00 | 4.4 | 0.0 | 1.5 | 3.0 |
| 3 | 0.00 | 8.1 | 24.1 | 3.0 | 3.0 |
| 4 | 0.44 | 16.6 | 50.3 | 1.0 | 0.0 |
All values are expressed as medians.
0 = no rejection, 1 = mild rejection, 2 = moderate rejection, and 3 = severe rejection on the basis of inflammation.
0 = normal histology, 1 = less than half empty lacunae and decreased peritrabecular osteoblast lining, 2 = more than half empty lacunae and no peritrabecular osteoblast lining, and 3 = severe necrosis with empty lacunae and fragmentation.
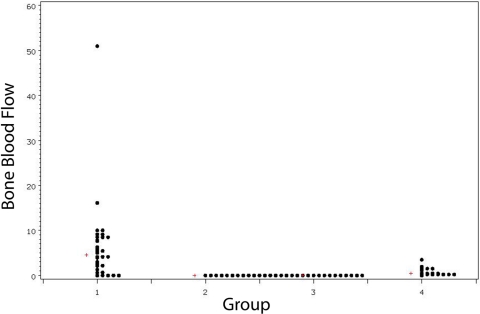
The median bone blood flow was 4.58 mL/min/100 g in allotransplants with a patent AV bundle (Group 1). This was significantly higher than that seen in the isotransplants (Group 4, 0.44 mL/min/100 g), the allotransplants with ligated AV bundles (Group 2, no flow), or the frozen allograft bone (Group 3, no flow) (p < 0.001 for each two-sample Wilcoxon rank-sum test) (Fig. 3).
Fig. 3.
Bone blood flow (mL/min/100 g) per group. The median value for each group is denoted by a plus sign (+).
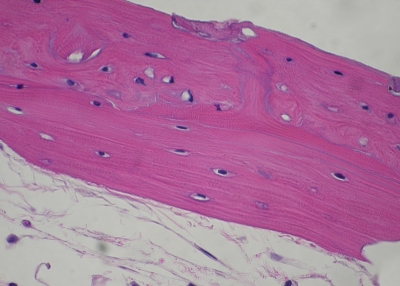
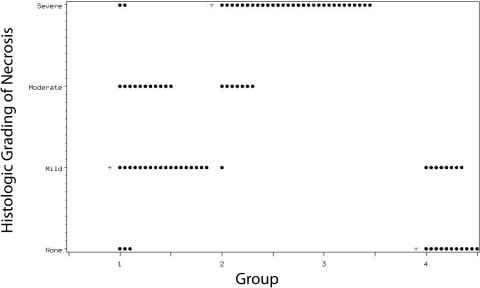
In Group 1, bone viability was grade 0 (normal) in 8.8%, grade 1 in 52.9%, grade 2 in 32.4%, and grade 3 (severe necrosis) in 5.9% (Figs. 4-A and 4-B). The respective results for Group 2 were 0.0%, 3.6%, 25.0%, and 71.4%. Purely grade-3 histology was found in Group 3. Group 4 bones had either normal histology (57.9%) or grade-1 necrosis (42.1%). Group 1 showed greater viability than Groups 2 and 3 did, and less viability than Group 4 did (p < 0.001, Cochran-Mantel-Haenszel test) (Fig. 5). Groups 2 and 3 did not differ statistically in bone viability (p = 0.06), but both had significantly fewer viable osteocytes than Group 4 had (p < 0.001).
Fig. 4-A Fig. 4-B.
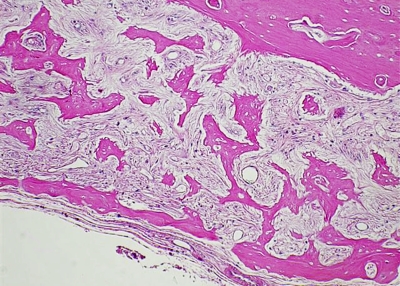
Figs. 4-A and 4-B Hematoxylin-eosin sections (original magnification, ×400). Fig. 4-A Representative sample from Group 1, in which more than 50% of the lacunae are filled with normal osteocytes and the sample exhibits normal bone morphology. Fig. 4-B Representative sample from Group 2, showing very few osteocyte-holding lacunae and grossly altered bone morphology with cortical necrosis and absent peritrabecular lining.
