Abstract
Photodynamic therapy (PDT) using topical 5-aminolevulinic acid (ALA) is currently used as a clinical treatment for nonmelanoma skin cancers. In order to optimize PDT treatment, vascular disruption early in treatment must be identified and prevented. We present blood flow responses to topical ALA-PDT in a preclinical model and basal cell carcinoma patients assessed by diffuse correlation spectroscopy (DCS). Our results show that ALA-PDT induced early blood flow changes and these changes were irradiance dependent. It is clear that there exists considerable variation in the blood flow responses in patients from lesion to lesion. Monitoring blood flow parameter may be useful for assessing ALA-PDT response and planning.
OCIS codes: (170.0170) Medical optics and biotechnology; (170.3660) Light propagation in tissues; (170.6480) Spectroscopy, speckle; (170.3880) Medical and biological imaging
1. Introduction
Photodynamic therapy (PDT) is an evolving cancer treatment modality that involves administration of a photosensitizer which reacts with oxygen when exposed to light, forming reactive oxygen species and causing subsequent tissue damage [1]. The photosensitizer can be applied systemically or topically. The most commonly used systemic clinical photosensitizer is porfimer sodium (Photofrin) [2]. A downside of this agent is the skin photosensitivity that lasts long after treatment. PDT with topical 5-aminolevulinic acid (ALA), a pro-drug that induces accumulation of the endogenous photosensitizer protoporphyrin IX (PpIX), is an approach in treatment of many cancer types [3]. The application of topical ALA followed by light is advantageous in that it does not cause body-wide skin photosensitization [3]; it also is highly selective, causing negligible damage to connective tissue and minimal scarring. Topical ALA-PDT has become an attractive treatment option for non-melanoma skin carcinomas, especially when treating large areas at a single session or multiple lesions on the same patient [2–6].
The efficacy of PDT is highly dependent on the tissue oxygen level, which in turn can be affected by blood flow [7,8]. Many photosensitizers lead to destruction of vasculature which is one of the mechanisms by which PDT is effective at destroying tumors. Blood flow may change during PDT and these changes can be useful early biomarkers for therapy outcome and planning. Measurement of the cutaneous microcirculation is of clinical importance for monitoring and determining the efficacy of PDT treatment. In order to optimize ALA-PDT, it is necessary to identify and prevent the vascular shutdown that can occur early in treatment. A temporary shutdown in vasculature will decrease the delivery of oxygen which will severely limit the effectiveness of the remaining PDT treatment. Early identification of vascular disruption will allow adjustment of treatment parameters in real time to improve the effectiveness of therapy. It will be important to find the optimal time at which these vascular effects provide improved efficacy [9]. Diffuse correlation spectroscopy (DCS), relying on the intrinsic dynamic contrast of moving blood cells, does not require contrast agent administration, and is highly suitable for monitoring blood flow during PDT. To date DCS has been successfully employed in monitoring of therapies, such as PDT [8,10–12], chemo-radiation [13], and neoadjuvant chemotherapy [14]. Using DCS, Yu et al. [8] showed early blood flow changes correlated well with the Photofrin-mediated PDT efficacy in a murine tumor model.
The reports on blood flow responses due to ALA-PDT are few, mainly focused on post treatment times and the results showed great variability [15–21]. The variability is likely due to differences in tumor characteristics (type, depth, size), PpIX concentration, treatment protocols and dosimetry [15,20–23]. Here we present blood flow responses monitored from the beginning to the end of ALA-PDT using DCS. We investigated the effects of different irradiances since irradiance is known to be an important determinant for PDT responses, including vascular responses. Our results from Colon-26 bearing mice show that topical ALA induced early blood flow decreases at all irradiances (10 mW/cm2, 35 mW/cm2, 75 mW/cm2), followed by a rapid increase and eventually a gradual decrease. Lower irradiance resulted in a smaller initial blood flow drop, followed by a blood flow increase. We also collected clinical data of ALA-PDT of superficial and nodular basal cell carcinomas (BCC). In contrast to results from mice, we observed a distinct pattern of early increase in blood flow in superficial lesions. The elevated blood flow was persistent throughout the treatment. In nodular lesions, blood flow stayed relatively constant throughout the treatment.
