Abstract
The timber and pulp industries of Finland rely heavily on importations from Russia as source of raw timber. These imports raise the risk of accidentally importing forest pests and pathogens, especially bark beetles and their associated fungi, into Finland. Although ophiostomatoid fungi have previously been reported from Finland and Russia, the risks of accidentally moving these fungi has prompted a first survey to compare the diversity of conifer-infesting bark beetles and associated fungi from boreal forests on both sides of the Finnish-Russian border. The aim of the present study was to identify and characterise Ophiostoma species isolated in association with 11 bark beetle species infesting Pinus sylvestris and Picea abies during this survey in the eastern parts of Finland and neighbouring Russia. Fungal isolates were grouped based on morphology and representatives of each morphological group were subjected to DNA sequence comparisons of the internal transcribed spaced region (ITS1, 5.8S, ITS2) and β-tubulin gene region. A total of 15 species of Ophiostoma were identified, including seven known species, five new species, and three species for which the identity remains uncertain. In the O. piceae-complex we identified O. canum, O. floccosum, O. karelicum and O. rachisporum sp. nov., and related to these, some isolates belonging to the European clade of O. minus in the O. minus-complex. Ophiostoma bicolor and O. fuscum sp. nov. were identified in the O. ips-complex, while O. ainoae, O. brunneo-ciliatum, O. tapionis sp. nov. and O. pallidulum sp. nov. were shown to group close to, but not in a strict monophyletic lineage with species of the O. ips-complex. Together with a single O. abietinum-like isolate, the only species that grouped close to the Sporothrix schenckii- O. stenoceras complex, was O. saponiodorum sp. nov.
Keywords: bark beetle, insect-fungus relationship, Ophiostoma, Ophiostomatales, symbiosis
INTRODUCTION
Global trade in untreated timber and wood products seriously raises the risk of introducing forest pathogens and pests into new environments (Tkacz 2002). One of the groups of wood infesting fungi that are easily transported through the movement of untreated wood products is the Ascomycete genus Ophiostoma. These fungi include many species that are agents of blue stain in timber (Seifert 1993) and some, such as the Dutch Elm Disease pathogens O. ulmi, O. himal-ulmi and O. novo-ulmi, are serious tree pathogens (Gibbs 1978, Brasier 1991). Bark beetles (Coleoptera: Scolytinae) are well-known vectors of Ophiostoma spp. and greatly facilitate the spread of these fungi (Wingfield et al. 1993, Kirisits 2004). The teleomorph and anamorph structures of these fungi are specifically adapted for insect dispersal (Malloch & Blackwell 1993), and many of the species are relatively specific symbionts of their insect vectors (Six 2003, Kirisits 2004).
The forestry, timber and paper industry contributes to approximately 10 % of the gross domestic product (GDP) of Finland and close to 30 % of total industrial output of the country (Finnish Forest Industries Federation 2010). Finnish forests cannot supply the demand of the industry and the country thus relies heavily on Russia as source of raw timber. This raises concerns relating to the risks of introducing pests and pathogens on timber imported from Russia to Finland. For example, a large number of bark beetle species, including potential pest species not native to Finland, were identified by Siitonen (1990). However, studies on the fungal associates of these beetles in Finland and Russia are limited and the species identification was made at a time when DNA sequence comparisons were not commonly applied. Furthermore, we are not aware of any study where the bark beetles and their fungal flora in the two countries have been compared.
Despite the occurrence of several native bark beetle species in Finland, previous studies (Table 1) have mainly focused on fungal associates of the spruce bark beetle, Ips typographus (Savonmäki 1990, Viiri 1997). The only Ophiostoma spp. from Finland of which identities have been confirmed by DNA sequences, are associates of the birch bark beetle, Scolytus ratzeburgi (Linnakoski et al. 2008, Kamgan et al. 2010). Reports of Ophiostoma species from Russia are more extensive, but apart from O. karelicum reported from S. ratzeburgi (Linnakoski et al. 2008), all identifications were based only on morphology (Table 1). Due to the relatively simple morphology and overlapping features between different species, it is often difficult to distinguish between these species and the accuracy of names that have been used in these studies is thus questionable. Thus, DNA sequence-based identification has become essential for reliable identification and the recognition of cryptic taxa amongst morphologically similar species of ophiostomatoid fungi (Gorton et al. 2004, Grobbelaar et al. 2009).
Table 1.
Ophiostoma spp. previously reported from different beetles and/or host trees in Finland (F) and Russia (R). Identifications in all these studies were based on morphology, and only those marked with * included DNA sequence comparisons.
| Fungus | Beetle | Host tree | Origin | Reference |
|---|---|---|---|---|
| O. ainoae | Ips acuminatus | Pinus sylvestris | R | Pashenova et al. 2004 |
| I. cembrae | Larix sibirica, P. sylvestris | R | Pashenova et al. 1995 | |
| I. typographus | – | R | Pashenova et al. 2001, 2004 | |
| I. typographus | Picea abies | F | Viiri 1997 | |
| O. bicolor | I. amitinus | P. abies, P. sylvestris | F | Savonmäki 1990 |
| I. cembrae | L. sibirica, P. sylvestris | R | Pashenova et al. 1995, 2004 | |
| I. typographus | P. abies | F | Savonmäki 1990, Viiri 1997 | |
| I. typographus | Picea obovata | R | Pashenova et al. 2001, 2004 | |
| Pityogenes chalcographus | P. abies | F | Savonmäki 1990 | |
| O. borealis* | Scolytus ratzeburgi | Betula pendula | F | Linnakoski et al. 2008, Kamgan et al. 2010 |
| O. curvicolle (= O. nigrum) | Monochamus urussovi | Abies sibirica | R | Pashenova et al. 2007 |
| Trypodendron lineatum | A. sibirica | R | Pashenova et al. 2004 | |
| O. fagi (= O. quercus) | – | Fagus orientalis | R | Potlajczuk & Schekunova 1985 |
| – | P. sylvestris | R | Potlajczuk & Schekunova 1985 | |
| – | P. abies | R | Potlajczuk & Schekunova 1985 | |
| O. ips | I. acuminatus | P. sylvestris | R | Pashenova et al. 2004 |
| I. cembrae | P. sylvestris | R | Pashenova et al. 1995 | |
| Pityogenes sp. | P. sylvestris | R | Pashenova et al. 2004 | |
| O. karelicum* | S. ratzeburgi | B. pendula | F, R | Linnakoski et al. 2008 |
| O. kubanicum (= O. quercus) | – | Quercus robur | R | Sherbin-Parfenenko 1953 |
| – | Quercus sp. | R | Potlajczuk 1957, Minkevich 1965, Kryukova & Terehova 1974, Kryukova & Plotnikova 1979a, b, Potlajczuk & Schekunova 1985, Selochnik 1998a, b | |
| S. intricatus | – | R | Ivanchenko 1957 | |
| O. minus | I. acuminatus | P. sylvestris | R | Pashenova et al. 2004 |
| I. cembrae | L. sibirica, P. sylvestris | R | Pashenova et al. 1995, 2004 | |
| – | P. abies, P. sylvestris | R | Potlajczuk & Schekunova 1985 | |
| Pityogenes sp. | P. sylvestris | R | Pashenova et al. 2004 | |
| Tomicus piniperda | P. sylvestris | R | Pashenova et al. 2004 | |
| O. novo-ulmi | – | – | R | Storozhenko et al. 2000 |
| Scolytid beetles | Ulmus spp. | R | Brasier 1990 | |
| O. piceae | Hylurgops palliatus | P. abies, P. sylvestris | F | Savonmäki 1990 |
| I. amitinus | P. abies, P. sylvestris | F | Savonmäki 1990 | |
| I. typographus | P. abies | F | Savonmäki 1990, Viiri 1997 | |
| M. urussovi | A. sibirica | R | Pashenova et al. 2007 | |
| – | – | F, R | Wegelius 1938, Meyer 1946, Storozhenko et al. 2000 | |
| – | P. abies, Pinus sp. | R | Potlajczuk & Schekunova 1985 | |
| P. chalcographus | P. abies | F | Savonmäki 1990 | |
| T. piniperda | P. sylvestris | F | Savonmäki 1990 | |
| T. lineatum | P. abies, P. sylvestris | F | Savonmäki 1990 | |
| O. piliferum | I. cembrae | L. sibirica, P. sylvestris | R | Pashenova et al. 1995, 2004 |
| I. typographus | P. abies | F | Savonmäki 1990 | |
| – | – | R | Rodigin 1947 | |
| – | Quercus sp. | R | Liubarski 1937 | |
| T. piniperda | P. sylvestris | R | Pashenova et al. 2004 | |
| O. quercus* | S. ratzeburgi | B. pendula | F | Linnakoski et al. 2008 |
| O. roboris (= O. quercus) | Mesosa myops | Quercus sp. | R | Minkevich 1965 |
| – | Betula sp. | R | Minkevich 1965 | |
| – | Malus domestica | R | Potlajczuk & Schekunova 1985 | |
| – | – | R | Storozhenko et al. 2000 | |
| – | Quercus robur | R | Selochnik 1998a,b | |
| – | Quercus sp. | R | Sherbin-Parfenenko 1953, Potlajczuk 1957, Potlajczuk & Schekunova 1985, Vorontsov 1986 | |
| S. intricatus | Quercus sp. | R | Ivanchenko 1957, Minkevich 1965 | |
| O. tetropii | I. typographus | P. abies | F | Savonmäki 1990, Viiri 1997 |
| P. chalcographus | P. abies | F | Savonmäki 1990 | |
| O. ulmi | – | Fraxinus sp. | R | Potlajczuk & Schekunova 1985 |
| – | – | R | Storozhenko et al. 2000 | |
| – | Quercus sp. | R | Vorontsov 1986 | |
| – | Ulmus sp. | R | Dudina 1936, 1938, Potlajczuk & Schekunova 1985 | |
| Scolytid beetles | Ulmus spp. | R | Brasier 1990 | |
| O. valachicum | – | Q. robur | R | Selochnik 1998a, b |
| – | Quercus sp. | R | Sherbin-Parfenenko 1953, Potljaczuk 1957, Potlajczuk & Schekunova 1985 | |
| S. intricatus | Quercus sp. | R | Ivanchenko 1957 |
A large number of ophiostomatoid fungi have been reported from mainland Europe and Scandinavia that exist in symbiosis with bark beetle species occupying different niches (Kirisits 2004). Consistent with other studies, it is likely that the species diversity for these fungi is more extensive in the boreal forests of Finland and Russia than has previously been recognised. Consequently, an extended survey of bark beetle-associated fungi is currently being undertaken at 12 different sites in the boreal forests of Karelia, on both the Finnish and Russian sides of the border. As part of this survey, fungal collections have been made from the most common bark beetle species infesting the two dominant conifer species in the boreal forests, Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). The aims of this study were to identify all species of Ophiostoma collected during this survey, and to describe new species found among them. These identifications were based on morphological characteristics and DNA sequence comparisons for the internal transcribed spacer (ITS) regions of the ribosomal RNA and part of the β-tubulin gene.