Fig. 5.
Bone viability per group, as measured by histologic grading of necrosis. The median value for each group is denoted by a plus sign (+).
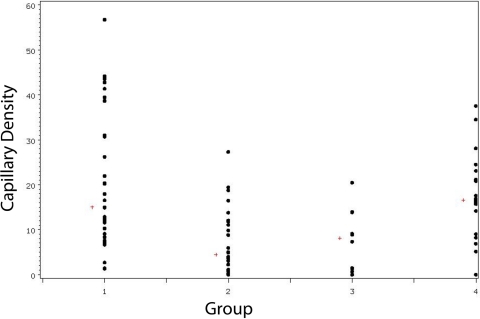
The median capillary density in Group-1 allotransplants was 15.0%, compared with 4.4% in Group-2 allotransplants with a ligated AV bundle (p < 0.001). Neoangiogenesis occurred within the Group-3 allografts to a lesser extent, at 8.1% (p = 0.01). The Group-4 isotransplants had the highest levels, at 16.6% (p = 0.96) (Wilcoxon rank-sum tests) (Fig. 6). Figure 7 shows the difference in capillary density in a fully revascularized allotransplant from Group 1 as compared with that in an allotransplant from Group 2. Groups 2 and 3 did not significantly differ in capillary density (p = 0.83), but had less than Group 4 (p < 0.001 and p = 0.008, respectively).
Fig. 6.
Capillary density (%) per group. The median value for each group is denoted by a plus sign (+).
Fig. 7.
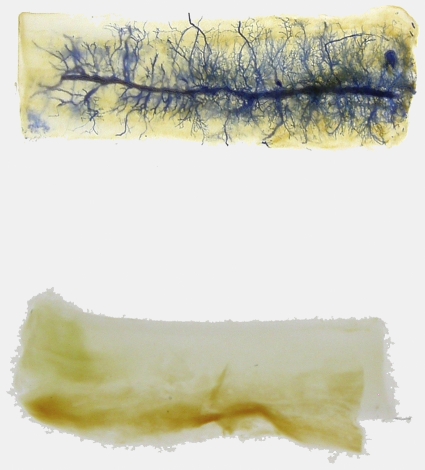
Cropped images showing a representative decalcified and cleared allotransplant from the Group-1 (top) and Group-2 (bottom) AV bundle groups. Polymerized Microfil infusion permits visualization of neovascularization (which is extensive in the Group-1 sample) from the implanted host-derived AV bundle.
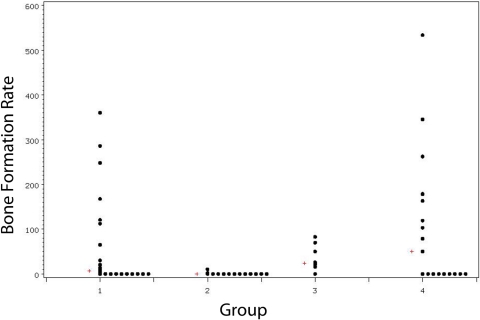
The median bone formation rate per bone surface area (μm3/μm2/yr) was 6.4 in Group-1 allotransplants, 0 in Group-2 allotransplants (p = 0.002), 24.1 in Group-3 allografts (p = 0.097), and 50.3 in Group-4 isotransplants (p = 0.63, Wilcoxon rank-sum tests) (Fig. 8). Group-2 allotransplants had significantly less bone formation than did Group-3 allografts or Group-4 isotransplants (p < 0.001 and p < 0.01, respectively). Groups 3 and 4 did not significantly differ in bone formation rates (p = 0.81). Figures 9-A and 9-B show representative images from Group-1 samples with use of fluorescent microscopy and dual-layer calcein and tetracycline labels.
Fig. 8.
Bone formation rate per bone surface area (μm3/μm2/yr) per group. The median value for each group is denoted by a plus sign (+).
Fig. 9-A Fig. 9-B.