2. Methods
2.1 Measurement protocol
Animal experiments were approved by the Institutional Animal Care and Use Committee at Roswell Park Cancer Institute (RPCI). Female BALB/c mice 7-14 weeks of age were inoculated subcutaneously (s.c.) with Colon-26 (1 x 106 cells in 50 µL) on the right posterior shoulder. The mice underwent treatment when tumors reached 6.5-8.5 mm in diameter. In order to keep the mice immobile, they were injected intraperitoneally with ketamine-xylazine (KX) 10 minutes before measurements were started and placed on a 36 °C heating pad. A thermocouple was inserted under the mouse skin to monitor temperature throughout treatment, which remained fairly constant throughout treatment. Mice were shaved and depilated with Nair® the day before injection of tumor cells. The treatment light source was an argon pumped dye laser set for 635 nm output. Treatment light irradiances were set to 75 mW/cm2, 35 mW/cm2, or 10 mW/cm2 and the treatment field diameter was ~16 mm, covering each tumor and surrounding normal tissue. The total light doses for 35 mW/cm2 and 75 mW/cm2 were 80 J/cm2. For the 10 mW/cm2 of irradiance, the total light dose was 37.5 J/cm2. This was decided due to the increased difficulty in keeping the mice anesthetized for longer than 75 minutes. ALA (Sigma, Missouri), 20% in topical vehicle solution (Dusa, Massachusetts), was applied to the tumors ~3 hours before treatment. Control mice had PDT light only without applied ALA. Five mice were measured for each irradiance and for both treated and control groups; therefore, the total of 30 mice were measured in this study.
The patient treatment and measurement protocol was approved by the RPCI Institutional Review Board. Patients with basal cell carcinomas were accrued for the measurement of blood flow during ALA-PDT. There was a ~4 hour incubation time between the application of the ALA and the start of light treatment. ALA (20%) in topical vehicle solution was applied to the lesions. The light source for the PDT treatment was a Coherent dye laser pumped by an Argon ion laser (Spectra Physics), and the light was delivered by a single quartz lens fiber. The treatment beam was centered on the lesion with the beam diameter slightly larger than lesion diameter. Nodular BCCs (nBCCs, N = 2) were treated with 150 mW/cm2, and superficial BCCs (sBCCs, N = 4) were treated initially with 40 mW/cm2 and followed by 150 mW/cm2 after reaching the 90% photobleaching point (measured concurrently with fluorescence spectroscopy [24–26]. Patients treated using the initial irradiances of 150 mW/cm2 (nBCCs) received local injections of lidocaine prior to the start of treatment in order to prevent pain [27]. For all treatments, the room temperature was maintained around 26 °C.
2.2 Diffuse optical blood flow
Diffuse Correlation Spectroscopy (DCS) was utilized to monitor blood flow as described previously [5,9–17]. The DCS instrument was described in our recent published report [11]. Briefly, the instrument had a long coherence length laser (785nm, CrystaLaser), four single photon-counting detectors (SPCD, Perkin-Elmer), and an autocorrelator board (Correlator.com) (Fig. 1a ). The autocorrelator board calculated intensity autocorrelation functions and photon arrival times that were recorded by a computer [5,9]. From the normalized intensity autocorrelation function, g2(r,τ), the diffuse electric field temporal autocorrelation function (g1(r,τ)), was extracted by using Siegert relation [28], g2(r,τ) = 1 + β|g1(r,τ)|2. Here, r is the source detector separation, τ is the autocorrelation time delay, and β is a constant related to detected number of speckles. It was shown that the electric field autocorrelation function satisfies the correlation diffusion equation and analytical solutions can be obtained under special conditions [21,22]. It can be shown that the temporal decay rate of the electric field autocorrelation function is a function of optical parameters and αDB, if one assumes the effective blood flow speed is characterized by effective diffusion coefficient (DB) for the blood cells [13,14,29,30]. Here, α is the probability that a scattering event in tissue is from a moving scatterer, and is related to blood volume fraction. Therefore, αDB characterizes blood flow [11,12,22]. The system was assumed to be ergodic. More generalized schemes for non-ergodic systems closely representing living tissue have been reported recently [23–25].
