Abstract
Background:
The prevalence of knee osteoarthritis is traditionally based on radiographic findings, but magnetic resonance imaging is now being used to provide better visualization of bone, cartilage, and soft tissues as well as the patellar compartment. The goal of this study was to estimate the prevalences of knee features defined on magnetic resonance imaging in a population and to relate these abnormalities to knee osteoarthritis severity scores based on radiographic findings, physical functioning, and reported knee pain in middle-aged women.
Methods:
Magnetic resonance images of the knee were evaluated for the location and severity of cartilage defects, bone marrow lesions, osteophytes, subchondral cysts, meniscal and/or ligamentous tears, effusion, and synovitis among 363 middle-aged women (724 knees) from the Michigan Study of Women's Health Across the Nation. These findings were related to Kellgren-Lawrence osteoarthritis severity scores from radiographs, self-reported knee pain, self-reported knee injury, perception of physical functioning, and physical performance measures to assess mobility. Radiographs, physical performance assessment, and interviews were undertaken at the 1996 study baseline and again (with the addition of magnetic resonance imaging assessment) at the follow-up visit during 2007 to 2008.
Results:
The prevalence of moderate-to-severe knee osteoarthritis changed from 3.7% at the baseline assessment to 26.7% at the follow-up visit eleven years later. Full-thickness cartilage defects of the medial, lateral, and patellofemoral compartments were present in 14.5% (105 knees), 4.6% (thirty-three knees), and 26.2% (190 knees), respectively. Synovitis was identified in 24.7% (179) of the knees, and joint effusions were observed in 70% (507 knees); 21.7% (157) of the knees had complex or macerated meniscal tears. Large osteophytes, marked synovitis, macerated meniscal tears, and full-thickness tibial cartilage defects were associated with increased odds of knee pain and with 30% to 40% slower walking and stair-climbing times.
Conclusions:
Middle-aged women have a high prevalence of moderate-to-severe knee osteoarthritis corroborated by strong associations with cartilage defects, complex and macerated meniscal tears, osteophytes and synovitis, knee pain, and lower mobility levels.
Level of Evidence:
Prognostic Level II. See Instructions to Authors for a complete description of levels of evidence.
Osteoarthritis is a highly prevalent disease whose impact is expected to increase markedly worldwide because of the aging of populations1,2 and the increasing prevalence of obesity. Recent data from Johnson County, North Carolina, indicate that one of every two elderly persons has symptomatic knee osteoarthritis (a Kellgren-Lawrence score of ≥2 and symptoms in the same knee)2. Osteoarthritis is a major reason for disability in women3, and osteoarthritis-related symptoms are one of the most common reasons for visits to health-care providers2,4.
Historically, knee radiographs and a report of pain have been used to describe the prevalence of osteoarthritis, although there are limitations to the use of each5-8. There are substantial between-person differences in responses to the same level of pain stimulus9, but articular cartilage is without nocireceptors10. Anteroposterior radiographs exclude adequate visualization of the patellofemoral compartment, and certain abnormalities such as ligamentous injury or synovitis are not well visualized on a radiograph. To address some of these limitations, high-resolution and tissue-contrast magnetic resonance imaging (MRI) is being used to display bone, cartilage, and soft tissue with greater sensitivity.
Our previous work demonstrated that MRI-defined knee damage was evident in a group of 120 middle-aged women11. However, because the sample selection was based on the presence of knee osteoarthritis and pain, accurate population-based prevalences of MRI-defined knee damage could not be estimated. Thus, the purposes of this study were to identify the prevalence of radiographic osteoarthritis of the knee and of multiple MRI-defined abnormalities in a population-based sample of women and to relate the MRI-defined features to radiographically defined knee osteoarthritis scores, measures of physical functioning, and self-report of knee pain and knee injury.
Materials and Methods
The Michigan Study of Women's Health Across the Nation (SWAN) is one of seven sites for SWAN, a multiethnic cohort study characterizing endocrinological, physiological, and behavioral changes occurring during the menopausal transition. The Michigan SWAN population, established in 1996, is a population-based sample of eligible women from two Detroit-area communities. The population was identified with a community census with use of electrical utility records that listed every residential household in the eligible communities. Households were contacted by telephone or in person to identify and recruit study-eligible women in residence.
Study eligibility criteria for SWAN at the 1996 baseline included an age of forty-two to fifty-two years; the presence of an intact uterus and at least one ovary; no use of exogenous hormones; menses in the three months prior to enrollment; and self-identification with a site's designated race or ethnic group (either African American or Caucasian at the Michigan site). Eligibility for participation in SWAN was not predicated on the presence or absence of pain, knee osteoarthritis, or physical functioning.
The Michigan SWAN site recruited 543 eligible women to the SWAN Core Longitudinal Study. By study design, at baseline, 60% of the women were self-declared African American and 40% were Caucasian, a proportion that has been sustained across the eleven-year follow-up period. At the 1996 baseline, Michigan SWAN women completed the assessment protocol common to all SWAN sites; then, only at the Michigan site, a supplemental protocol including radiographs and functional assessments was implemented. Women were seen for annual follow-up visits, although the supplemental imaging protocol was not included at all visits. Participation at the annual assessments has been excellent, with >80% seen at follow-up visit 11.
All Michigan SWAN women were eligible for inclusion in the osteoarthritis and physical functioning protocols; however, women with artificial joints, pacemakers, defibrillators, or other implanted metal considered incompatible with MRI were excluded from the MRI protocol. A table in the Appendix shows the 1996 baseline characteristics of the women who had been retained after ten years of follow-up, with and without MRI. Of the 419 women seen at the time of follow-up visit 11, 361 had an MRI of both knees, two participants had an MRI of one knee, and twenty-nine other women had radiographs and a physical functioning assessment but no MRI. Further, twenty-seven women completed only the interview portion of the protocol. There was no difference in body-mass index (BMI) and baseline radiographic knee osteoarthritis scores in women with and without MRI.