MATERIALS AND METHODS
Isolation of fungi from bark beetles and galleries
Bark beetles and their galleries were collected from spruce and pine logs and naturally infested trees at four different sites in Finland and eight in Russia (Table 2). All sampling sites were located in the eastern parts of the Finland and in the Karelia region on the Russian side of the border. In July 2004, beetles were collected from the Ohtama region in Russia, at a location of extensive spruce bark beetle (Ips typographus) damage. In July–August 2005, more extensive isolations were done at the other research sites. Fungi were isolated from the bark beetles as well as their galleries (Fig. 1). Additional fungal isolates obtained in July 2007 from Tomicus piniperda infesting Pinus sylvestris in Russia were included in the study. Samples were collected from natural wounds but also using a trap-tree method. Methods of fungal isolation were the same as those described by Linnakoski et al. (2008).
Table 2.
Fungal isolates obtained from different bark beetle species infesting pine and spruce in Russia and Finland, as well as isolates of known species included for reference purposes. Accession numbers in bold type are for sequences obtained in the present study.
| Species identity | Lineage | Isolate numbers1 |
Herbarium KUO | Origin | Host | Insect vector | Collector | GenBank no. |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CBS | CMW | VTT/JCM | ITS | β-tubulin | |||||||
| Isolates obtained in the present study | |||||||||||
| O. ainoae | H | – | 23123 | – | – | Ohtama, Russia | Picea abies | Ips typographus | Ahtiainen | HM031496 | HM031550 |
| – | 23167 | – | – | Lisino-Corpus, Russia | P. abies | I. typographus | Linnakoski | – | HM031551 | ||
| O. bicolor | K | – | 23175 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | – | HM031562 |
| – | 23179 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | HM031505 | HM031560 | ||
| – | 23189 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | HM031561 | |||
| O. brunneo-ciliatum | G | – | 23115 | – | – | Lisino-Corpus, Russia | Pinus sylvestris | Pityogenes chalcographus | Linnakoski | HM031499 | HM031557 |
| 128127 | 23143 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | – | HM031554 | ||
| – | 23146 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | – | HM031556 | ||
| – | 23153 | – | – | Ohtama, Russia | P. abies | I. typographus | Ahtiainen | – | HM031555 | ||
| O. canum | C1 | – | 23229 | – | – | Punkaharju, Finland | P. sylvestris | Tomicus minor | de Beer | – | HM031519 |
| – | 23230 | – | – | Punkaharju, Finland | P. sylvestris | Trypodendron lineatum | de Beer | – | HM031522 | ||
| – | 23241 | – | – | Punkaharju, Finland | P. sylvestris | T. minor | de Beer | – | HM031524 | ||
| – | 23253 | – | – | Lisino-Corpus, Russia | P. sylvestris | T. minor | Linnakoski | – | HM031523 | ||
| – | 23254 | – | – | Lisino-Corpus, Russia | P. sylvestris | T. minor | Linnakoski | – | HM031525 | ||
| – | 23260 | – | – | Lisino-Corpus, Russia | P. sylvestris | T. minor | Linnakoski | HM031488 | HM031520 | ||
| – | 23261 | – | – | Lisino-Corpus, Russia | P. sylvestris | Hylurgops palliatus | Linnakoski | – | HM031521 | ||
| – | 28158 | – | – | Lisino-Corpus, Russia | P. sylvestris | H. palliatus | Linnakoski | – | HM031526 | ||
| O. canum-like | C2 | 127077 | 34938 | D-101402 | – | Volosovo, Russia | P. sylvestris | Tomicus piniperda | Sidorov | – | HM031527 |
| – | 34939 | D-101403 | – | Volosovo, Russia | P. sylvestris | T. piniperda | Sidorov | – | HM031528 | ||
| – | 23208 | D-101404 | – | Punkaharju, Finland | P. sylvestris | T. lineatum | de Beer | HM031487 | HM031532 | ||
| – | 23219 | – | – | Punkaharju, Finland | P. sylvestris | T. lineatum | Linnakoski | – | HM031531 | ||
| – | 23255 | – | – | Lisino-Corpus, Russia | P. sylvestris | H. palliatus | Linnakoski | – | HM031529 | ||
| – | 23259 | D-101405 | – | Lisino-Corpus, Russia | P. sylvestris | H. palliatus | Linnakoski | – | HM031530 | ||
| O. floccosum | E | – | 23287 | – | – | Lisino-Corpus, Russia | P. abies | I. typographus | Linnakoski | HM031492 | HM031538 |
| – | 23288 | – | – | Lisino-Corpus, Russia | P. sylvestris | H. palliatus | Linnakoski | – | HM031537 | ||
| O. fuscum sp. nov. | J | 128124 | 23195a,b | D-101410 | 021876 | Lisino-Corpus, Russia | P. abies | I. typographus | Linnakoski | – | HM031565 |
| – | 23196a,b,T | D-101411 | 021877 | Pyhäselkä, Finland | P. abies | P. chalcographus | de Beer | HM031504 | HM031563 | ||
| – | 28019a,b | D-101412 | 021878 | Kivennapa, Russia | P. abies | P. chalcographus | Linnakoski | HM031503 | HM031564 | ||
| O. karelicum | A | 127073 | 34935 | – | – | Punkaharju, Finland | P. abies | I. typographus | de Beer | HM031540 | |
| 127074 | 34936 | – | – | Punkaharju, Finland | P. abies | I. typographus | de Beer | HM031498 | HM031541 | ||
| – | 23244 | – | – | Punkaharju, Finland | P. sylvestris | T. minor | de Beer | – | HM031539 | ||
| – | 34937 | – | – | Kivennapa, Russia | P. sylvestris | T. minor | Linnakoski | – | HM031542 | ||
| O. minus Europe | D | – | 27992 | – | – | Kivennapa, Russia | P. sylvestris | T. piniperda | Linnakoski | – | HM031533 |
| – | 28117 | – | – | Uuksujärvi, Russia | P. abies | T. minor | Linnakoski | HM031497 | HM031535 | ||
| – | 28154 | – | – | Punkaharju, Finland | P. sylvestris | T. piniperda | Linnakoski | – | HM031536 | ||
| – | 28171 | – | – | Lisino-Corpus, Russia | P. sylvestris | T. piniperda | Linnakoski | – | HM031534 | ||
| O. pallidulum sp. nov. | L | 127075 | 34941a,b | D-101413 | – | Punkaharju, Finland | P. sylvestris | Hylastes brunneus | de Beer | – | HM031567 |
| 128118 | 23278a,b,T | D-101414 | 021879 | Punkaharju, Finland | P. sylvestris | H. brunneus | de Beer | HM031510 | HM031566 | ||
| 128125 | 23279a,b | D-101415 | 021880 | Punkaharju, Finland | P. sylvestris | H. brunneus | de Beer | HM031509 | – | ||
| 127076 | 23280a,b | D-101416 | 021881 | Punkaharju, Finland | P. sylvestris | H. palliatus | de Beer | – | – | ||
| O. rachisporum sp. nov. | B | – | 23271 | – | – | Ilomantsi, Finland | P. sylvestris | T. lineatum | de Beer | – | HM031515 |
| 128119 | 23272a,b,T | D-101400 | 021869 | Punkaharju, Finland | P. sylvestris | T. lineatum | de Beer | HM031490 | HM031513 | ||
| – | 23273a | – | 021871 | Punkaharju, Finland | P. sylvestris | H. palliatus | Linnakoski | – | – | ||
| 128123 | 23274a,b | D-101401 | 021870 | Punkaharju, Finland | P. sylvestris | T. lineatum | de Beer | – | HM031514 | ||