Fig. 1.
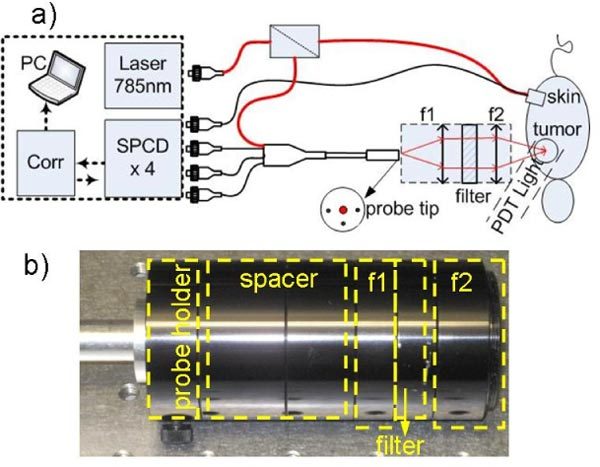
a) Schematic diagram of the instrument and noncontact and contact probes. The DCS setup consists of 785 nm laser, single photon counting detector (SPCD), correlator (Corr), and laptop (PC). Laser light is split in two; one for contact probe, and another for noncontact probe. In the probe tip, the larger red dot at the center represents source fiber, and other three black dots represent detector fibers. The separations between source and detector fibers were 3mm. The open circle is the source position. Lens setup for noncontact probe is also shown with f1 = 60mm, f2 = 40mm. Filter was 785/10 nm band-pass. b) Picture of the noncontact probe.
During experiments in mice, laser light was split (90:10) into two with 10% of light directed into a contact probe where source and one detector fiber were set at a distance of 2 mm at the hip of each animal to monitor the systemic blood flow changes, while 90% laser light was directed into a custom non-contact probe that allowed assessing blood flow response continuously at the tumor site during PDT light irradiation (Fig. 1a). In noncontact probe (Fig. 1a, b), a relay lens allowed projecting the probe tip onto the tumor. Two NIR coated plano-convex lenses (Edmund Optics) with 30 mm diameter and focal points of 60 mm (f1) and 40 mm (f2) were used to construct the relay lens. A bandpass filter (785/10 nm, Chroma) was placed between the lenses to block the PDT light from reaching the detectors. Source fiber and three detector fibers were set at equal distance of 3 mm at the probe tip. Our relay lens setup demagnified the source detector spacing from 3 mm to 2 mm at the tumor surface. A source detector spacing of 2 mm allowed approximate penetration depth of 0.67 mm to 1 mm [11]. All data was normalized to the contact probe located away from the PDT irradiation site to eliminate systemic reaction effects such as changes in skin temperature.
The noncontact probe was not practical for continuous measurements during PDT in the clinic since patients could move causing changes in the position and focus of the probe. This necessitated a less bulky probe that could be taped to the patient. We also collimated the source and detector fibers as Gisler et al. [31] showed that a setup is much less sensitive to misalignment or disturbances if the observation beam is collimated to a diameter of ~1 mm instead of being focused. Source and two detector fibers were collimated with source-detector separations of 2 mm and 3 mm and 700 nm long pass (700LP) filters were installed into detection channels to remove treatment light (Fig. 2a ). The collimated probe was validated against a contact probe in an Intralipid phantom (absorption coefficient, µa = 0.021 cm−1 and
Fig. 2.
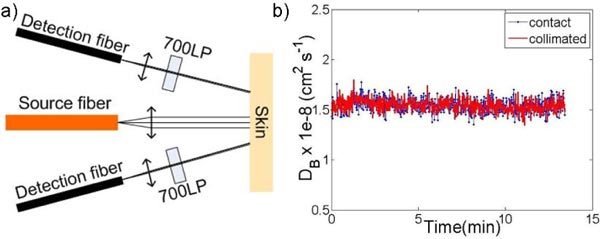
a) Schematic diagram of the noncontact probe in the clinical instrument. Source and detector fibers were collimated with source-detector separations of 2 mm and 3 mm. 700nm long-pass (700LP) filters were installed into detection channels to remove treatment light. b) Particle diffusion coefficient (DB ) of Intralipid obtained with a contact and a collimated probe.