An institutional review board approved the study protocol, and written informed consent was obtained from each participant.
This report is based on radiographs from baseline (which occurred from 1996 to 1997) and follow-up visit 11 (which occurred from 2007 to 2008) as well as MRI, pain, and physical functioning data from follow-up visit 11.
Osteoarthritis Measures
Weight-bearing anteroposterior radiographs with semiflexed positioning12 were obtained with use of General Electric radiographic equipment at baseline (model X-GE MPX-80; General Electric Medical Systems, Milwaukee, Wisconsin). The AXIOM Aristos radiographic system with integrated digital flat detector technology (Siemens, Erlangen, Germany) was used in 2007. The anteroposterior radiograph characterizes knee osteoarthritis in the medial and lateral femorotibial compartments, excluding the patellofemoral compartment. The Kellgren-Lawrence scale is a five-level osteoarthritis severity score (with 0 indicating normal; 1, doubtful; 2, mild or minimal; 3, moderate; and 4, severe) based on a pictorial guide13 depicting the degree of osteophyte formation, joint space narrowing, sclerosis, and joint deformity. Knee osteoarthritis was defined as at least one knee with a score of ≥2, and moderate-to-severe osteoarthritis was defined as a score of 3 or 4. The three right knees and the two left knees with a knee replacement were assigned a Kellgren-Lawrence score of 4.
Magnetic Resonance Imaging
Knees were imaged at the time of follow-up visit 11. Each knee was assessed, globally and by compartment, for cartilage defects, subchondral bone marrow lesions, osteophytes, subchondral cysts, meniscal abnormalities, ligamentous abnormalities (including anterior cruciate [ACL], posterior cruciate [PCL], medial collateral [MCL], and lateral collateral [LCL] ligaments), joint effusions, Baker cysts, and synovitis. A description of the MRI sequences protocol and information about the MRI reading and scoring procedures are available in the Appendix.
Knee Pain, Knee Injury, and Physical Functioning
Participant interview and performance-based physical functioning data were collected during follow-up visit 11. Knee pain was characterized by asking if the participant had experienced knee pain in the past month. A report of pain in one knee is highly correlated with a report of pain in the other knee, so results for the right knee are presented in the text and tables; the left knee profiles were similar. Knee injury was defined as a report by the participant of a serious knee injury in the past year. Self-reported knee functioning was characterized with the seventeen-item Western Ontario and McMaster Universities Arthritis Index14,15 (WOMAC) scaled to values from 0 to 100. Performance-based measures of physical functioning included a timed 40-ft (12.19-m) walk (in seconds) and a timed stair climb (in seconds). For the timed walk, participants walked down a carpeted hallway at a “brisk, purposeful pace” for a distance of 40 ft. For the timed stair climb, participants climbed up and down a set of three standard stairs three times, beginning with the right leg. Total movement time was recorded as the amount of time required to ascend the stairs, turn, and descend the stairs three consecutive times. Times were measured by a trained staff member using a stopwatch and were recorded to the tenth of a second.
Statistical Analysis
Baseline information refers to data (interviews and knee radiographs) collected during the study visit that occurred from 1996 to 1997. Year-11 follow-up information refers to data (interviews, physical functioning assessments, knee radiographs, and knee MRI) collected during the follow-up visit that occurred from 2007 to 2008. The frequencies (percentage) or prevalence of radiographic scores were calculated for baseline and the year-11 follow-up. Likewise, the frequency (percentage) of MRI findings was calculated globally and by compartment (medial, lateral, and patellofemoral). Comparisons between frequencies were based on chi-square tests or Fisher exact tests.
Magnetic resonance imaging variables were correlated (Spearman correlations) by knee with the corresponding (left and right knee) osteoarthritis scores (Kellgren-Lawrence scores 0 or 1, 2, and 3 or 4). The three-level Kellgren-Lawrence scores and the MRI-feature scores by compartment were associated with pain and injury measures with use of logistic regression analyses. The measure of association, the odds ratio, was estimated from the beta coefficient and accompanied by 95% confidence intervals (95% CI). Analysis of variance was used to estimate the group means of the physical functioning performance measures according to the three-level Kellgren-Lawrence scores and MRI scores.
Source of Funding
The recruitment and annual assessment of SWAN participants at all sites were funded under the U.S. National Institutes of Health cooperative agreements AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495. The Michigan SWAN participants were recruited and assessed under cooperative agreement NR004061, with the radiographs, MRI, and functional evaluations funded by AG017104.
Results
Prevalence of Radiographically Defined Knee Osteoarthritis
The prevalence of radiographically defined knee osteoarthritis changed from 18.1% (ninety-eight women) at the baseline assessment to 62.4% (225) at follow-up visit 11, at which time the women were fifty-three to sixty-three years old (Table I). The prevalence of moderate-to-severe knee osteoarthritis (Kellgren-Lawrence scores of 3 or 4) was 3.7% (twenty women) at baseline and 26.7% (ninety-six) at the time of follow-up visit 11 (Table I).
TABLE I.
Characteristics of Women at the Baseline Visit (1996-1997) and Among Those with Knee MRI and Knee Radiographs at the Year-Eleven Follow-up Evaluation (2007-2008)
| Baseline (N = 543) | Follow-up Visit 11 (N = 360) | |
| Age*(yr) | 45 (5) | 56 (5) |
| Body mass index*(kg/m2) | 31.1 (11.0) | 33.0 (11.5) |
| Kellgren-Lawrence severity score from radiographs† | ||
| 0 or 1 (normal or doubtful osteoarthritis) | 81.9 (79, 85) | 37.6 (33, 43) |
| 2 (minimal osteoarthritis) | 14.4 (11, 17) | 35.7 (31, 41) |
| 3 or 4 (moderate to severe osteoarthritis) | 3.7 (2, 5) | 26.7 (22, 31) |
| Knee pain in the last month (% of participants) | 29.0 | 41.1 |
| Knee injury (% of participants) | ||
| Ever | 19.6 | NA |
| In the previous year | NA | 4.1 |
The values are given as the median with the interquartile range in parentheses.