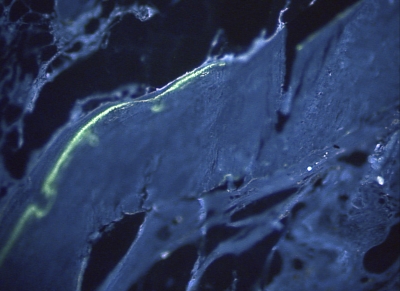
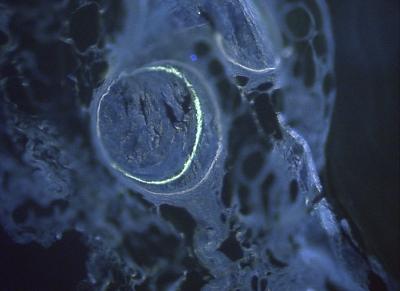
Figs. 9-A and 9-B Histomorphometric analysis of calcein and tetracycline-labeled, methylmethacrylate-embedded 5-μm sections (original magnification, ×200). Both samples are from Group 1. Double fluorescence was discernible both on the perimeter of the transplant (Fig. 9-A) and within the cortical boundaries (Fig. 9-B).
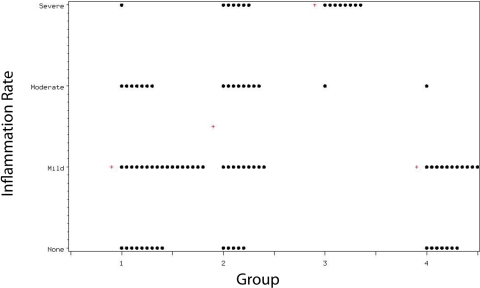
In Group-1 allotransplants, inflammation in the medullary canal was absent in 26.5%, mild in 50.0%, moderate in 20.6%, and severe in 2.9%. The respective values for Group-2 allotransplants were 17.9%, 32.1%, 28.6%, and 21.4%, which were significantly greater (p = 0.04) than the values in Group 1. Significantly more inflammation was seen in Group 3 than in Groups 1, 2, or 4 (p < 0.001). Group 1 compared favorably (p = 0.16) with Group 4, which had inflammation rates of 36.8% (none), 57.9% (mild), 5.3% (moderate), and no severe inflammation (Fig. 10).
Fig. 10.
Inflammation rate in the medullary canal per group. The median value for each group is denoted by a plus sign (+).
All DA skin grafts were fully rejected, demonstrating that donor-specific tolerance had not developed. PVG-to-PVG skin grafts showed good acceptance overall (84% good, 14% fair, and 2% poor), demonstrating successful skin-grafting technique. LEW-to-PVG skin grafts were all fully rejected, demonstrating a healthy immune system in the graft recipients.
The percentage of AV bundles that remained patent at the end of the study from among those that were initially patent was 75%. Trichrome-stained sections showed clear evidence of osteoblasts and osteoclasts adjacent to the mineral bone and osteocytes inside the mineral bone, with new bone formation around the AV bundle and at the perimeter of the bone. This was clearly more evident in the samples from Groups 1 and 4.
Discussion
The ideal replacement for large bone defects is one that behaves as the missing bone did; that is, it should be viable, nonimmunogenic to the host, and able to provide sound structural support. This might be accomplished best by transplantation rather than the use of structural allografting, if the appreciable risks of long-term immune modulation can be mitigated. The current study applies the methods of therapeutic angiogenesis to the problem of transplanting composite-tissue allografts, creating a novel method of tissue transplantation that requires neither immune suppression nor a state of tolerance. Instead, we have created a transplant chimera with an autogenous blood supply. This is accomplished by microsurgical transplantation and two weeks of drug immunosuppression to maintain short-term viability while angiogenesis from an autogenous AV bundle is occurring. This ultimately makes the nutrient pedicle superfluous and permits drug-free tissue viability as measured by blood flow, capillary density, osteocyte viability, and active bone-remodeling. We used four experimental groups to study this problem. We evaluated the effect of the AV bundle (by comparing allotransplants that had either ligated or patent AV bundles), bone allogenicity (by comparing revascularized isogeneic versus allogeneic transplants), and initial osteocyte viability (by comparing allotransplantation versus allografting).