scattering coefficient, μ’s = 10 cm−1). The source-detector separation for both the collimated probe and the contact probe was 2.8 mm. Intralipid has a suspension of particles ranging in diameter from 260 nm to 300 nm. For 300 nm diameter polystyrene beads, the particle diffusion coefficient (DB) was extracted to be ~1.5 × 10−8 cm2 s−1 [32]. As can be seen from Fig. 2b, our extracted DB values are very close to each other (1.545 ± 0.66 10−8 cm2 s−1, for contact vs 1.543 ± 0.62 10−8 cm2 s−1 for collimated), matching well with the expected DB for a 300 nm particle diameter.
3. Results and discussion
Figure 3 summarizes our preclinical results. It is clear from Fig. 3a that ALA-PDT induced acute early vascular changes: Initial quick drop was followed by an increase and a final gradual change towards initial levels for all irradiances. Lowest fluence rate results showed a very early increase. Error bars represents the standard error over five animals (N = 5). Lower irradiance resulted in a lower initial blood flow drop and a higher subsequent increase. Fluence-rate dependent differences in blood flow decreases were statistically significant (p<0.05, Wilcoxon rank sum test). The reasons for these blood flow patterns are not well understood. A possible explanation for early decrease may be due to constriction of the blood vessels arising from a lack of nitric oxide, since it has been shown that nitric oxide production
decreases in deoxygenated conditions [13] and deoxygenation can be caused by PDT consuming the oxygen. Then there may have been a temporary burst of blood flow causing an increase trend. Figure 3b shows that the control group (light only, no ALA) showed rather constant blood flow values throughout PDT treatment suggesting the changes in blood flow in treated mice were due to PDT effects as opposed to systemic effects due to anesthesia, temperature or light.
Fig. 3.
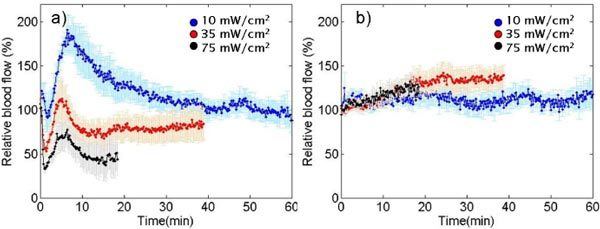
a) Relative blood flow changes in mice during ALA-PDT with different irradiances. b) Relative blood flow changes for light-only controls.
Henderson et al. observed that ALA induced blood perfusion reduction using fluorescein methods in Colon-26 tumor (75 mW/cm2, 135 J/cm2) at 1 hr, 3 hr, 5 hr, and 24 hr post-PDT [18]. Van der Veen et al. [19] also observed a reduction at 4 hr and 10 hr post-PDT using the same methods in UVB-treated skin models (100 mW/cm2, 100 J/cm2). Leveckis et al. [16] studied blood flow during ALA PDT on the cremaster muscle in rats. They investigated irradiances of 105 mW/cm2, 178 mW/cm2, and 300 mW/cm2 using a 390-460 nm light source. They found a rapid artery diameter decrease within 4 minutes to about 20% of the original value in the 178 mW/cm2, and 300 mW/cm2 cases followed a gradual increase. Schacht et al. [22] found a large decrease in red blood cell velocity and in functional vessel density at “high dose” (100 J/cm2) but not in “low dose” (10 J/cm2). This study was done in a skin fold chamber where the ALA was applied to the underside of the tumor and did not need to penetrate the epidermis so that levels may have been higher than typical. Herman et al. [15] did not see a blood flow decrease in Colon-26 tumor bearing mice treated at 55 to 68 mW/cm2 with 633 nm PDT light. They administered ALA by tail vein injection rather than topically as in our study; Schact et al. [22] showed that topically administered ALA resulted in much more microcirculatory effects than intravenously administered ALA.