The values are given as the percentage with the 95% confidence interval in parentheses. NA = not available.
MRI Prevalence Findings
The prevalences of MRI-defined features are summarized in Tables II, III, and IV, globally, by compartment (medial, lateral, and patellofemoral), and for specific articular surfaces, respectively.
TABLE II.
MRI-Defined Cartilage and Bone Marrow Edema Lesions Among 724 Knees by Compartment and Surface
| Medial Compartment* |
Lateral Compartment* |
Patellofemoral Compartment* |
|||||||||
| Femur | Tibia | Overall† | Femur | Tibia | Overall† | Trochlea | Medial Facet | Lateral Facet | Overall† | Any Location*† | |
| Cartilage defects | |||||||||||
| Normal | 81 (11.2) | 126 (17.4) | 70 (9.7) | 138 (19.1) | 197 (27.2) | 98 (13.5) | 194 (26.8) | 78 (10.8) | 175 (24.2) | 43 (5.9) | 5 (0.7) |
| Internal signal alteration only | 62 (8.6) | 153 (21.1) | 48 (6.6) | 91 (12.6) | 222 (30.7) | 88 (12.2) | 109 (15.1) | 50 (6.9) | 126 (17.4) | 34 (4.7) | 12 (1.7) |
| Defect of <50% | 318 (43.9) | 306 (42.3) | 331 (45.7) | 356 (49.2) | 208 (28.7) | 366 (50.6) | 231 (31.9) | 251 (34.7) | 218 (30.1) | 217 (30) | 197 (27.2) |
| Defect of 50%-99% | 163 (22.5) | 74 (10.2) | 170 (23.5) | 114 (15.8) | 70 (9.7) | 139 (19.2) | 104 (14.4) | 231 (31.9) | 122 (16.9) | 240 (33.2) | 254 (35.1) |
| 100% defect with no bone ulceration | 89 (12.3) | 57 (7.9) | 93 (12.9) | 24 (3.3) | 23 (3.2) | 29 (4.0) | 82 (11.3) | 107 (14.8) | 77 (10.6) | 175 (24.2) | 227 (31.4) |
| 100% defect with bone ulceration | 11 (1.5) | 8 (1.1) | 12 (1.7) | 1 (0.1) | 4 (0.6) | 4 (0.6) | 4 (0.6) | 7 (1) | 6 (0.8) | 15 (2.1) | 29 (4.0) |
| Bone marrow edema | |||||||||||
| Normal | 627 (86.6) | 603 (83.3) | 570 (78.7) | 681 (94.1) | 642 (88.7) | 627 (86.6) | 569 (78.6) | 512 (70.7) | 527 (72.8) | 395 (54.6) | 318 (43.9) |
| Small (largest diameter <1 cm) | 75 (10.4) | 101 (14) | 121 (16.7) | 34 (4.7) | 67 (9.3) | 78 (10.8) | 136 (18.8) | 211 (29.1) | 188 (26) | 303 (41.9) | 340 (47) |
| Large (largest diameter 1.01-2.0 cm) | 14 (1.9) | 15 (2.1) | 21 (2.9) | 3 (0.4) | 10 (1.4) | 11 (1.5) | 17 (2.4) | 1 (0.1) | 9 (1.2) | 24 (3.3) | 46 (6.4) |
| Very large (largest diameter >2.0 cm) | 8 (1.1) | 5 (0.7) | 12 (1.7) | 6 (0.8) | 5 (0.7) | 8 (1.1) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 2 (0.3) | 20 (2.8) |
The values are given as the number of knees, with the percentage in parentheses.
Based on the most severe score across compartments.
TABLE III.
MRI-Defined Osteophytes, Subchondral Cysts, and Meniscal Tears Among 724 Knees by Compartment
| Medial Compartment* | Lateral Compartment* | Patellofemoral Compartment* | Overall (Any Compartment)* | |
| Osteophytes | ||||
| No osteophytes | 284 (39.2) | 314 (43.4) | 317 (43.8) | 203 (28.0) |
| Osteophyte of ≤5 mm | 299 (41.3) | 301 (41.6) | 344 (47.5) | 341 (47.1) |
| Osteophyte of >5 mm | 141 (19.5) | 109 (15.1) | 63 (8.7) | 180 (24.9) |
| Subchondral cysts | ||||
| Normal | 636 (87.9) | 661 (91.3) | 554 (76.5) | 472 (65.2) |
| Small (≤1 cm) | 79 (10.9) | 61 (8.4) | 164 (22.7) | 236 (32.6) |
| Large (>1 cm) | 9 (1.2) | 2 (0.3) | 6 (0.8) | 16 (2.2) |
| Meniscal abnormalities | ||||
| Normal | 110 (15.2) | 219 (30.3) | NA | 82 (11.4) |
| Intrasubstance abnormality only | 390 (54.0) | 378 (52.4) | NA | 355 (49.2) |
| Simple, nondisplaced tear | 108 (15.0) | 69 (9.6) | NA | 128 (17.7) |
| Complex, macerated tear | 114 (15.8) | 56 (7.8) | NA | 157 (21.7) |
| Meniscal extrusion | ||||
| None | 27 (3.7) | 340 (47.1) | NA | 15 (2.1) |
| <5 mm | 587 (81.3) | 362 (50.1) | NA | 580 (80.3) |
| ≥5 mm | 108 (15.0) | 20 (2.8) | NA | 127 (17.6) |
The values are given as the number of knees with the percentage in parentheses. NA = not applicable.
TABLE IV.