Therapeutic angiogenesis for the purpose of treating or preventing tissue ischemia may be accomplished by surgical transfer of well-vascularized autogenous tissue; the therapy may be used alone or may be augmented by simultaneous administration of vasculogenic cytokines22-24. Surgical angiogenesis in autogenous bone grafts was first demonstrated by implantation of a vascular bundle in a canine model22. Implantation of vessels has been shown to induce neovascularization and new bone formation in conventional rabbit and canine autografts23,25,26, canine nonvascularized allografts27, and dog-to-rabbit nonvascularized xenografts28. The method has been applied clinically in the treatment of Kienböck disease29, talar osteonecrosis22,30, and scaphoid nonunion with osteonecrosis19,31,32, and in prefabricated bone free flaps33-35. It also improves the results of necrotic structural allografts36 and revascularizes massive structural allografts (intramedullary bone autograft)37.
The potential of surgical angiogenesis to maintain viability of vascularized bone and joint allotransplants with and without short-term immunosuppression has been the focus of our previous research13,38. In one study, we compared fresh nonvascularized allografts with vascularized allotransplanted bone, and found significantly more blood flow and viability in the transplants38. In another study, we showed that short-term immunosuppression is a prerequisite to retain viability in the long term13.
In this study, we demonstrated that the behavior of isotransplants was similar to that of autotransplants, with measurable blood flow in the cortex, new vascular networks being formed radially from the implanted AV bundle, new bone formation, absence of signs of rejection, and normal osteocyte-filled lacunae.
The allograft group (Group 3) had minimal viable bone due to initial necrosis and incomplete creeping substitution at the time of final follow-up. There was a strong inflammatory response due to the MHC mismatch, and bone remodeling was only seen adjacent to neoangiogenic blood vessels. These new vessels, forming from the AV bundle within the medullary canal, did not reach the cortex, leading to no measurable cortical blood flow.
The vascularized allotransplants with a ligated AV bundle (Group 2) were expected to thrombose their nutrient vessels following withdrawal of tacrolimus immunosuppression. The neoangiogenesis and resulting blood flow was small as a result; any new vessels that formed were the result of either AV bundle inosculation or the ingrowth of new vessels following in the path of the AV bundle. The hypovascularity is further reflected in the low bone-viability score and absence of bone formation in this group.
Finally, the allotransplanted femora with a patent AV bundle (Group 1) experienced enough time for capillaries to sprout from the implanted AV bundles, traverse the medullary canal, and penetrate cortical bone; this growth was reflected by measurable cortical blood flow and maintained despite the withdrawal of immunosuppression and the rejection of the transplant pedicle. The extent of capillary proliferation compared favorably to that seen in the isotransplant controls. The vasculature provided a source of host-derived osteoblasts and osteocytes, which maintained bone turnover and bone viability (i.e., measurable bone formation and high bone-viability grade, respectively). In the long term, the only immunogenic tissue remaining was sequestered within the bone (leading to a low inflammation grade).
Some differences in blood flow, capillary density, and histomorphometry were observed between groups; this is attributable to the independent variables by which they differ. Neoangiogenic capillaries reached the cortical edge more frequently in Group-1 bones than in Group-4 bones (Fig. 4). This resulted in higher cortical blood-flow values, which are only detected near the cortical bone surface by the hydrogen-sensing electrode. The overall capillary density was not different. As capillary density is measured within the entire bone volume, this demonstrates that the robust angiogenesis from Group-4 AV bundles had simply not advanced through the endosteal surface to the same extent as in Group 1.
The femoral allotransplants (Groups 1 and 2) had greater immunogenicity than the allograft Group 3, where deep freezing resulted in values that were between those of the allotransplanted (Groups 1 and 2) and the isotransplanted (Group 4) femora. Immune response within the bone is in turn dependent on the circulation of immune cells and antibodies, which can only occur when the blood supply to the bone is adequate. Differences in both initial blood flow (which was present in transplant Groups 1, 2, and 4 and absent in allograft Group 3), blood flow resulting from patent AV bundle angiogenesis, and tissue immunogenicity would all be expected to modulate the rejection response.