We also initiated blood flow measurements in our ongoing clinical trial of ALA-PDT for treatment of BCCs [25]. Although the current patient number is low and the treatment regimen is not the same for all of the lesions, we divided the data into two groups: nodular BCCs (nBCCs, lesions nBCC1 & nBCC2) treated with 150 mW/cm2, and superficial BCCs (lesions sBCC1 to sBCC4) treated initially with 40 mW/cm2 and followed by 150 mW/cm2 after reaching the 90% photobleaching point (measured concurrently with fluorescence spectroscopy [24–26]. Figure 4 summarizes our results from these lesions (sBCC1 divided by 4 to be on the same scale). It is clear that there exists considerable variation in the blood flow responses from lesion to lesion. It is also clear that low irradiance caused a more pronounced increase in the early stage of the treatment in sBCCs (Fig. 4a), indicating more blood flow during treatment that should result in increased oxygenation and a therapeutic benefit. The elevated blood flow responses were persistent until completion of PDT treatment. In nodular lesions, blood flow did not show such a high increase trend but stayed relatively constant during the treatment (Fig. 4b). nBCC2 showed a decrease trend, which is more similar to preclinical findings.
Fig. 4.
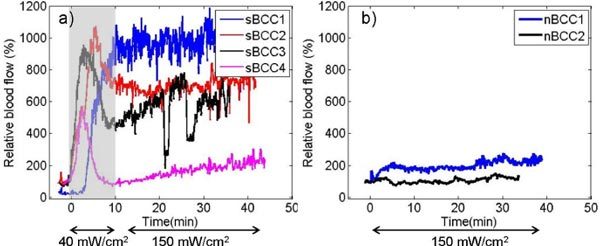
Relative blood flow changes during clinical ALA-PDT of a) sBCC and b) nBCC lesions. Shaded area represents 40mW/cm2, otherwise 150mW/cm2.
Blood flow changes due to clinical ALA-PDT have been discussed in several studies with differing results. Wang et al. [20] and Enejder et al. [21] reported blood flow increase after ALA-PDT in human BCCs by using laser Doppler imaging. These studies were done at varying unstated irradiances under 100 mW/cm2 and their estimated blood flow probing depth was in the range of 150-350 µm; meaning the signal originated primarily from the superficial layer. Interestingly they also observed elevated blood flow responses immediately after PDT for all of the sBCCs compared with the nBCCs. This difference in blood flow response may be due to epidermal layer, which usually covers nodular tumors and results in less of the dynamically scattered light from moving blood cells to be detected [21]. The range of thicknesses of the epidermis and dermis in human skin are 0.03-1.4 mm and 0.86-3 mm respectively [33,34], showing great variation from patient to patient, which may affect individual blood flow quantification significantly in clinical settings. This point also necessitates the use of a high resolution imaging modality such as ultrasound [35] so that probe could be adjusted if the thickness and depth were known as a priori information. Local injections of lidocaine during treatments of nBCCs might also have affected blood flow changes in nBCCs. In contradiction to these blood flow increases after PDT, Herman et al. [15] showed a blood flow decrease in patients, but their treatment irradiance was 200 mW/cm2 and patients had different types of tumors: colon or rectum adenocarcinomas. Differences in irradiance, accumulated PDT dose and tumor vascularity may affect observed blood flow patterns [36]. PpIX content in the vasculature was unknown for these patients, but there is a possibility of more heterogeneity of PpIX distribution in nBCCs compared to sBCCs. This may be because the nodular tumors generally have larger regions of differentiation. Thickness and depth of tumors might have an effect on the response. Superficial tumors are generally more oxygenated due to possibility of getting oxygen from the surrounding air.
Topical ALA-PDT causes very significant pain during treatment at conventional light irradiances (~75-150 mW/cm2). The pain appears to be due to direct photodynamic activity on the cutaneous nerve endings, and thus is a mark of normal tissue damage. Our group published earlier that the pain level is dependent on the irradiance [27]. Fluence rates above 50 mW/cm2 are increasingly painful and fluence rates below that cause no or lesser pain. We have found that the use of painless irradiance of 40 mW/cm2 to photobleach the photosensitizer PpIX to 80-90% of its initial level with a subsequent irradiance of 150 mW/cm2 to be painless in most patients. Our current protocol is designed to spare our patients from pain and excessive exposure to analgesics. Patients with superficial basal cell carcinomas accrued with the current protocol, which was the main reason for the difference in irradiances (40 mW/cm2 vs 150 mW/cm2) at the beginning of the treatment.