MRI-Defined Ligamentous Abnormalities, Synovitis, Effusion, and Baker Cysts Among Left and Right Knees
| Left Knee* (N = 363) | Right Knee* (N = 361) | Overall* (N = 724) | |
| Anterior cruciate ligament | |||
| Normal | 305 (84.0) | 296 (82.0) | 601 (83.0) |
| Edematous and/or sprain | 52 (14.3) | 61 (16.9) | 113 (15.6) |
| Complete tear | 6 (1.7) | 4 (1.1) | 10 (1.4) |
| Posterior cruciate ligament | |||
| Normal | 361 (99.5) | 356 (98.6) | 717 (99.0) |
| Edematous and/or sprain | 2 (0.6) | 5 (1.4) | 7 (1.0) |
| Complete tear | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Medial collateral ligament | |||
| Normal | 247 (68.0) | 250 (69.6) | 497 (68.8) |
| Grade-1 sprain | 110 (30.3) | 104 (29.0) | 214 (29.6) |
| Grade-2 sprain | 6 (1.7) | 5 (1.4) | 11 (1.5) |
| Complete tear | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lateral collateral ligament | |||
| Normal | 248 (68.3) | 229 (63.8) | 477 (66.1) |
| Grade-1 sprain | 6 (1.7) | 6 (1.7) | 12 (1.7) |
| Grade-2 sprain | 107 (29.5) | 122 (34.0) | 229 (31.7) |
| Complete tear | 2 (0.6) | 2 (0.6) | 4 (0.6) |
| Synovitis | |||
| Normal | 272 (74.9) | 273 (75.6) | 545 (75.3) |
| Mild | 71 (19.6) | 63 (17.5) | 134 (18.5) |
| Moderate to marked | 20 (5.5) | 25 (6.9) | 45 (6.2) |
| Effusion | |||
| Normal physiological fluid | 107 (29.5) | 110 (30.5) | 217 (30.0) |
| Small (≤10 mm) | 224 (61.7) | 219 (60.7) | 443 (61.2) |
| Moderate or large (>10 mm) | 32 (8.8) | 32 (8.9) | 64 (8.8) |
| Baker cyst | |||
| Normal | 226 (62.3) | 240 (66.5) | 466 (64.4) |
| Small | 111 (30.6) | 103 (28.5) | 214 (29.6) |
| Large | 26 (7.2) | 18 (5.0) | 44 (6.1) |
The values are given as the number of knees with the percentage in parentheses.
Cartilage Defects
The prevalence (and standard deviation) of knee cartilage defects was high in the medial (83.7% ± 37.0%; 606 knees), lateral (74.3% ± 43.7%; 538 knees), and patellofemoral compartments (89.4% ± 30.9%; 647 knees). When aggregated across compartments and surfaces, 97.7% (707 knees) had some MRI-identified cartilage defect. Importantly, the prevalence of full-thickness cartilage defects was 14.5% (105 knees), 4.6% (33 knees), and 26.2% (190 knees) in the medial, lateral, and patellofemoral compartments, respectively.
Bone Marrow Lesions
Most bone marrow lesions were in the patellofemoral compartment. The prevalences of bone marrow lesions in the medial, lateral, and patellofemoral compartments were 21.3% (154 knees), 13.4% (ninety-seven knees), and 45.4% (329 knees), respectively.
Osteophytes and Cysts
The frequency of osteophytes was congruent with the prevalence of radiographically defined osteoarthritis in this sample; prevalences can be found by compartment in Table III. Subchondral cysts were found in approximately one-third of the knees (Table III).
Menisci
Meniscal tears, both simple and complex, occurred with greater frequency in the medial compartment compared with the lateral compartment (Table III). Notably, 21.7% (157) of all knees had a complex or macerated meniscal tear. Most knees had some degree of meniscal extrusion, and the meniscus was extruded ≥5 mm in 17.6% (127) of the knees; this was more common in the medial compartment compared with the lateral compartment.
Synovitis, Effusions, and Baker Cysts
Synovitis was present in 24.7% (179) of the knees; synovitis was rated as moderate to marked in 6.2% (forty-five knees). Joint effusions were detected in 70% (507) of the knees. Large Baker cysts were observed in 6.1% of the knees.
Ligamentous Tissues
There were few complete tears of the ACL or LCL and no tears of the PCL or MCL (Table IV).
Correlations Between Three-Level Kellgren-Lawrence Scores and MRI Scores
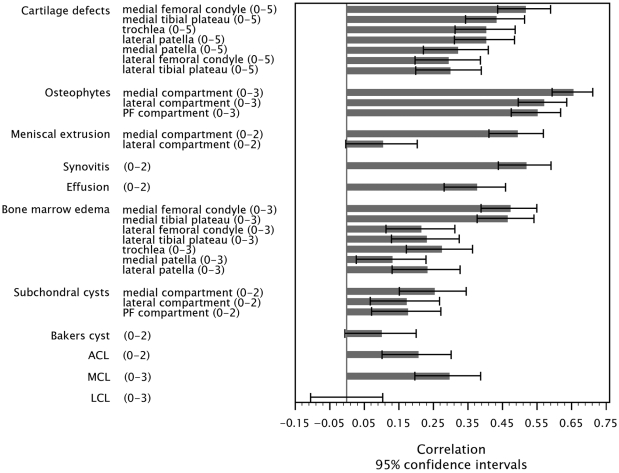
The three-level Kellgren-Lawrence scores (0 or 1, 2, and 3 or 4) for severity of knee osteoarthritis based on radiographic findings were most highly correlated with cartilage defects, osteophytes, meniscal extrusion, and synovitis, particularly in the medial compartment (Fig. 1).
Fig. 1.
Spearman rank correlations, and 95% confidence intervals, for the magnetic resonance imaging (MRI)-feature scores with the Kellgren-Lawrence scores (0 or 1, 2, and 3 or 4) for the left knee. Possible scores for each MRI feature are included in parentheses with the labels on the left side of the figure. PF = patellofemoral, ACL = anterior cruciate ligament, MCL = medial collateral ligament, and LCL = lateral collateral ligament.