Osteocyte viability is dependent on either the survival of transplanted allogeneic cells or the replacement of those cells, resulting from later bone remodeling. The lineage of viable cells within long-term surviving bone allotransplants is of interest in transplanted tissues. We have previously demonstrated high levels of graft chimerism in rat femoral allotransplants with use of semiquantitative polymerase chain reaction analysis39. A more accurate evaluation of osteocytes suggests that many of these cells are of recipient origin40. Such chimeric replacement is made possible by the neoangiogenic capillary network, through which osteogenic cells migrate.
We used skin-grafting as a means for evaluation of immune competence and to demonstrate the absence of donor-specific tolerance. The method is a good indicator of class-I antigen mismatch. While flow cytometry and mixed lymphocyte reactions were not used in our study, others have shown that medullary washout before placing the transplant does not statistically reduce the class-II MHC load in experimental composite-tissue allotransplantations, which would indicate that our model is clinically relevant41,42. Skin has been shown to be the most immunogenic tissue in composite-tissue allotransplantations43. Our results demonstrate that the animals were immunocompetent at twenty-one weeks and that they had no donor-specific tolerance. Survival of the transplants is therefore explainable only by the development of a neoangiogenic circulation. If clinically applicable, this method would greatly increase the safety of allotransplantation.
Methods to produce a state of tolerance recently have been at least partially successful, using systemic irradiation44, monoclonal antibodies against adhesion molecules45,46, or short-term FK-506 combined with 15-deoxyspergualin47-49. Tissue survival associated with donor-specific tolerance has only recently been satisfactorily demonstrated in studies that made use of rat hind limb and vascularized skin transplant models, monoclonal αβ T-cell receptor antibodies, and a short course of either FK-506 or cyclosporine A50-52. Although mixed chimerism has been considered a promising method of tolerance induction, the method remains problematic for non-life-critical transplants53. The prevalence of graft-versus-host disease after bone-marrow transplantation has been reported to be 30% to 50% or more54,55. Transplantation of living musculoskeletal allotransplants containing bone marrow may carry some risk as well, although no evidence of graft-versus-host disease has been found following hand transplantation56. From the practical point of view, the limitations and complications of tolerance are currently as onerous as those of immunosuppression. Neither is acceptable if widespread use of allotransplantation of living musculoskeletal tissue is to become practical or ethical.
Using the rat model, we have described a novel method to maintain viability of bone allotransplants without the development of donor-specific tolerance or the need for long-term immunosuppression. In the present experimental study, we have demonstrated that bone allotransplants may maintain their bone blood flow through the development of an autogenous neocirculation, and that this may be accomplished without the sustained use of immunosuppression or the development of donor-specific tolerance. Ongoing research must focus in detail on the role of transplant chimerism as well as on the evaluation of mechanical properties after orthotopic transfer. Before the widespread use of composite tissue allotransplantation becomes a clinical reality, additional issues must be dealt with. The two most poignant hurdles are the availability of suitable donors and evaluating the precise risk of disease transmission through composite tissue allotransplantation. These practical considerations are similar to those for any organ transplant. Ultimately, we expect vascularized bone allotransplantation to become a viable and ethical method to reconstruct large bone defects.