4. Conclusion
We presented preliminary results of changes in blood flow during ALA-PDT in a preclinical model and recently initiated clinical studies for BCC patients. It can be concluded that in preclinical studies blood flow decreased during treatment in an irradiance dependent manner. The pattern of these blood flow changes is an initial early sharp decrease followed by an increase and then a more gradual decrease. ALA-PDT induced significant blood flow changes. We observed significant variation in each patient and lesion, thus emphasizing the importance of monitoring the treatment response on an individual basis. Extracted blood flow should be valuable for understanding and assessing the PDT dose adjustment and clinical response and feedback. The ongoing clinical trial and collection of data generating statistically significant data sets may show a clear trend in blood flow responses.
Acknowledgments
This research is supported by NCI CA55791 (B. W. Henderson) and RPCI Startup Grant (U. Sunar). We thank Dan Muffoletto and Bill Potter for technical assistance for the DCS instrument. We also thank Dan Rohrbach for editing the manuscript.
References and Links
- 1.Henderson B. W., Dougherty T. J., “How does photodynamic therapy work?” Photochem. Photobiol. 55(1), 145–157 (1992). 10.1111/j.1751-1097.1992.tb04222.x [DOI] [PubMed] [Google Scholar]
- 2.Szeimies R. M., Morton C. A., Sidoroff A., Braathen L. R., “Photodynamic therapy for non-melanoma skin cancer,” Acta Derm. Venereol. 85(6), 483–490 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Ericson M. B., Wennberg A. M., Larkö O., “Review of photodynamic therapy in actinic keratosis and basal cell carcinoma,” Ther Clin Risk Manag 4(1), 1–9 (2008). [PMC free article] [PubMed] [Google Scholar]
- 4.Kalka K., Merk H., Mukhtar H., “Photodynamic therapy in dermatology,” J. Am. Acad. Dermatol. 42(3), 389–413, quiz 414–416 (2000). 10.1016/S0190-9622(00)90209-3 [DOI] [PubMed] [Google Scholar]
- 5.Morton C. A., Whitehurst C., McColl J. H., Moore J. V., MacKie R. M., “Photodynamic therapy for large or multiple patches of Bowen disease and basal cell carcinoma,” Arch. Dermatol. 137(3), 319–324 (2001). [PubMed] [Google Scholar]
- 6.Kennedy J. C., Pottier R. H., Pross D. C., “Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience,” J. Photochem. Photobiol. B 6(1-2), 143–148 (1990). 10.1016/1011-1344(90)85083-9 [DOI] [PubMed] [Google Scholar]
- 7.Georgakoudi I., Foster T. H., “Singlet oxygen- versus nonsinglet oxygen-mediated mechanisms of sensitizer photobleaching and their effects on photodynamic dosimetry,” Photochem. Photobiol. 67(6), 612–625 (1998). [PubMed] [Google Scholar]
- 8.Yu G., Durduran T., Zhou C., Wang H. W., Putt M. E., Saunders H. M., Sehgal C. M., Glatstein E., Yodh A. G., Busch T. M., “Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy,” Clin. Cancer Res. 11(9), 3543–3552 (2005). 10.1158/1078-0432.CCR-04-2582 [DOI] [PubMed] [Google Scholar]
- 9.Major A., Kimel S., Mee S., Milner T. E., Smithies D. J., Srinivas S. M., Chen Z., Nelson J. S., “Microvascular photodynamic effects determined in vivo using optical doppler tomography,” IEEE J. Sel. Top. Quantum Electron. 5(4), 1168–1175 (1999). 10.1109/2944.796343 [DOI] [Google Scholar]
- 10.Yu G., Durduran T., Zhou C., Zhu T. C., Finlay J. C., Busch T. M., Malkowicz S. B., Hahn S. M., Yodh A. G., “Real-time in situ monitoring of human prostate photodynamic therapy with diffuse light,” Photochem. Photobiol. 82(5), 1279–1284 (2006). 10.1562/2005-10-19-RA-721 [DOI] [PubMed] [Google Scholar]