Among cartilage defects, those in the medial femoral condyle were most highly correlated with radiographically defined knee osteoarthritis (r = 0.52; 95% CI, 0.44 to 0.59). Correlations of MRI-identified osteophytes and radiographically defined knee osteoarthritis ranged from r = 0.66 (95% CI, 0.59 to 0.71) in the medial compartment to r = 0.55 (95% CI, 0.47 to 0.62) in the patellofemoral compartment.
The magnitude of the associations between radiographically defined Kellgren-Lawrence scores and MRI-defined meniscal extrusion varied by compartment; correlations were strong for medial meniscal extrusions (r = 0.49) and much weaker for lateral extrusions (r = 0.10). The correlations between Kellgren-Lawrence scores and MRI-defined synovitis (r = 0.52; 95% CI, 0.44 to 0.59) and effusion (r = 0.38; 95% CI, 0.28 to 0.46) were high.
While the Kellgren-Lawrence scores were highly correlated with bone marrow lesions in the medial femoral and tibial compartments, associations were much weaker with bone marrow lesions in the trochlea and the lateral and patellar compartments. Ligamentous abnormalities were also highly variable in their correlation with Kellgren-Lawrence scores (Fig. 1).
Associations of Knee Pain with Imaging
The prevalence of knee pain (during the previous month) changed from 29% at baseline to 41.1% at the time of follow-up visit 11 (Table I). Compared with women with Kellgren-Lawrence scores of 0 or 1, there was an increased odds of knee pain in women with a Kellgren-Lawrence score of 2 (odds ratio [OR] = 1.6; 95% CI, 0.9 to 2.9) and a significantly increased odds with Kellgren-Lawrence scores of 3 or 4 (OR = 11.3; 95% CI, 6.2 to 20.4) (see Appendix).
While cartilage defects were significantly associated with increased pain (see Appendix), the odds of reporting pain were frequently not significant unless the cartilage defects were full thickness. The odds of reporting pain with 100% (full-thickness) cartilage defects in the medial and lateral compartments were greater than with defects in the patella. Compared with women without osteophytes, those with either large osteophytes (>5 mm) or small osteophytes (≤5 mm), medially or laterally, were more likely to report pain (see Appendix). Likewise, the presence of meniscal tears or meniscal abnormalities was associated with a report of pain.
For some anatomical sites, the odds of reported pain with smaller bone marrow lesions (<1 cm) was greater than that with the larger lesions (see Appendix). However, large bone marrow lesions in the medial femoral condyle or the medial or lateral tibial plateaus were associated with substantially increased odds of reported pain. Mild or moderate-to-marked synovitis and moderate-to-large effusions were associated with increased odds of reported pain, but smaller effusions were not.
Associations of Knee Injury and Imaging
Knee injury in the previous year was reported by approximately 4% of the women at the time of follow-up visit 11 (Table I) and was associated with Kellgren-Lawrence osteoarthritis severity scores of 3 or 4 (OR = 5.9; 95% CI, 1.2 to 29.7). Knee injury was also associated with the presence of a 100% (full-thickness) cartilage defect in the lateral femoral condyle or lateral tibial plateau, medial or lateral osteophytes of >5 mm, and small Baker cysts (see Appendix).
Associations of Functional Performance and Imaging
The amount of time needed to complete the 40-ft walk and the stair climb was highly associated with the Kellgren-Lawrence osteoarthritis scores, including a mean increase of approximately 20% in the timed walk and a mean increase of 34% in the timed stair climb in a comparison of those with Kellgren-Lawrence scores of 3 or 4 and those with a score of 0 or 1 (Table V). There was a decrease in functional performance in both walking and stair-climbing with some anatomical features, particularly those in the medial aspect of the knee. Full-thickness cartilage defects in the medial femoral condyle and the medial tibial plateau were associated with 15% to 30% decreases in walking and stair-climbing performance (p < 0.001). The presence of medial or lateral osteophytes (>5 mm), moderate-to-marked synovitis, and moderate or large effusion were associated with physical functioning decreases of >20% to 40% (p < 0.001). Bone marrow lesions in the medial femoral condyle and the medial tibial plateau were also associated with marked decreases in walking and stair-climbing performance (p < 0.001).
TABLE V.