Acknowledgments
Note: FK-506 was provided by the Fujisawa Pharmaceutical Company, Ltd.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (AR49718). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Bishop AT. Vascularized bone grafting. : Green DP, Hotchkiss RN, Pederson WC, editors Green's operative hand surgery. 4th ed New York: Churchill Livingstone; 1999. p 1221-50 [Google Scholar]
- 2.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86-97 [DOI] [PubMed] [Google Scholar]
- 3.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123-42 [PubMed] [Google Scholar]
- 4.Heck DA, Chao EY, Sim FH, Pritchard DJ, Shives TC. Titanium fibermetal segmental replacement prostheses. A radiographic analysis and review of current status. Clin Orthop Relat Res. 1986;204:266-85 [PubMed] [Google Scholar]
- 5.Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br. 1996;78:652-7 [PubMed] [Google Scholar]
- 6.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res. 1996;324:233-43 [DOI] [PubMed] [Google Scholar]
- 7.Schürmann M, Gradl G, Andress HJ, Kauschke T, Hertlein H, Lob G. Metastatic lesions of the humerus treated with the isoelastic diaphysis prosthesis. Clin Orthop Relat Res. 2000;380:204-14 [DOI] [PubMed] [Google Scholar]
- 8.Rose PS, Shin AY, Bishop AT, Moran SL, Sim FH. Vascularized free fibula transfer for oncologic reconstruction of the humerus. Clin Orthop Relat Res. 2005;438:80-4 [DOI] [PubMed] [Google Scholar]
- 9.Hofmann GO, Kirschner MH, Wagner FD, Brauns L, Gonschorek O, Bühren V. Allogeneic vascularized transplantation of human femoral diaphyses and total knee joints—first clinical experiences. Transplant Proc. 1998;30:2754-61 [DOI] [PubMed] [Google Scholar]
- 10.Doi K, Kawai S, Shigetomi M. Congenital tibial pseudoarthrosis treated with vascularised bone allograft. Lancet. 1996;347:970-1 [DOI] [PubMed] [Google Scholar]
- 11.Diefenbeck M, Wagner F, Kirschner MH, Nerlich A, Mückley T, Hofmann GO. Outcome of allogeneic vascularized knee transplants. Transpl Int. 2007;20:410-8 [DOI] [PubMed] [Google Scholar]
- 12.Schoofs M, Cariou JL, Amarante J, Panconi B, Bovet JL, Baudet J. Microvascular free bone transfer: experimental technique on rat's femur. Microsurgery. 1984;5:19-23 [DOI] [PubMed] [Google Scholar]
- 13.Ohno T, Pelzer M, Larsen M, Friedrich PF, Bishop AT. Host-derived angiogenesis maintains bone blood flow after withdrawal of immunosuppression. Microsurg. 2007;27:657-63 [DOI] [PubMed] [Google Scholar]
- 14.Larsen M, Pelzer M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique-part II: validation by comparison to microsphere entrapment. J Orthop Res. 2008;26:746-52 [DOI] [PubMed] [Google Scholar]
- 15.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique-part I: quantitative evaluation of tissue perfusion in the laboratory rat. J Orthop Res. 2008;26:741-5 [DOI] [PubMed] [Google Scholar]
- 16.Aukland K, Bower BF, Berliner RW. Measurement of local blood flow with hydrogen gas. Circ Res. 1964;14:164-87 [DOI] [PubMed] [Google Scholar]
- 17.Whiteside LA, Lesker PA, Simmons DJ. Measurement of regional bone and bone marrow blood flow in the rabbit using the hydrogen washout technique. Clin Orthop Relat Res. 1977;122:340-6 [PubMed] [Google Scholar]
- 18.Spalteholz K. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. Nebst Anhang: über Knochenfärbung. Leipzig: S. Hirzel; 1914 [Google Scholar]
- 19.Sheetz KK, Bishop AT, Berger RA. The arterial blood supply of the distal radius and ulna and its potential use in vascularized pedicled bone grafts. J Hand Surg Am. 1995;20:902-14 [DOI] [PubMed] [Google Scholar]
- 20.Büttemeyer R, Jones NF, Min Z, Rao U. Rejection of the component tissues of limb allografts in rats immunosuppressed with FK-506 and cyclosporine. Plast Reconstr Surg. 1996;97:139-51 [DOI] [PubMed] [Google Scholar]