| – | 28021 | – | – | Kivennapa, Russia | P. sylvestris | T. lineatum | Linnakoski | HM031491 | HM031512 | ||
| O. saponiodorum sp. nov. | M | 127081 | 34942a,b | D-101417 | 021883 | Pyhäselkä, Finland | P. abies | P. chalcographus | de Beer | – | – |
| 127080 | 34943 | D-101418 | 021885 | Pyhäselkä, Finland | P. abies | P. chalcographus | de Beer | – | HM031570 | ||
| 127079 | 34944a,b | D-101419 | 021887 | Pyhäselkä, Finland | P. abies | P. chalcographus | de Beer | – | HM031572 | ||
| – | 28135 | – | – | Roikonkoski, Russia | P. abies | P. chalcographus | Linnakoski | HM031508 | – | ||
| 127078 | 34945b,T | D-101420 | 021884 | Pyhäselkä, Finland | P. abies | I. typographus | de Beer | HM031507 | HM031571 | ||
| 128126 | 30883a,b | D-101421 | 021886 | Pyhäselkä, Finland | P. abies | I. typographus | de Beer | – | HM031569 | ||
| O. tapionis sp. nov. | F | 128120 | 23265a,b,T | D-101406 | 021872 | Punkaharju, Finland | P. abies | H. palliatus | de Beer | – | HM031545 |
| 128122 | 23266a,b | D-101407 | 021873 | Punkaharju, Finland | P. sylvestris | H. brunneus | Linnakoski | HM031493 | HM031544 | ||
| – | 23263 | – | 021874 | Punkaharju, Finland | P. abies | H. palliatus | Linnakoski | – | HM031546 | ||
| – | 34940 | – | – | Punkaharju, Finland | P. abies | H. palliatus | Linnakoski | – | HM031543 | ||
| – | 23264 | – | – | Punkaharju, Finland | P. sylvestris | T. minor | Linnakoski | – | HM031548 | ||
| 128121 | 23269a,b | D-101408 | 021875 | Lisino-Corpus, Russia | P. sylvestris | H. palliatus | Linnakoski | HM031494 | HM031547 | ||
| Ophiostoma sp. I | I | – | 23194 | D-101409 | – | Punkaharju, Finland | P. abies | Dryocoetes autographus | de Beer | HM031502 | HM031549 |
| Ophiostoma sp. N | N | – | 28030 | – | – | Kivennapa, Russia | P. sylvestris | T. minor | Linnakoski | HM031511 | HM031568 |
| Isolates of reference species | |||||||||||
| O. abietinum | 125.89T | 22310 | – | – | Mexico | Abies vejari | Pseudohylesinus sp. | Marmolejo | AF4844532 | HM067820 | |
| O. ainoae | 205.83T | 1037 | – | – | Norway | P. abies | I. typograhus | Solheim | – | HM031552 | |
| 118672 | 1903 | – | – | Norway | P. abies | – | Olsen | HM031495 | HM031553 | ||
| O. bicolor | – | 5031 | – | – | Austria | Pinus sp. | I. amitinus | Kirisits | HM031506 | – | |
| O. breviusculum | – | – | JCM 12500T | – | Japan | Larix kaempferi | D. baikalicus | Yamaoka | – | HM031516 | |
| – | – | JCM 12501T | – | Japan | L. kaempferi | D. baikalicus | Yamaoka | AB2004233 | HM031517 | ||
| O. brunneo-ciliatum | – | 5212 | – | – | Scotland | Larix sp. | – | Kirisits | HM031500 | HM031559 | |
| – | 5214 | – | – | Scotland | Larix sp. | – | Kirisits | HM031501 | HM031558 | ||
| O. canum | 133.51A | – | – | – | Sweden | P. sylvestris | – | Mathiesen | HM031489 | HM031518 | |
| O. narcissi | 138.50T | 1096 | – | – | Netherlands | Narcissus sp. | – | Limber | AF4844512 | HM067821 | |
| O. rostrocoronatum | 434.77A | 456 | – | – | USA | pulpwood chips | – | Eslyn | AY1945094 | HM067822 | |
1CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; VTT: Culture Collection of the Technical Research Centre of Finland, Espoo, Finland; JCM: Japan Collection of Micro-organisms, Saitama, Japan. KUO: Kuopio Museum of Natural History, Kuopio, Finland.
2From the study of de Beer et al. (2003a); 3 Chung et al. (2006); and 4 Jacobs et al. (2003).
aIsolates used in growth studies; b Isolates used in morphological descriptions.
TEx-type isolates; A Authentic isolates from the original collections.
Fig. 1.

a. Part of a typical boreal forest in Finland; b. exposed galleries of Hylurgops palliatus on Pinus sylvestris; c. trapping logs in Ilomantsi, Finland; d. galleries of Trypodendron lineatum on Picea abies; e. galleries of Tomicus minor on P. sylvestris.
Purified cultures were grouped according to culture morphology. From each morphological group, isolates representing different sites, beetle and host tree species were selected for DNA sequencing. They were deposited in the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands and the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa. Isolates of the new taxa are also maintained in the VTT Culture collection, VTT Technical Research Centre of Finland, Espoo, herbarium specimens were deposited in Kuopio Museum of Natural History (KUO), Kuopio, Finland, and taxonomic novelties in MycoBank (Crous et al. 2004). Several isolates of known species for which DNA sequences were not available, were also included in the study for comparative purposes (Table 2).
DNA extraction and PCR
Fungal isolates were grown on malt extract agar (MEA; 20 g Difco BactoTM malt extract from Becton, Dickinson & Company, Sparks, USA, 20 g Difco BactoTM agar, and 1 L Milli-Q water) plates. DNA was extracted using PrepMan Ultra Sample preparation reagent (Applied Biosystems, Foster City, CA, USA) using a slight modification on the manufacturer’s protocol (Linnakoski et al. 2008). The ITS 1 and 2 regions, including the 5.8S gene, were amplified using primers ITS1-F (Gardes & Bruns 1993) and ITS4 (White et al. 1990). Part of the β-tubulin gene region was amplified using primers Bt2a and Bt2b (Glass & Donaldson 1995). Primer Bt2b was replaced in some cases with primer T10 (O’Donnell & Cigelnik 1997).
Gene fragments were amplified in 25 μL reaction mixture containing 0.25 μL of Super-Therm DNA polymerase mixture (250 U) (Hoffman-La Roche, Nutley, USA), 2.5 μL of reaction buffer (10×) and 2.5 μL of MgCl2 (25 mM) (supplied with the enzyme), 2.5 μL of dNTPs (2 mM each) and 0.25 μL of each primer (10 μM). Amplification reactions were performed using an Eppendorf Mastercycler® Personal (Perkin-Elmer, Hamburg, Germany). The PCR conditions for ITS gene region were: an initial denaturation step at 95 °C for 2 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 54 °C and 1 min at 72 °C, and a final chain elongation at 72 °C for 8 min. The β-tubulin gene was amplified using a denaturation step at 95 °C for 2 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 56 °C and 1 min at 72 °C, and a final chain elongation at 72 °C for 8 min. Amplified products were purified using the High Pure PCR Product Purification Kit (Roche Molecular Biochemicals, Indianapolis, USA) and sequenced with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on the ABI Prism 377 Autosequencer (Applied Biosystems, Foster City, CA, USA), using the same primers used for the PCR.