Results of Timed Walk, Timed Stair Climb, and Western Ontario and McMaster Universities Arthritis Index (WOMAC) Functioning Score According to Kellgren-Lawrence Three-Level Score and Magnetic Resonance Imaging Anatomical Features*
| Timed Walk (sec) |
Timed Stair Climb (sec) |
WOMAC Score |
||||||||
| No. of Knees | Mean | Standard Error | P Value | Mean | Standard Error | P Value | Mean | Standard Error | P Value | |
| Kellgren-Lawrence score | <0.001 | <0.001 | <0.001 | |||||||
| 0 or 1 | 143 | 10.22 | 0.19 | 18.79 | 0.44 | 2.85 | 0.30 | |||
| 2 | 138 | 10.63 | 0.20 | 20.45 | 0.49 | 4.75 | 0.51 | |||
| 3 or 4 | 105 | 12.20 | 0.26 | 25.09 | 0.72 | 11.88 | 1.50 | |||
| Cartilage | ||||||||||
| Medial femoral condyle | <0.001 | <0.001 | <0.001 | |||||||
| Normal or internal signal alteration only | 34 | 10.59 | 0.41 | 19.72 | 0.99 | 3.04 | 0.68 | |||
| Defect of ≤}99% | 258 | 10.56 | 0.15 | 19.85 | 0.37 | 4.08 | 0.34 | |||
| 100% defect | 71 | 11.92 | 0.32 | 24.55 | 0.89 | 11.35 | 1.78 | |||
| Medial tibial plateau | <0.001 | <0.001 | <0.001 | |||||||
| Normal or internal signal alteration only | 81 | 10.45 | 0.26 | 19.63 | 0.64 | 3.36 | 0.48 | |||
| Defect of ≤99% | 234 | 10.63 | 0.15 | 20.20 | 0.39 | 4.38 | 0.38 | |||
| 100% defect | 48 | 12.50 | 0.41 | 25.41 | 1.14 | 15.22 | 2.91 | |||
| Lateral patella | 0.05 | 0.02 | <0.001 | |||||||
| Normal or internal signal alteration only | 103 | 10.32 | 0.23 | 19.28 | 0.58 | 3.27 | 0.44 | |||
| Defect of ≤99% | 202 | 11.01 | 0.18 | 21.05 | 0.45 | 5.22 | 0.50 | |||
| 100% defect | 58 | 11.03 | 0.34 | 21.84 | 0.89 | 7.50 | 1.34 | |||
| Lateral femoral condyle | 0.01 | <0.001 | <0.001 | |||||||
| Normal or internal signal alteration only | 64 | 10.45 | 0.29 | 19.60 | 0.71 | 3.36 | 0.56 | |||
| Defect of ≤99% | 277 | 10.77 | 0.15 | 20.31 | 0.36 | 4.80 | 0.39 | |||
| 100% defect | 22 | 12.50 | 0.61 | 29.65 | 1.87 | 17.45 | 5.19 | |||
| Lateral tibial plateau | 0.05 | <0.001 | <0.001 | |||||||
| Normal or internal signal alteration only | 158 | 10.54 | 0.19 | 19.75 | 0.47 | 4.09 | 0.44 | |||
| Defect of ≤99% | 181 | 10.91 | 0.18 | 20.76 | 0.47 | 4.86 | 0.49 | |||
| 100% defect | 24 | 11.89 | 0.56 | 26.83 | 1.69 | 15.55 | 4.44 | |||
| Osteophytes | ||||||||||
| Medial compartment | <0.001 | <0.001 | <0.001 | |||||||
| No osteophyte | 93 | 10.10 | 0.23 | 19.05 | 0.57 | 2.84 | 0.38 | |||
| Osteophyte of ≤5 mm | 174 | 10.58 | 0.18 | 19.72 | 0.43 | 4.19 | 0.41 | |||
| Osteophyte of >5 mm | 96 | 12.06 | 0.27 | 24.54 | 0.75 | 10.84 | 1.45 | |||
| Lateral compartment | <0.001 | <0.001 | <0.001 | |||||||
| No osteophyte | 105 | 10.14 | 0.22 | 18.88 | 0.52 | 2.79 | 0.35 | |||
| Osteophyte of ≤5 mm | 178 | 10.61 | 0.17 | 19.71 | 0.41 | 4.62 | 0.45 | |||
| Osteophyte of >5 mm | 80 | 12.29 | 0.30 | 26.19 | 0.86 | 11.38 | 1.67 | |||
| Meniscal abnormalities | ||||||||||
| Medial compartment | 0.08 | <0.001 | <0.001 | |||||||
| Normal | 27 | 10.67 | 0.47 | 20.45 | 1.21 | 2.89 | 0.74 | |||
| Intrasubstance meniscal abnormality only | 186 | 10.56 | 0.18 | 19.43 | 0.42 | 3.70 | 0.36 | |||
| Nondisplaced or displaced tear | 150 | 11.17 | 0.21 | 22.39 | 0.56 | 7.60 | 0.84 | |||
| Lateral compartment | 0.12 | <0.001 | 0.003 | |||||||
| Normal | 63 | 10.55 | 0.30 | 20.10 | 0.76 | 3.49 | 0.60 | |||
| Intrasubstance meniscal abnormality only | 205 | 10.69 | 0.17 | 19.97 | 0.43 | 4.48 | 0.43 | |||
| Nondisplaced or displaced tear | 95 | 11.26 | 0.27 | 22.59 | 0.71 | 7.21 | 1.02 | |||
| Synovitis | <0.001 | <0.001 | <0.001 | |||||||
| Normal | 239 | 10.40 | 0.15 | 19.40 | 0.36 | 3.61 | 0.31 | |||
| Mild | 88 | 11.34 | 0.27 | 22.10 | 0.69 | 7.38 | 1.03 | |||
| Moderate to marked | 36 | 12.45 | 0.46 | 26.92 | 1.34 | 13.35 | 3.07 | |||
| Effusion | <0.001 | <0.001 | <0.001 | |||||||
| Normal physiological fluid | 61 | 10.03 | 0.28 | 18.64 | 0.69 | 2.82 | 0.46 | |||
| Small (≤}10 mm) | 252 | 10.75 | 0.15 | 20.28 | 0.37 | 4.44 | 0.36 | |||
| Moderate or large (>10 mm) | 50 | 12.25 | 0.40 | 26.15 | 1.14 | 14.99 | 2.79 | |||
| Bone marrow edema | ||||||||||
| Medial femoral condyle | <0.001 | <0.001 | <0.001 | |||||||
| Normal | 288 | 10.59 | 0.14 | 20.01 | 0.36 | 4.06 | 0.32 | |||
| Small (largest diameter of ≤1 cm) | 54 | 11.57 | 0.36 | 23.00 | 0.95 | 8.52 | 1.55 | |||
| Large or very large (largest diameter of >1 cm) | 21 | 12.10 | 0.62 | 24.08 | 1.64 | 14.58 | 4.55 | |||
| Medial tibial plateau | <0.001 | <0.001 | <0.001 | |||||||
| Normal | 270 | 10.48 | 0.14 | 19.83 | 0.36 | 4.12 | 0.34 | |||
| Small (largest diameter of ≤1 cm) | 73 | 11.99 | 0.32 | 23.25 | 0.83 | 7.11 | 1.13 | |||
| Large or very large (largest diameter of >1 cm) | 20 | 11.36 | 0.58 | 23.13 | 1.53 | 11.07 | 3.41 | |||
| Lateral patella | 0.09 | 0.29 | 0.002 | |||||||
| Normal | 230 | 10.60 | 0.16 | 20.26 | 0.41 | 3.98 | 0.36 | |||
| Small (largest diameter of ≤1 cm) | 124 | 11.18 | 0.23 | 21.27 | 0.59 | 6.75 | 0.83 | |||
| Large or very large (largest diameter of >1 cm) | 9 | 11.45 | 0.92 | 22.19 | 2.37 | 7.31 | 3.49 | |||
| Lateral femoral condyle | <0.001 | <0.001 | 0.005 | |||||||