- 21.Mehta CR, Patel NR, Tsiatis AA. Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics. 1984;40:819-25 [PubMed] [Google Scholar]
- 22.Hori Y, Tamai S, Okuda H, Sakamoto H, Takita T, Masuhara K. Blood vessel transplantation to bone. J Hand Surg Am. 1979;4:23-33 [DOI] [PubMed] [Google Scholar]
- 23.Busa R, Adani R, Castagnetti C, Zaffe D, Mingione A. Neovascularized bone grafts: experimental investigation. Microsurgery. 1999;19:289-95 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki O, Bishop AT, Sunagawa T, Katsube K, Friedrich PF. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery. 2004;24:85-91 [DOI] [PubMed] [Google Scholar]
- 25.Sunagawa T, Bishop AT, Muramatsu K. Role of conventional and vascularized bone grafts in scaphoid nonunion with avascular necrosis: a canine experimental study. J Hand Surg Am. 2000;25:849-59 [DOI] [PubMed] [Google Scholar]
- 26.Nagi ON. Revascularization of diaphyseal bone segments by vascular bundle implantation. Clin Orthop Relat Res. 2005;440:233-41 [DOI] [PubMed] [Google Scholar]
- 27.Carneiro R, Malinin T. Vascularized bone allografts: an experimental study in dogs. J Reconstr Microsurg. 1991;7:101-3 [DOI] [PubMed] [Google Scholar]
- 28.Ikebe S, Masumi S, Yano H, Fukunaga T, Shimizu K, Shin S. Immunosuppressive effect of tacrolimus (FK-506). Bone xenografts in rabbits. Acta Orthop Scand. 1996;67:389-92 [DOI] [PubMed] [Google Scholar]
- 29.Tamai S, Yajima H, Ono H. Revascularization procedures in the treatment of Kienböck's disease. Hand Clin. 1993;9:455-66 [PubMed] [Google Scholar]
- 30.Doi K, Hattori Y. Vascularized bone graft from the supracondylar region of the femur. Microsurgery. 2009;29:379-84 [DOI] [PubMed] [Google Scholar]
- 31.Shin AY, Bishop AT. Pedicled vascularized bone grafts for disorders of the carpus: scaphoid nonunion and Kienbock's disease. J Am Acad Orthop Surg. 2002;10:210-6 [DOI] [PubMed] [Google Scholar]
- 32.Jones DB, Jr, Bürger H, Bishop AT, Shin AY. Treatment of scaphoid waist nonunions with an avascular proximal pole and carpal collapse. Surgical technique. J Bone Joint Surg Am. 2009;91 Suppl 2(Pt 2):169-83 [DOI] [PubMed] [Google Scholar]
- 33.Vinzenz KG, Holle J, Würinger E, Kulenkampff KJ. Prefabrication of combined scapula flaps for microsurgical reconstruction in oro-maxillofacial defects: a new method. J Craniomaxillofac Surg. 1996;24:214-23 [DOI] [PubMed] [Google Scholar]
- 34.Hirasé Y, Valauri FA, Buncke HJ. Neovascularized bone, muscle, and myo-osseous free flaps: an experimental model. J Reconstr Microsurg. 1988;4:209-15 [DOI] [PubMed] [Google Scholar]
- 35.Khouri RK, Upton J, Shaw WW. Prefabrication of composite free flaps through staged microvascular transfer: an experimental and clinical study. Plast Reconstr Surg. 1991;87:108-15 [DOI] [PubMed] [Google Scholar]
- 36.Han CS, Wood MB, Bishop AT, Cooney WP., 3rd Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441-9 [PubMed] [Google Scholar]
- 37.Ceruso M, Falcone C, Innocenti M, Delcroix L, Capanna R, Manfrini M. Skeletal reconstruction with a free vascularized fibula graft associated to bone allograft after resection of malignant bone tumor of limbs. Handchir Mikrochir Plast Chir. 2001;33:277-82 [DOI] [PubMed] [Google Scholar]
- 38.Pelzer M, Larsen M, Chung YG, Ohno T, Platt JL, Friedrich PF, Bishop AT. Short-term immunosuppression and surgical neoangiogenesis with host vessels maintains long-term viability of vascularized bone allografts. J Orthop Res. 2007;25:370-7 [DOI] [PubMed] [Google Scholar]
- 39.Muramatsu K, Bishop AT. Microchimerism following vascularized bone allotransplantation. Transplant Proc. 2002;34:2722-4 [DOI] [PubMed] [Google Scholar]