Sequence analyses
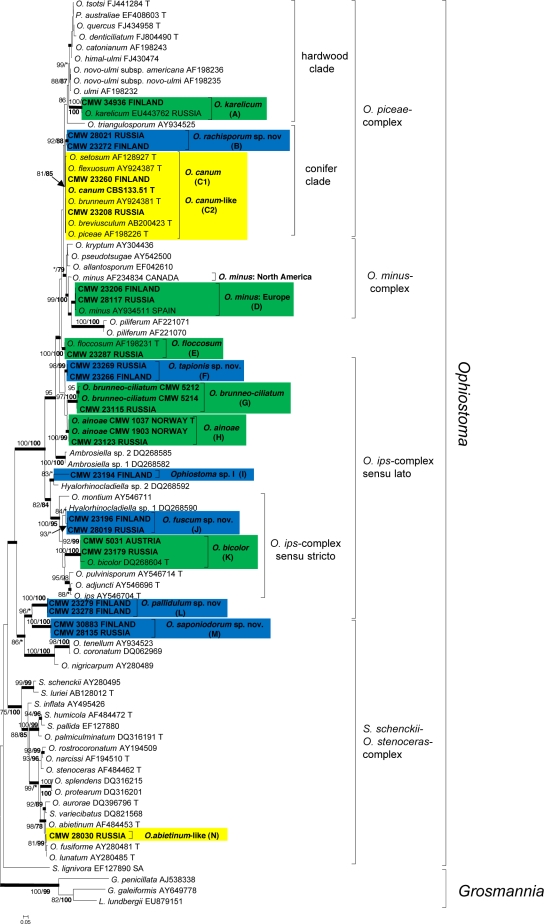
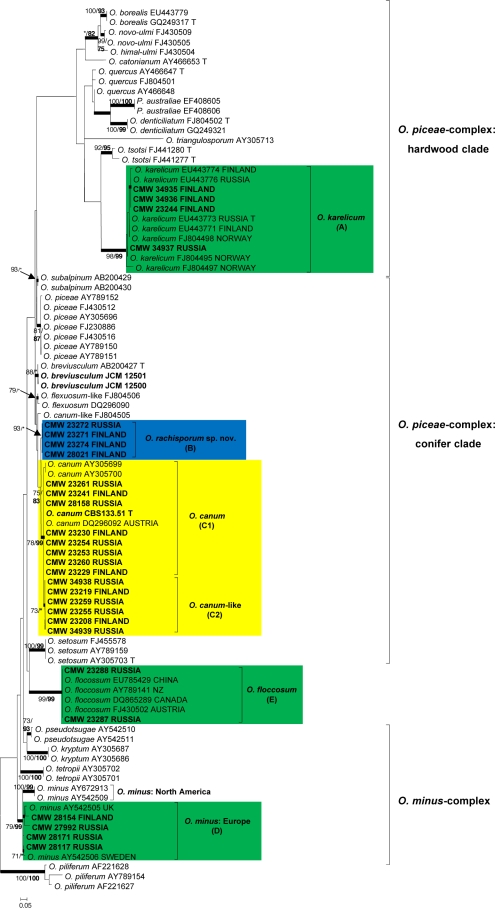
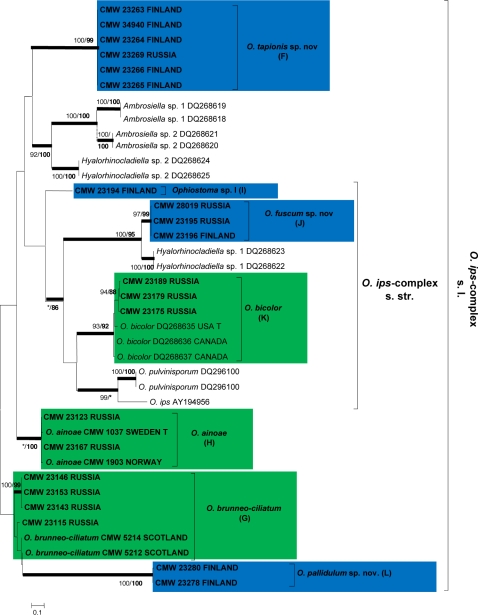
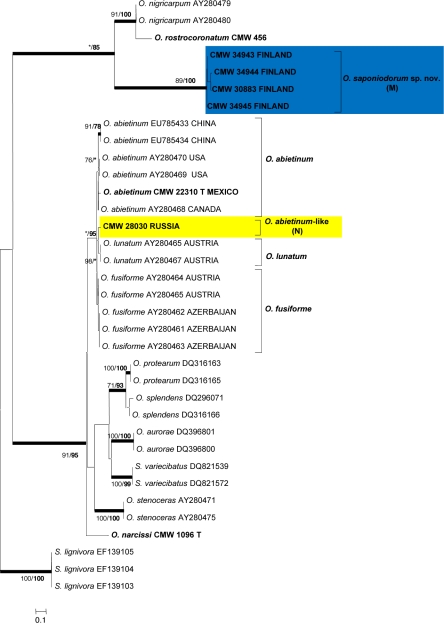
For each isolate, sequences obtained using the forward and reverse primers were aligned and consensus sequences determined using Geneious Pro v4.0.4 for MacIntosh (Biomatters, Auckland, New Zealand). BLAST searches were conducted for preliminary identifications, after which datasets that included published GenBank sequences were compiled in Molecular Evolutionary Genetic Analyses (MEGA) v3.1 (Kumar et al. 2004). Sequences were aligned online with MAFFT v6 (Katoh et al. 2002), using the FFT-NS-i option with a gap opening penalty of 1.53 and an offset value of 0.00. All sequences generated in this study were deposited in GenBank (Table 2). Accession numbers for sequences from reference isolates obtained from GenBank are presented in the phylogenetic trees (Fig. 2, 3, 4 and 5).
Fig. 2.
Phylogram obtained from ML analyses of the ITS region. Novel sequences obtained in this study are printed in bold type. ML bootstrap support values (1 000 replicates) (normal type) and MP Jackknife values (10 000 replicates) (bold type) above 75 % are indicated at the nodes. Posterior probabilities (above 90 %) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 75 %. T = ex-type isolates. Scale bar = total nucleotide difference between taxa. Green = known species; blue = new species; yellow = species of uncertain identity.
Fig. 3.
Phylogram obtained from ML analyses of the β-tubulin gene of the O. piceae- and O. minus complexes. Novel sequences obtained in this study are printed in bold type. ML bootstrap support values (1 000 replicates) (normal type) and MP Jackknife values (10 000 replicates) (bold type) above 75 % are indicated at the nodes. Posterior probabilities (above 90 %) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 75 %. T = ex-type isolates. Scale bar = total nucleotide difference between taxa. Green = known species; blue = new species; yellow = species of uncertain identity.
Fig. 4.
Phylogram obtained from ML analyses of the β-tubulin gene of species in the O. ips-complex s.l. Novel sequences obtained in this study are printed in bold type. ML bootstrap support values (1 000 replicates) (normal type) and MP Jackknife values (10 000 replicates) (bold type) above 75 % are indicated at the nodes. Posterior probabilities (above 90 %) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 75 %. T = ex-type isolates. Scale bar = total nucleotide difference between taxa. Green = known species; blue = new species.
Fig. 5.
Phylogram obtained from ML analyses of the β-tubulin gene of species in the S. schenckii-O. stenoceras-complex. Novel of sequences obtained in this study are printed in bold type. ML bootstrap support values (1 000 replicates) (normal type) and MP Jackknife values (10 000 replicates) (bold type) above 75 % are indicated at the nodes. Posterior probabilities (above 90 %) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 75 %. T = ex-type isolates. Scale bar = total nucleotide difference between taxa. Blue = new species; yellow = species of uncertain identity.
Datasets were analysed using maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI). MP analyses were conducted using TNT v1.1 (Goloboff et al. 2008) run on the computer clusters of the CSC, IT Centre for Science, Espoo, Finland. Heuristic searches with 10 000 replicates of random addition sequences (RAS) and tree bisection and reconnection (TBR) branch swapping were carried out. Gaps were treated as a fifth character for all datasets (Odgen & Rosenberg 2007). A Jackknife test (JK) with 10 000 replicates was used for counting the support values. ML analyses were performed using RAxML v7.0.4 (Stamakis et al. 2008) assuming the GTR+G substitution model, run on the CIPRES cluster at the San Diego Supercomputing Center. Support for the nodes was estimated from 1 000 bootstrap replicates (Felsenstein 1985). BI analyses based on a Markov Chain Monte Carlo (MCMC) were carried out with MrBayes v3.1.2 (Ronquist & Huelsenbeck 2003). The MCMC chains were run for five million generations using the best fitting model selected by the AIC in MrModeltest v2.3 (http://www.abc.se/~nylander/). Trees were sampled every 100 generations resulting in 50 000 trees from both runs. From these, the first 200, 250, 100 and 100 trees were discarded as burn-in, as calculated for the respective datasets. The remaining trees were used to construct majority rule consensus trees.