- 11.Sunar U., Rohrbach D., Rigual N., Tracy E., Keymel K., Cooper M. T., Baumann H., Henderson B. H., “Monitoring photobleaching and hemodynamic responses to HPPH-mediated photodynamic therapy of head and neck cancer: a case report,” Opt. Express 18(14), 14969–14978 (2010). 10.1364/OE.18.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch T. M., Xing X., Yu G., Yodh A., Wileyto E. P., Wang H. W., Durduran T., Zhu T. C., Wang K. K., “Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy,” Photochem. Photobiol. Sci. 8(12), 1683–1693 (2009). 10.1039/b9pp00004f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunar U., Quon H., Durduran T., Zhang J., Du J., Zhou C., Yu G., Choe R., Kilger A., Lustig R., Loevner L., Nioka S., Chance B., Yodh A. G., “Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: a pilot study,” J. Biomed. Opt. 11(6), 064021 (2006). 10.1117/1.2397548 [DOI] [PubMed] [Google Scholar]
- 14.Zhou C., Choe R., Shah N., Durduran T., Yu G., Durkin A., Hsiang D., Mehta R., Butler J., Cerussi A., Tromberg B. J., Yodh A. G., “Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy,” J. Biomed. Opt. 12(5), 051903 (2007). 10.1117/1.2798595 [DOI] [PubMed] [Google Scholar]
- 15.Herman M. A., Fromm D., Kessel D., “Tumor blood-flow changes following protoporphyrin IX-based photodynamic therapy in mice and humans,” J. Photochem. Photobiol. B 52(1-3), 99–104 (1999). 10.1016/S1011-1344(99)00109-8 [DOI] [PubMed] [Google Scholar]
- 16.Leveckis J., Brown N. J., Reed M. W., “The effect of aminolaevulinic acid-induced, protoporphyrin IX-mediated photodynamic therapy on the cremaster muscle microcirculation in vivo,” Br. J. Cancer 72(5), 1113–1119 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedwell J., MacRobert A. J., Phillips D., Bown S. G., “Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model,” Br. J. Cancer 65(6), 818–824 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson B. W., Vaughan L., Bellnier D. A., van Leengoed H., Johnson P. G., Oseroff A. R., “Photosensitization of murine tumor, vasculature and skin by 5-aminolevulinic acid-induced porphyrin,” Photochem. Photobiol. 62(4), 780–789 (1995). 10.1111/j.1751-1097.1995.tb08730.x [DOI] [PubMed] [Google Scholar]
- 19.van der Veen N., Hebeda K. M., de Bruijn H. S., Star W. M., “Photodynamic effectiveness and vasoconstriction in hairless mouse skin after topical 5-aminolevulinic acid and single- or two-fold illumination,” Photochem. Photobiol. 70(6), 921–929 (1999). 10.1111/j.1751-1097.1999.tb08303.x [DOI] [PubMed] [Google Scholar]
- 20.Wang I., Andersson-Engels S., Nilsson G. E., Wårdell K., Svanberg K., “Superficial blood flow following photodynamic therapy of malignant non-melanoma skin tumours measured by laser Doppler perfusion imaging,” Br. J. Dermatol. 136(2), 184–189 (1997). 10.1111/j.1365-2133.1997.tb14893.x [DOI] [PubMed] [Google Scholar]
- 21.Enejder A. M. K., af Klinteberg C., Wang I., Andersson-Engels S., Bendsoe N., Svanberg S., Svanberg K., “Blood perfusion studies on basal cell carcinomas in conjunction with photodynamic therapy and cryotherapy employing laser-Doppler perfusion imaging,” Acta Derm. Venereol. 80(1), 19–23 (2000). 10.1080/000155500750012441 [DOI] [PubMed] [Google Scholar]
- 22.Schacht V., Szeimies R. M., Abels C., “Photodynamic therapy with 5-aminolevulinic acid induces distinct microcirculatory effects following systemic or topical application,” Photochem. Photobiol. Sci. 5(5), 452–458 (2006). 10.1039/b514128a [DOI] [PubMed] [Google Scholar]
- 23.Svanberg K., Andersson T., Killander D., Wang I., Stenram U., Andersson-Engels S., Berg R., Johansson J., Svanberg S., “Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation,” Br. J. Dermatol. 130(6), 743–751 (1994). 10.1111/j.1365-2133.1994.tb03412.x [DOI] [PubMed] [Google Scholar]
- 24.Cottrell W. J., Oseroff A., Foster T. H., “Portable instrument that integrates irradiation with fluorescence and reflectance spectroscopies during clinical photodynamic therapy of cutaneous disease,” Rev. Sci. Instrum. 77, 064302 (2006). 10.1063/1.2204617 [DOI] [Google Scholar]
- 25.T. L. Becker, “Irradiance: A parameter determining oxygenation during topical photodynamic therapy (PDT),” Department of Molecular and Cellular Biophysics and Biochemistry, SUNY at Buffalo, 2010).