- 40.Pelzer M, Larsen M, Friedrich PF, Aleff RA, Bishop AT. Repopulation of vascularized bone allotransplants with recipient-derived cells: detection by laser capture microdissection and real-time PCR. J Orthop Res. 2009;27:1514-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deijkers RL, Bouma GJ, van der Meer-Prins EM, Huysmans PE, Taminiau AH, Claas FH. Human bone allografts can induce T cells with high affinity for donor antigens. J Bone Joint Surg Br. 1999;81:538-44 [DOI] [PubMed] [Google Scholar]
- 42.Elves MW. Cell mediated immunity to allografts of fresh and treated bone. Int Orthop. 1978;2:171-5 [Google Scholar]
- 43.Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401-11 [DOI] [PubMed] [Google Scholar]
- 44.Ayhan S, Tugay C, Porvasnik S, Crownover R, Siemionow M. Effect of low-dose donor radiation on acute rejection of composite limb allografts. Transplant Proc. 2000;32:588-90 [DOI] [PubMed] [Google Scholar]
- 45.Gudemez E, Turegun M, Carnevale K, Zins J, Siemionow M. Effect of anti-ICAM-1 antibodies on macromolecular leakage and leukocyte activation: a study of hindlimb allografts in the rat. Plast Reconstr Surg. 1999;104:161-70 [PubMed] [Google Scholar]
- 46.Ozer K, Siemionow M. Combination of anti-ICAM-1 and anti-LFA-1 monoclonal antibody therapy prolongs allograft survival in rat hind-limb transplants. J Reconstr Microsurg. 2001;17:511-8 [DOI] [PubMed] [Google Scholar]
- 47.Muramatsu K, Doi K, Kawai S. Limb allotransplantation in rats: combined immunosuppression by FK-506 and 15-deoxyspergualin. J Hand Surg Am. 1999;24:586-93 [DOI] [PubMed] [Google Scholar]
- 48.Min Z, Jones NF. Limb transplantation in rats: immunosuppression with FK-506. J Hand Surg Am. 1995;20:77-87 [DOI] [PubMed] [Google Scholar]
- 49.Arai K, Hotokebuchi T, Miyahara H, Arita C, Mohtai M, Sugioka Y, Kaibara N. Limb allografts in rats immunosuppressed with FK506. I. Reversal of rejection and indefinite survival. Transplantation. 1989;48:782-6 [DOI] [PubMed] [Google Scholar]
- 50.Siemionow MZ, Izycki DM, Zielinski M. Donor-specific tolerance in fully major histocompatibility major histocompatibility complex-mismatched limb allograft transplants under an anti-alphabeta T-cell receptor monoclonal antibody and cyclosporine A protocol. Transplantation. 2003;76:1662-8 [DOI] [PubMed] [Google Scholar]
- 51.Ozer K, Izycki D, Zielinski M, Siemionow M. Development of donor-specific chimerism and tolerance in composite tissue allografts under alphabeta-T-cell receptor monoclonal antibody and cyclosporine A treatment protocols. Microsurgery. 2004;24:248-54 [DOI] [PubMed] [Google Scholar]
- 52.Demir Y, Ozmen S, Klimczak A, Mukherjee AL, Siemionow MZ. Strategies to develop chimerism in vascularized skin allografts across MHC barrier. Microsurgery. 2005;25:415-22 [DOI] [PubMed] [Google Scholar]
- 53.Larsen M, Habermann TM, Bishop AT, Shin AY, Spinner RJ. Epstein-Barr virus infection as a complication of transplantation of a nerve allograft from a living related donor. Case report. J Neurosurg. 2007;106:924-8 [DOI] [PubMed] [Google Scholar]
- 54.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA, et al. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689-91 [DOI] [PubMed] [Google Scholar]
- 55.Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, Gajewski J, Hansen JA, Henslee-Downey J, McCullough J, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593-602 [DOI] [PubMed] [Google Scholar]
- 56.Granger DK, Briedenbach WC, Pidwell DJ, Jones JW, Baxter-Lowe LA, Kaufman CL. Lack of donor hyporesponsiveness and donor chimerism after clinical transplantation of the hand. Transplantation. 2002;74:1624-30 [DOI] [PubMed] [Google Scholar]