Morphological studies
DNA sequence analyses suggested that some of the isolates considered in this study represented undescribed species of Ophiostoma. In an attempt to obtain sexual structures and to determine the thallism of the purportedly unknown taxa discovered, mating experiments were conducted. For each species, a single conidial culture was prepared from each of the available isolates, that was then crossed in all possible combinations with each other. The method described by Grobbelaar et al. (2010) was used where sterilised pine and spruce twigs (depending on the origin of isolate) were placed on the agar plates to encourage sporulation. For controls, isolates were crossed against themselves. Mating experiments were conducted on three different media including water agar (WA; 15 g Difco BactoTM agar and 1 L Milli-Q water), MEA and oatmeal agar (OA; 15 g oatmeal, 15 g Difco BactoTM agar and 1 L Milli-Q water). Cultures were grown in an incubator at 20 °C and inspected regularly for fruiting structures. The viability of ascospores produced from the crosses was confirmed by transferring a drop of ascospores from an ascoma from each positive cross to fresh MEA. These plates were then incubated and monitored. Culture morphology was used to confirm their identity.
For the species descriptions, anamorph and teleomorph (where present) fruiting structures were mounted in 85 % lactic acid on glass slides and observed using a Nikon Eclipse 50i phase contrast microscope (Nikon Corporation Tokyo, Japan). A Nikon DS-Fi1 camera system (Nikon Corporation, Tokyo, Japan) was used to capture photographic images. For the description of new species, measurements were made of 50 each of the relevant anamorph and teleomorph structures. Micronematal anamorph structures were studied using slide cultures (Riddel 1950). Averages, ranges and standard deviation of the measurements were computed. The measurements are presented in the format (minimum–) mean minus standard deviation – mean plus standard deviation (–maximum).
For scanning electron microscopy (SEM), agar disks were cut from the sporulating colonies and fixed in 2.5 % glutaraldehyde in a 0.15 M phosphate buffer overnight and 1 % osmium tetroxide, and dehydrated in an ethanol series. The samples were critical-point dried in liquid carbon dioxide with a Balzers Union CPD 010 (Balzers Union AG, Balzers, Liechtenstein) and sputter-coated with copper or gold at minimum 20 nm thickness in an Emitech K675X sputter coater (Emitech, Ashford, UK). A LEO 1550 scanning electron microscope (Beamtech Nordiska AB, Gnesta, Sweden) operating at 5 kV was used to examine the specimens.
Three representative isolates (Table 2) of each unknown Ophiostoma species were chosen to determine the optimal temperature for growth in culture. Agar plugs 8 mm diam bearing mycelium of isolates were cut from actively growing margins of colonies, and placed at the centres of 9 cm Petri dishes containing 2 % MEA. Five replicate plates were prepared for each isolate at each temperature considered. The growth rates of isolates were determined at temperatures ranging from 5 to 35 °C (± 0.5 °C) at 5 °C intervals in the dark. For one species, the growth rate was determined also at 40 and 45 °C. Colony diameter (three colony diameter measurements per plate) on each plate was measured after 4 and 8 d of incubation in each temperature. Mean radial growth rates (mm/day) at 25 °C were calculated as an average of readings.
RESULTS
Collections of bark beetles and fungi
Altogether 11 bark beetle species were collected from Finland and Russia during the course of this study (Table 3). The majority of bark beetle species occurred on both pine and spruce. Ips sexdentatus, Tomicus minor and T. piniperda were found only on pine, while Ips typographus and Ips sp. were encountered only on spruce. The isolations from bark beetles and their galleries yielded a total of 717 isolates resembling Ophiostoma spp. (Table 3), producing typical Pesotum, Hyalorhinocladiella and/or Sporothrix-like anamorph structures in culture. Isolates representing other genera of the Ophiostomatales were excluded from the present study.
Table 3.
Number of Ophiostoma isolates obtained from 11 bark beetle species and their galleries during the course of this study.
| Picea abies (spruce) (species 1–8) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pinus sylvestris (pine) (species 3–11) | |||||||||||||||||||||||
| Beetle species* → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | |||||||||||
| b | g | b | g | b | g | b | g | b | g | b | g | b | g | b | g | b | g | b | g | b | g | ||
| Fungus species ↓ | |||||||||||||||||||||||
| Finland | |||||||||||||||||||||||
| O. abietinum-like | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. ainoae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. bicolor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. brunneo-ciliatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. canum | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 13 |
| O. canum-like | 17 | 0 | 0 | 0 | 15 | 0 | 1 | 1 | 15 | 0 | 9 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 8 | 0 | 27 | 0 | 100 |
| O. floccosum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. fuscum sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| O. karelicum | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| O. minus European | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| O. pallidulum sp. nov. | 0 | 0 | 0 | 0 | 1 | 0 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 9 |
| O. rachisporum sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| O. saponiodorum sp. nov. | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| O. tapionis sp. nov. | 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 10 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 21 |
| Ophiostoma sp. I | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total isolates | 28 | 1 | 0 | 0 | 17 | 0 | 8 | 1 | 29 | 0 | 19 | 2 | 0 | 0 | 19 | 0 | 0 | 0 | 9 | 0 | 41 | 0 | 174 |
| Russia | |||||||||||||||||||||||
| O. abietinum-like | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| O. ainoae | 26 | 25 | 0 | 0 | 0 | 2 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 63 |
| O. bicolor | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| O. brunneo-ciliatum | 50 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 71 |
| O. canum | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 14 | 21 | 65 |
| O. canum-like | 5 | 11 | 1 | 0 | 25 | 23 | 0 | 0 | 27 | 14 | 18 | 15 | 0 | 4 | 0 | 0 | 6 | 7 | 24 | 30 | 7 | 3 | 220 |
| O. floccosum | 0 | 3 | 4 | 1 | 0 | 0 | 0 | 0 | 13 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 33 |
| O. fuscum sp. nov. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| O. karelicum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| O. minus European | 0 | 4 | 0 | 0 | 1 | 4 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 15 | 5 | 11 | 51 |
| O. pallidulum sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O. rachisporum sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| O. saponiodorum sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| O. tapionis sp. nov. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Ophiostoma sp. I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total isolates | 109 | 54 | 5 | 1 | 26 | 29 | 0 | 0 | 60 | 23 | 38 | 20 | 1 | 6 | 1 | 1 | 9 | 11 | 29 | 50 | 30 | 40 | 543 |
* Bark beetle species: 1 = Ips typographus; 2 = Ips sp.; 3 = Dryocoetes autographus; 4 = Hylastes brunneus; 5 = Hylurgops palliatus; 6 = Pityogenes chalcographus; 7 = Pityogenes sp.; 8 = Trypodendron lineatum; 9 = Ips sexdentatus; 10 = Tomicus piniperda; 11 = Tomicus minor.
b = beetles; g = galleries.
DNA sequence analyses
The preliminary analyses indicated that all isolates selected for this study represented Ophiostoma spp., even though sexual structures were not observed for all groups. Due to the large number of isolates collected and sequenced, representative isolates of different beetles, hosts and countries were selected for each taxon for the final sequence datasets. In several cases, the ITS data did not distinguish clearly between closely related species, but they were useful to assign isolates to species complexes within Ophiostoma (Fig. 2). The β-tubulin gene region was used to identify isolates to the species level. Due to differences in the presence and absence of β-tubulin introns between species complexes in Ophiostoma (Zipfel et al. 2006), β-tubulin datasets for the different complexes were analyzed separately from each other (Fig. 3, 4 and 5).
Aligned DNA sequences for the ITS gene region yielded 839 characters, including gaps (Fig. 2). Alignments of the three β-tubulin data subsets consisted respectively of 305, 690 and 276 characters, including gaps (Fig. 3, 4 and 5). The Bayesian analyses for ITS and β-tubulin gene regions produced trees with topologies similar to those of the ML and MP analyses. The best fitting substitution models selected for Bayesian analyses were GTR+I+G (Fig. 2, 4, 5) and GTR+G (Fig. 3).