| Normal | 328 | 10.68 | 0.13 | 20.04 | 0.33 | 4.49 | 0.34 | |||
| Small (largest diameter of ≤1 cm) | 27 | 11.79 | 0.52 | 25.53 | 1.42 | 10.35 | 2.75 | |||
| Large or very large (largest diameter of >1 cm) | 8 | 13.21 | 1.05 | 32.19 | 3.28 | 8.98 | 4.59 | |||
| Lateral tibial plateau | 0.09 | <0.001 | <0.001 | |||||||
| Normal | 299 | 10.70 | 0.14 | 20.00 | 0.35 | 4.23 | 0.33 | |||
| Small (largest diameter of ≤1 cm) | 51 | 11.10 | 0.36 | 23.14 | 0.96 | 7.91 | 1.49 | |||
| Large or very large (largest diameter of >1 cm) | 13 | 12.21 | 0.77 | 26.34 | 2.16 | 16.71 | 6.45 | |||
| Baker cysts | 0.53 | 0.17 | 0.008 | |||||||
| Normal | 180 | 10.87 | 0.19 | 20.30 | 0.46 | 4.15 | 0.42 | |||
| Small | 145 | 10.66 | 0.20 | 20.60 | 0.53 | 5.03 | 0.57 | |||
| Large | 38 | 11.14 | 0.42 | 22.53 | 1.13 | 8.91 | 2.01 | |||
| Anterior cruciate ligament | 0.21 | <0.001 | 0.09 | |||||||
| Normal | 255 | 10.74 | 0.15 | 20.10 | 0.38 | 4.52 | 0.39 | |||
| Edematous and/or sprain | 100 | 10.89 | 0.25 | 21.51 | 0.66 | 5.37 | 0.74 | |||
| Complete tear | 8 | 12.38 | 1.00 | 30.17 | 3.39 | 12.05 | 5.82 | |||
| Medial collateral ligament | 0.01 | <0.001 | <0.001 | |||||||
| Normal | 218 | 10.54 | 0.16 | 19.74 | 0.40 | 3.92 | 0.36 | |||
| Sprain | 145 | 11.23 | 0.21 | 22.12 | 0.56 | 6.73 | 0.77 | |||
| Complete tear | 0 | – | – | – | – | – | – | |||
The values represent the maximum Kellgren-Lawrence score or MRI-feature score from the right and left knee. Cartilage (medial patella and trochlea), bone marrow edema (medial patella and trochlea), subchondral cysts, and lateral collateral ligament are not included in the table because they were not significantly associated with timed walk, timed stair climb, or WOMAC.
Women with Kellgren-Lawrence scores of 3 or 4, indicating moderate-to-severe osteoarthritis, had WOMAC scores that were more than double the WOMAC functioning scores for women with a Kellgren-Lawrence score of 2 (mild osteoarthritis). Increasing WOMAC scores were also significantly associated with certain MRI features including cartilage defects, osteophytes, meniscal tears, synovitis, effusion, bone marrow lesions, and MCL abnormalities (Table V).
Discussion
Current estimates of the prevalence of knee osteoarthritis with corroboration among findings from radiographs, MRI, and reports of pain as well as measures of functional capacity in a population sample are important to identify the nature and magnitude of the problem, to consider windows of opportunity to forestall progression to more severe knee osteoarthritis, and to determine whether certain anatomical features of the knee can be more successful targets for treatment. We identified a surprisingly high prevalence of radiographically defined knee osteoarthritis and corresponding MRI-defined abnormalities in a comprehensive evaluation of 363 women from fifty-four to sixty-four years old. In the eleven-year period following their initial 1996 examination, the prevalence of radiographically defined knee osteoarthritis in these women tripled to 62% (225 women). More than one-quarter (ninety-six) of the women had moderate-to-severe knee osteoarthritis as evidenced by measures from either radiography or MRI. The magnitude of these prevalences, the potential for current and continued economic and social contributions from these women, as well as the specter of the cost of the treatment of knee osteoarthritis for an average of thirty additional years suggest this is an apt time to more aggressively address severity-specific and feature-specific interventions.
By extending the evaluation to include MRI, the extent, nature, and impact of the knee osteoarthritis became more evident and may help to determine the priority for feature-specific interventions. While all women had some evidence of cartilage abnormalities, >25% (ninety-nine) had a full-thickness cartilage defect. Nearly 20% of the knees (127) had menisci extruded ≥5 mm and a similar number (157 knees) had a complex, macerated meniscal tear. One in three women (124 of 363) had evidence of synovitis, and nearly one in ten women (thirty-six) had marked or severe synovitis. Moderate to large effusions were identified in approximately 15% (fifty) of the women.
The impact of knee osteoarthritis based on radiographic or MRI-defined anatomical defects was corroborated with measures of physical performance. The average time required to walk 40 ft or ascend and descend three steps was greatest among women with full-thickness cartilage defects. Stair-climbing time appeared to be a particularly sensitive indicator of the impact of osteoarthritis. It has long been recognized that stair-climbing requires strength; the large torques at weight-bearing joints are considerably higher with stair-climbing than with walking, and stair descent may be particularly risky since joint torque is greater in descent than in ascent16. Stair ascent and descent also require dynamic balance control since the body weight is controlled from a single support limb during swing.