- 26.T. Sands, U. Sunar, T. H. Foster, and A. Oseroff, “Monitoring blood flow and photobleaching during topical ALA PDT treatment,” Proc. SPIE 7164, 71640U (2009). [Google Scholar]
- 27.Cottrell W. J., Paquette A. D., Keymel K. R., Foster T. H., Oseroff A. R., “Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas,” Clin. Cancer Res. 14(14), 4475–4483 (2008). 10.1158/1078-0432.CCR-07-5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.P. J. Berne and R. Pecora, Dynamic Light Scattering (Wiley, New York, 1976). [Google Scholar]
- 29.Cheung C., Culver J. P., Takahashi K., Greenberg J. H., Yodh A. G., “In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies,” Phys. Med. Biol. 46(8), 2053–2065 (2001). 10.1088/0031-9155/46/8/302 [DOI] [PubMed] [Google Scholar]
- 30.Sunar U., Makonnen S., Zhou C., Durduran T., Yu G., Wang H. W., Lee W. M. F., Yodh A. G., “Hemodynamic responses to antivascular therapy and ionizing radiation assessed by diffuse optical spectroscopies,” Opt. Express 15(23), 15507–15516 (2007). 10.1364/OE.15.015507 [DOI] [PubMed] [Google Scholar]
- 31.Gisler T., Rüger H., Egelhaaf S. U., Tschumi J., Schurtenberger P., Rička J., “Mode-selective dynamic light scattering: theory versus experimental realization,” Appl. Opt. 34(18), 3546–3553 (1995). 10.1364/AO.34.003546 [DOI] [PubMed] [Google Scholar]
- 32.D. A. Boas, “Diffuse photon probes of structural and dynamical properties of turbid media: theory and biomedical applications ” Ph.D. dissertation (University of Pennsylvania, 1996). [Google Scholar]
- 33.Gush R. J., King T. A., Jayson M. I., “Aspects of laser light scattering from skin tissue with application to laser Doppler blood flow measurement,” Phys. Med. Biol. 29(12), 1463–1476 (1984). 10.1088/0031-9155/29/12/001 [DOI] [PubMed] [Google Scholar]
- 34.Moore J. V., Allan E., “Pulsed ultrasound measurements of depth and regression of basal cell carcinomas after photodynamic therapy: relationship to probability of 1-year local control,” Br. J. Dermatol. 149(5), 1035–1040 (2003). 10.1111/j.1365-2133.2003.05558.x [DOI] [PubMed] [Google Scholar]
- 35.Gruber J. D., Paliwal A., Krishnaswamy V., Ghadyani H., Jermyn M., O’Hara J. A., Davis S. C., Kerley-Hamilton J. S., Shworak N. W., Maytin E. V., Hasan T., Pogue B. W., “System development for high frequency ultrasound-guided fluorescence quantification of skin layers,” J. Biomed. Opt. 15(2), 026028 (2010). 10.1117/1.3374040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin C. W., Foss A. J., Stevens A., Lowe J., “Differences in the vascular patterns of basal and squamous cell skin carcinomas explain their differences in clinical behaviour,” J. Pathol. 200(3), 308–313 (2003). 10.1002/path.1363 [DOI] [PubMed] [Google Scholar]