Comparisons of ITS sequences obtained for isolates in this study, with sequences from GenBank and the reference isolates (Fig. 2), showed that our isolates could be assigned to 14 taxa that are designated A to N. These resided in four species complexes (Harrington et al. 2001, de Beer et al. 2003a, Zipfel et al. 2006) within the genus Ophiostoma. These were the O. piceae-, O. minus-, O. ips- and S. schenckii-O. stenoceras-complexes (Fig. 2). In the ITS-tree (Fig. 2), the lineages representing O. karelicum (A), the European clade of O. minus (D), O. floccosum (E), O. brunneo-ciliatum (G), O. ainoae (H), O. bicolor (K) and five apparently undescribed taxa (B, F, J, L, M) were well-resolved. Isolates in lineages C and N could not be distinguished from closely related species in the ITS tree (Fig. 2).
The β-tubulin gene region amplified for all species in the O. piceae- and O. minus-complexes (Fig. 3) included exons 4, 5, and 6, as well as intron 4, but intron 5 was absent. Four of the groups of isolates in the study formed well-supported lineages, three of which included reference sequences of known species, confirming their identities as O. karelicum (A), the European clade of O. minus (D), and O. floccosum (E). The fourth lineage (B) did not include any isolates of known species, but was clearly distinct from its closest relative, O. canum. The isolates residing in phylogenetic lineage C could be separated in two sublineages (C1 and C2) based on morphology and these are further discussed in the morphology section. However, lineages C1 and C2 had ITS sequences identical to each other and an authentic isolate of O. canum (CBS 133.51). The β-tubulin sequences of lineage C1 were identical to that of the O. canum isolate, but differed only in a single base pair at the 30th position of exon 5, from the sequences of lineage C2.
The β-tubulin sequences of species in the O. ips-complex (Fig. 4) had an intron/exon composition similar to those of the O. piceae- and O. minus-complexes. However, they varied greatly from these complexes and were more appropriately aligned and analyzed as a separate dataset. Analyses of the β-tubulin data for isolates in the O. ips-complex confirmed the identities of three known species from amongst the isolates obtained in this study. These were O. brunneo-ciliatum (G), O. ainoae (H) and O. bicolor (K). The remaining isolates grouped in four lineages (F, I, J, L) with good statistical support, corresponding to the lineages revealed in the ITS tree and represented undescribed taxa.
The intron/exon arrangement of the β-tubulin data for the S. schenckii-O. stenoceras-complex (Fig. 5) differed from the other complexes, lacking intron 4 but with intron 5 present. Analyses of these data revealed that group M formed a well-supported lineage peripheral to the majority of species in the complex but most closely related to O. nigricarpum and O. rostrocoronatum. The single isolate representing group N, resided in a well-supported lineage that included O. abietinum, O. fusiforme and O. lunatum. However, within the larger group, this isolate formed a distinct lineage between the other three species and thus could not be assigned to any of the three known species with certainty.
Morphology
Morphological characters of the isolates identified and new species in the phylogenetic studies are described in detail with the species descriptions in the Taxonomy section. However, the morphological differences between the C1 and C2 groups of isolates that formed a monophyletic lineage (Fig. 2, 3) containing an authentic isolate of O. canum (CBS 133.51) are worth noting here. The morphology of lineage C1 isolates corresponded with that of O. canum (CBS 133.51) of which the Pesotum anamorph produces characteristic globose conidia (Mathiesen 1950, 1951, Harrington et al. 2001). The lineage C2 isolates were different from those of C1, producing obovoid conidia.
The two single isolates representing lineages I and N, both produced only anamorphs in culture. The isolate in lineage I (CMW 23194) primarily formed a Hyalorhinocladiella-like anamorph but also had a Pesotum-like synanamorph, while CMW 28030 (lineage N) produced a well-defined Sporothrix anamorph with denticulate conidiogenous cells.
Mating experiments
Matings between the single conidial cultures resulted in successful crosses, producing ascomata for isolates in lineages B and M, as well as the isolates identified as the European clade of O. minus (lineage D). All crosses, including those of the controls for lineages B and D produced ascomata, confirming that these isolates are homothallic. However, lineage M isolates only produced ascomata when different isolates were crossed, and in none of the control crosses, confirming heterothallism in this species. The ascomata resulting from all the crosses produced viable ascospores that gave rise to colonies typical of the parent isolates on MEA. Isolates representing lineages F, J and L did not form ascomata in culture.
Taxonomy
Based on phylogenetic analyses and morphological comparisons, the isolates collected in this study could be assigned to 15 different taxa, all in the genus Ophiostoma. Seven of these are of known species and these include O. karelicum, O. canum, representatives of the European clade of O. minus, O. floccosum, O. brunneo-ciliatum, O. ainoae and O. bicolor. One species (lineage C2) could not be distinguished from O. canum based on DNA sequences, but had a distinct morphology, and this is consequently treated as ‘O. canum-like’. Another species (lineage N), represented by a single isolate, could not be distinguished from O. abietinum, O. fusiforme and O. lunatum with confidence and we refer to it as ‘O. abietinum-like’. Lineage I, a distinct and undescribed taxon, was represented only by a single isolate, and we have chosen not to describe this species until additional isolates are available for study. This species is thus treated as Ophiostoma sp. ‘I’. Based on the results of this study, the remaining five lineages (B, F, J, L, M) clearly represent undescribed taxa that are described here as new species of Ophiostoma. Two of the novel taxa (lineages B and M) produced ascomata in culture, while the remaining three species (lineages F, J, L) are known only by their anamorph states.
Taxon B
Ophiostoma rachisporum Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB518881; Fig. 6
Fig. 6.
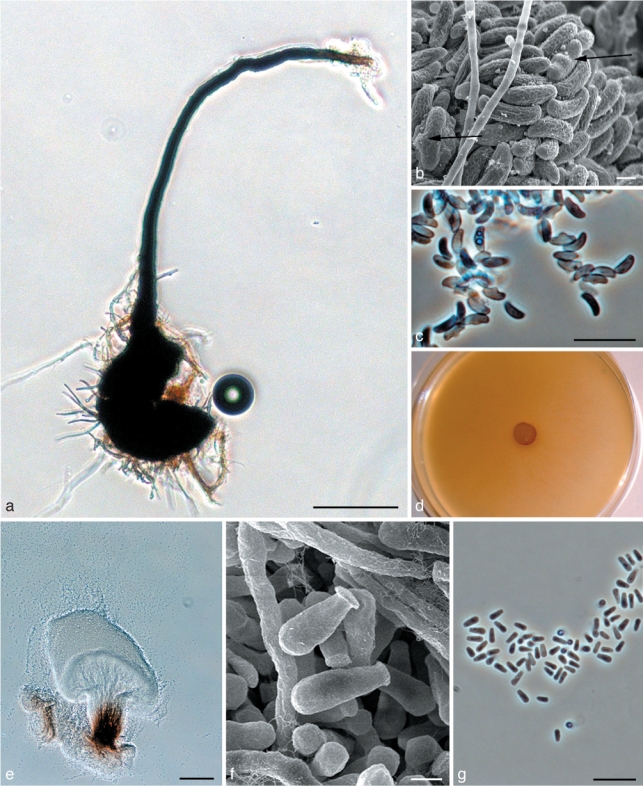
Morphological characters of teleomorph and anamorph structures of Ophiostoma rachisporum sp. nov. a. Ascoma; b. scanning electron micrograph (SEM) of ascospores; c. ascospores; d. fourteen day old culture on malt extract agar (MEA); e. Pesotum anamorph; f. SEM of conidia; g. conidia. — Scale bars: a, e = 100 μm; b, f = 1 μm; c, g = 10 μm.
Anamorph. Pesotum-like
Synanamorph. Sporothrix-like
Ascomata post 30 d in agaro cum farina avenae remulisque laricis sterilisatis evoluta, superficialia vel in medio subimmersa. Bases atrae globosae (97–)111–182(–228) μm diametro. Colla ascomatum nigra, recta vel subfalcata, (230–)282–530(–784) μm longa. Hyphae ostiolares absunt. Ascosporae hyalinae, unicellulares, lateraliter visae allantoidea medio cristata (3–)3.5–4(–4.5) × 1–1.5(–2) μm, a fronte visae cylindricae. Anamorphae Pesotum macronematalis et Sporothriciformis micronematalis adsunt. Mycelium hyalinum, in agaro superficiale. Crescit optime in 20 °C.
Etymology. The epithet rachisporum refers to the ridged ascospores in this species.