The degree to which patellar anatomical defects contribute to the impact of knee osteoarthritis is of current interest because this compartment is inaccessible with the standard anteroposterior radiograph. We found substantial evidence of bone marrow lesions and cartilage defects in the patellar compartment. However, there was much less congruence between self-reported pain and decreases in physical performance for patellar compartment defects compared with other compartments. This is an important observation because aggressive treatment, including surgery, would not be undertaken in patients without pain and a decline in physical functioning. Currently, the natural history of the progression of patellar defects is incompletely understood and needs further development so we can anticipate how abnormalities in this compartment affect disease progression in other compartments or influence pain and contribute to loss of function.
The contribution of bone marrow lesions to the impact of knee osteoarthritis is also of current interest. The degree of congruence between self-reported pain and the frequency or size of bone marrow lesions was compartment-specific. While pain was associated with bone marrow lesions in the medial or lateral compartment, it was poorly associated with bone marrow lesions of the patellar compartment. Further, larger bone marrow lesions were not strongly associated with pain; indeed, the smaller lesions were associated with a report of pain as much or more than the larger lesions. Notably, since these associations are based on cross-sectional MRI data, we could not determine whether the small lesions were developing or resolving.
The profile of women with increased odds of reporting knee pain included the presence of large osteophytes, synovitis, macerated meniscal tears, full-thickness (100%) cartilage defects, and small bone marrow lesions in the medial femoral condyle or large bone marrow lesions in the medial or lateral tibial plateau. Similar profiles were observed in the right and left knees.
This study has notable strengths and some limitations. The study included a panel of measures to define knee osteoarthritis including radiographs, MRI, self-report of pain and functioning, and performance-based measures of functioning. The study was population-based, and women were not selected on the basis of disease outcome or risk-factor profiles, thereby making the findings applicable to the general population of women. There was minimal loss to follow-up after eleven years. Since our unpublished data indicated that women who are more functionally challenged become less likely to participate, we surmise that our findings reflect a bias toward the participants in this sample who had fewer knee abnormalities than may be expected when considering the entire cohort. The quality of the MRI assessments is very good, with readings by experienced radiologists utilizing a rigorous quality assurance protocol.
Nevertheless, there are limitations. While it would be of interest to have patellar pain measures, especially in light of our interesting findings of disease in the patellofemoral compartment, our pain questions are not compartment-specific and did not differentiate between patellar pain and other knee pain. Although the same quality assurance protocols were implemented for image scoring, the radiographs from baseline and follow-up visit 11 were read by different radiologists. At the time of writing, we did not have full-length radiographs of the lower extremity that would allow us to consider varus or valgus alignment. Since this report is descriptive, we did not undertake any statistical modeling to test hypotheses and account for covariates or measurement error, or to address the nonparticipation of women who had become physically compromised over the eleven years of follow-up. The findings are not generalizable to men.
Appendix
The MRI scoring sheet, the description of the MRI sequences protocol and the MRI scoring procedures for Michigan SWAN; a table showing population characteristics as well as participation information for follow-up visit 11; and a table showing right knee pain or any knee injury associated with the three-level Kellgren-Lawrence score and MRI features are available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Supplementary Material
Acknowledgments
Note: The authors thank Suzan Rohrer, Tom Chenevert, Erin Butler, and the staffs of the University of Michigan Hospital and St. Joseph Mercy Hospital for their assistance with MRI and radiographic data collection.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health; National Institute on Aging; National Institute of Nursing Research; and Office of Research on Women's Health. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Centers for Disease Control and Prevention (CDC) Public health and aging: projected prevalence of self-reported arthritis or chronic joint symptoms among persons aged >65 years—United States, 2005-2030. MMWR Morb Mortal Wkly Rep. 2003;52:489-91 [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers M, Jannausch ML, Gross M, Karvonen-Gutierrez CA, Palmiere RM, Crutchfield M, Richards-McCullough K. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006;163:950-8 [DOI] [PubMed] [Google Scholar]
- 4.From the Centers for Disease Control and Prevention Prevalence and impact of arthritis among women—United States, 1989-1991. JAMA. 1995;273:1820, 1822 [PubMed] [Google Scholar]
- 5.Hodler J, Resnick D. Current status of imaging of articular cartilage. Skeletal Radiol. 1996;25:703-9 [DOI] [PubMed] [Google Scholar]
- 6.Braunstein EM, Brandt KD, Albrecht M. MRI demonstration of hypertrophic articular cartilage repair in osteoarthritis. Skeletal Radiol. 1990;19:335-9 [DOI] [PubMed] [Google Scholar]
- 7.Leouille D, Olivier P, Mainard D, Gillet P, Netter P, Blum A. Review: magnetic resonance imaging of normal and osteoarthritic cartilage. Arthritis Rheum. 1998;41:963-75 [DOI] [PubMed] [Google Scholar]
- 8.Peterfy CG, Genant HK. Emerging applications of magnetic resonance imaging in the evaluation of articular cartilage. Radiol Clin North Am. 1996;34:195-213, ix [PubMed] [Google Scholar]
- 9.Hawthorn J, Redmond K. Pain: causes and management. Oxford: Blackwell Science; 1998. The pain experience; p 70-80 [Google Scholar]
- 10.Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O'Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421-30 [DOI] [PubMed] [Google Scholar]
- 11.Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC, Sowers MR. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237:998-1007 [DOI] [PubMed] [Google Scholar]
- 12.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3 Suppl A:71-80 [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Atlas of standard radiographs. : Kellgren JH, editor The epidemiology of chronic rheumatism; a symposium organized by the Council for International Organizations of Medical Sciences. Vol 2. Oxford: Blackwell; 1963 [Google Scholar]
- 14.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40 [PubMed] [Google Scholar]
- 15.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S148-53 [PubMed] [Google Scholar]
- 16.Andriacchi TP, Andersson GBJ, Fermier RW, Stern D, Galante JO. A study of lower-limb mechanics during stair-climbing. J Bone Joint Surg Am. 1980;62:749-57 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.