Ascomata developing after 30 d on oatmeal agar with sterilised spruce twigs: superficial or partly embedded on media (Fig. 6a). Bases dark, globose, (97–)111–182(–228) μm diam; ornamented with brown hyphal hairs (17–)31–70(–103) μm long, 1–1.5(–2) μm wide at apex, (1–)1.5–2(–2.5) μm wide at base. Ascomatal necks black, straight or slightly curved, (230–)282–530(–784) μm long, (22–)30–43(–50) μm wide at base, (10–)13–20(–25) μm wide at the apex. Ostiolar hyphae absent. Asci not observed. Ascospores hyaline, 1-celled, allantoid in side view, with a ridge in the middle (3–)3.5–4(–4.5) × 1–1.5(–2) μm, cylindrical in frontal view, (2.5–)3–4(–4.5) × 1–1.5(–2) μm, globose in end view (Fig. 6b–c). Pesotum macronematal anamorph predominant (Fig. 6e). Synnemata hyaline, pigmented at the base, (135–)200–365(–547) μm long including capitulum, (22–)35–75(–110) μm wide at base; conidiogenous cells (10–)12–21(–30) × 1 μm; conidia hyaline, 1-celled, smooth, oblong, (2–)3–4(–4.5) × 1–1.5 μm, (Fig. 6f–g) aggregating into a cream-white mucilaginous spore drop. Synnematal anamorph usually abundant on oatmeal agar, malt extract agar and water agar. Attached to substratum by brown rhizoid-like hyphae. Sporothrix-like micronematal anamorph present. Conidiogenous cells arising directly from hyphae, (9–)15–27(–35) × 1–1.5 μm; conidia hyaline, smooth, obovoid to bacilliform, (3–)3.5–5(–7) × 1–1.5(–2) μm.
Culture characteristics — Mycelium superficial on the agar, hyaline, with white to greyish aerial mycelium (Fig. 6d). Colonies slow growing, reaching 31 mm diam in 8 d at 25 °C, mean radial growth rate 3 (± 0.3) mm/d. Growth reduced at 5 and 30 °C. No growth observed at 35 °C. Optimal temperature for growth 20 °C.
Sexuality — Homothallic.
Host range — Associated with Trypodendron lineatum and their galleries on Picea abies and Pinus sylvestris, and Hylurgops palliatus on P. sylvestris.
Distribution — Presently known from Ilomantsi and Punkaharju, Finland; Kivennapa, Russia.
Specimens examined. Finland, Southern Savonia, Punkaharju, on Trypodendron lineatum infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, holotype KUO 021868 (dried culture obtained from cross between CMW 23272 × CMW 23274, Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CBS 128119 = CMW 23272, CBS 128123 = CMW 23274; Southern Savonia, Punkaharju, on Hylurgops palliatus infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, paratype KUO 021871 (dried culture obtained from CMW 23273).
Taxon F
Ophiostoma tapionis Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB518882; Fig. 7
Fig. 7.
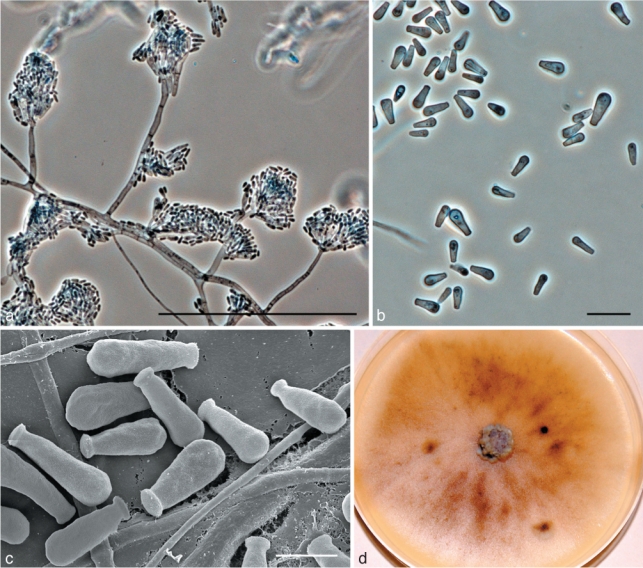
Morphological characters of anamorph structures of Ophiostoma tapionis sp. nov. a. Hyalorhinocladiella anamorph; b. conidia; c. SEM of conidia; d. fourteen day old culture on MEA. — Scale bars: a = 100 μm; b = 10 μm; c = 3 μm.
Anamorph. Hyalorhinocladiella-like
Teleomorpha ignota. Anamorpha micronematalis Hyalorhinocladiella adest. Cellulae conidiogenae (11–)15–25(–31) × 1–2 μm; conidia obovoidea vel bacilliformia (4–)4.5–6(–8) × (1–)1.5–2.5(–3) μm. Mycelium primo hyalinum, deinde medio brunnescens, in agaro superficiale. Crescit optime in 20 °C.
Etymology. The epithet tapionis refers to Tapio, a forest god of Finnish mythology. He figures prominently in the Kalevala, the national epic of Finland.
Teleomorph unknown. Hyalorhinocladiella micronematal anamorph present (Fig. 7a). Conidiogenous cells arising directly from hyphae, (11–)15–25(–31) × 1–2 μm; conidia hyaline, smooth, obovoid to bacilliform, (4–)4.5–6(–8) × (1–)1.5–2.5 (–3) μm (Fig. 7b–c).
Culture characteristics — Colonies at first hyaline, later becoming moderately brown at the centre (Fig. 7d). Mycelium superficial on the agar. Cultures slow growing, reaching 44 mm diam in 8 d at 25 °C, mean radial growth rate 4.5 mm (± 1) mm/d. Growth reduced at 5 and above 30 °C. No growth observed at 35 °C. Optimal temperature for growth 20 °C.
Sexuality — Unknown.
Host range — Associated with Hylurgops palliatus on Picea abies and Pinus sylvestris, Hylastes brunneus and Tomicus minor on P. sylvestris, Ips typographus and Pityogenes chalcographus on P. abies.
Distribution — Presently known from Punkaharju, Finland; Lisino-Corpus and Kivennapa, Russia.
Specimens examined. Finland, Southern Savonia, Punkaharju, on Hylurgops palliatus infesting Picea abies, Feb. 2006, R. Linnakoski, holotype KUO 021872 (dried culture obtained from CBS 128120 = CMW 23265), Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CBS 128120 = CMW 23265; Southern Savonia, Punkaharju, on Hylastes brunneus infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, paratype KUO 021873 (dried culture obtained from CBS 128122 = CMW 23266). – Russia, Lisino-Corpus, on Hylurgops palliatus infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, paratype KUO 021875 (dried culture obtained from CBS 128121 = CMW 23269).
Taxon J
Ophiostoma fuscum Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB518883; Fig. 8
Fig. 8.
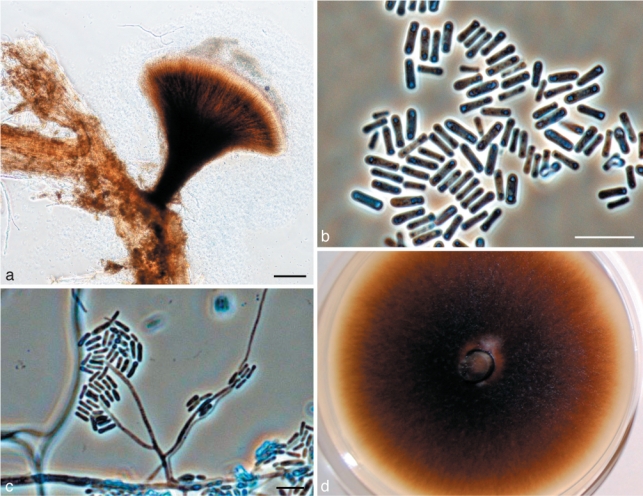
Morphological characters of anamorph structures of Ophiostoma fuscum sp. nov. a. Pesotum-like anamorph; b. conidia; c. Hyalorhinocladiella anamorph; d. fourteen day old culture on MEA. — Scale bars: a = 100 μm; b, c = 10 μm.
Anamorph. Hyalorhinocladiella-like
Synanamorph. Pesotum-like
Teleomorpha ignota. Anamorpha micronematalis Hyalorhinocladiella dominans. Cellulae conidiogenae (5–)15–23(–25) × 1–1.5 μm; conidia obovoidea vel bacilliformia (3.5–)4–5(–6) × 1–1.5 μm. Anamorpha macronematalis Pesotiformis adest. Synnemata atrobrunnea (240–)284–539(–954) μm longa capitulo incluso. Coloniae aequaliter brunneae, in agaro superficiale. Crescit optime in 25 °C.
Etymology. The epithet fuscum refers to the dark brown colour of the macronematal anamorph and colonies.
Teleomorph unknown. Hyalorhinocladiella micronematal anamorph predominant. Conidiogenous cells (5–)15–23(–25) × 1–1.5 μm; conidia hyaline, smooth, obovoid to bacilliform, (3.5–)4–5(–6) × 1–1.5 μm (Fig. 8c). Pesotum-like macronematal anamorph present (Fig. 8a–b). Synnemata dark brown, (240–)284–539(–954) μm long including capitulum, (26–)30–66(–109) μm wide at base; conidiogenous cells (9–)15–24(–30) × 1–1.5 μm (Fig. 8a); conidia hyaline, 1-celled, smooth, oblong, (4–)5–6(–7) × 1–1.5 μm, aggregating into a cream-white mucilaginous spore drop (Fig. 8b). Attached to substratum by brown rhizoid-like hyphae.
Culture characteristics — Colonies evenly brown (Fig. 8d). Mycelium superficial on the agar, greyish aerial mycelium present. Cultures rather slow growing, reaching 53 mm diam in 8 d at 25 °C, mean radial growth rate 6.1 mm (± 0.3) mm/d. Growth reduced at 5 and above 30 °C. Optimal temperature for growth 25 °C.
Sexuality — Unknown.
Host range — Associated with Ips typographus and Pityogenes chalcographus on Picea abies.
Distribution — Presently known from Pyhäselkä, Finland; Kivennapa and Lisino-Corpus, Russia.
Specimens examined. Finland, Pyhäselkä, on Pityogenes chalcographus infesting Picea abies, Aug. 2005, Z.W. de Beer, holotype KUO 021877 (dried culture obtained from CMW 23196, Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CMW 23196. – Russia, Lisino-Corpus, on Ips typographus infesting Picea abies, Feb. 2006, R. Linnakoski, paratype KUO 021876 (dried culture obtained from CBS 128124 = CMW 23195); Kivennapa, on Pityogenes chalcographus infesting Picea abies, Feb. 2006, R. Linnakoski, paratype KUO 021878 (dried culture obtained from CMW 28019).
Taxon L
Ophiostoma pallidulum Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB518884; Fig. 9
Fig. 9.
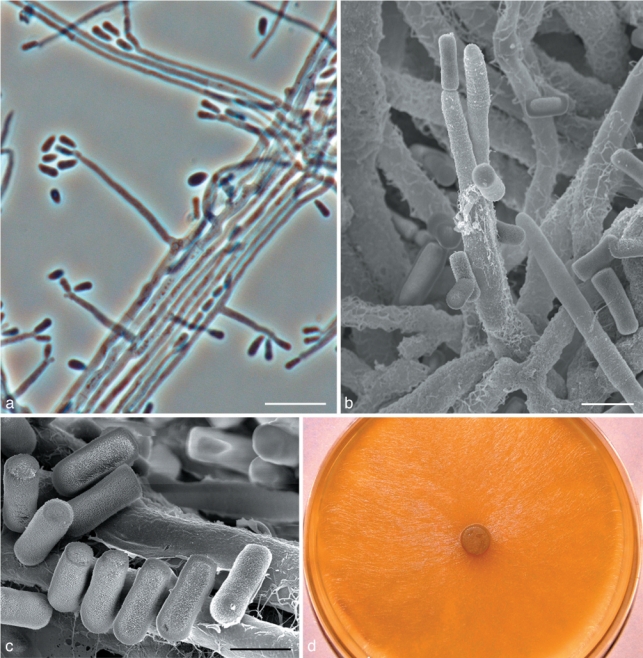
Morphological characters of Ophiostoma pallidulum sp. nov. anamorph structures. a. Hyalorhinocladiella anamorph; b. SEM of conidiogenous cells of Hyalorhinocladiella anamorph; c. SEM of conidia; d. fourteen day old culture on MEA. — Scale bars: a = 10 μm; b = 3 μm, c = 2 μm.
Anamorph. Hyalorhinocladiella-like
Teleomorpha ignota. Anamorpha micronematalis Hyalorhinocladiella adest. Cellulae conidiogenae (9–)11–19(–26) × 1–1.5 μm; conidia obovoidea vel bacilliformia (2–)2.5–3.5(–4) × 1–1.5 μm. Coloniae hyalinae, in agaro superficiale. Crescit optime in 30 °C.
Etymology. The epithet pallidulum refers to the pale colour of the colonies.
Teleomorph unknown. Hyalorhinocladiella micronematal anamorph present (Fig. 9a–c). Conidiogenous cells (9–)11–19(–26) × 1–1.5 μm (Fig. 9a–b); conidia hyaline, smooth, obovoid to bacilliform, (2–)2.5–3.5(–4) × 1–1.5 μm (Fig. 9c).
Culture characteristics — Colonies hyaline, white aerial mycelium present (Fig. 9d). Mycelium superficial on the agar. Rather slow growing, reaching 55 mm diam in 8 d at 25 °C, mean radial growth rate 6 mm (± 1) mm/d. Growth reduced at 5 and above 30 °C. No growth observed at 35 °C. Optimal temperature for growth 30 °C.
Sexuality — Unknown.
Host range — Associated with Hylastes brunneus, Hylurgops palliatus, Tomicus minor, Trypodendron lineatum and Dryocoetes autographus on Pinus sylvestris.
Distribution — Presently known from Punkaharju, Finland.
Specimens examined: Finland, Punkaharju, on Hylastes brunneus infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, holotype KUO 021879 (dried culture obtained from CBS 128118 = CMW 23278, Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CBS 128118 = CMW 23278; on Hylastes brunneus infesting Pinus sylvestris, Feb. 2006, R. Linnakoski, paratype KUO 021880 (dried culture obtained from CMW 23279); on Hylurgops palliatus infesting Pinus sylvestris, Feb. 2005, R. Linnakoski, paratype KUO 021881, (dried culture CBS 127076 = CMW 23280).
Taxon M
Ophiostoma saponiodorum Linnakoski, Z.W. de Beer & M.J. Wingf., sp. nov. — MycoBank MB518885; Fig. 10
Fig. 10.
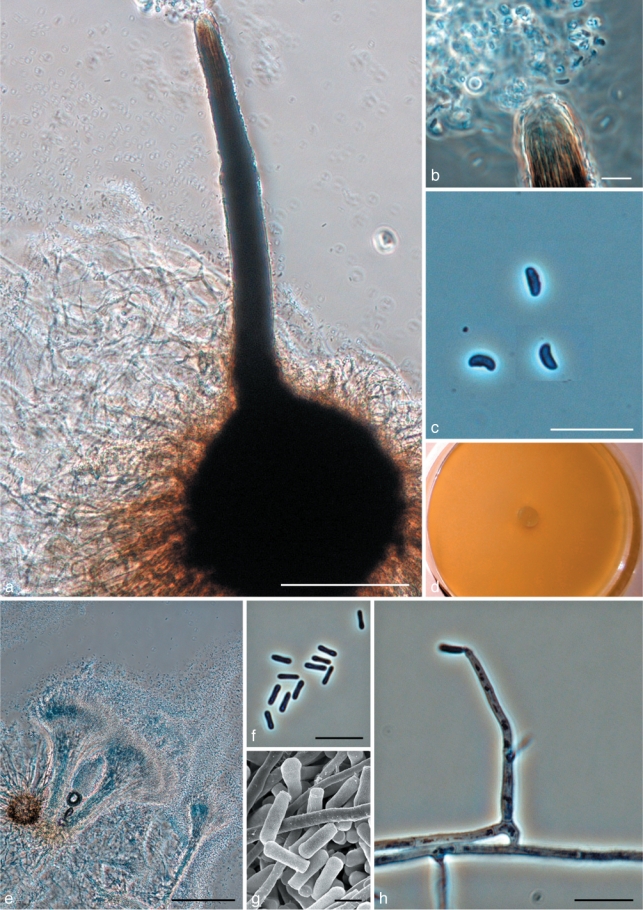
Morphological characters of Ophiostoma saponiodorum sp. nov. anamorph and teleomorph structures. a. Ascoma; b. tip of neck with ascospores; c. ascospores; d. fourteen day old culture on MEA; e. Pesotum-like anamorph; f. conidia; g. SEM of conidia; h. conidiogenous cell and conidia of Hyalorhinocladiella-like anamorph. — Scale bars: a, e = 100 μm; b, c, f, h = 10 μm; g = 2 μm.
Anamorph. Pesotum-like
Synanamorph. Hyalorhinocladiella-like
Ascomata post 30 d in agaro cum farina avenae remulisque laricis sterilisatis evoluta, superficialia vel in medio subimmersa. Bases atrae globosae (110–) 155–211(–263) μm diametro. Colla ascomatum nigra, recta vel subfalcata, (171–)315–449(–527) μm longa, interdum duo vel tres in quoque ascomate in cultura. Hyphae ostiolares absunt. Ascosporae hyalinae, unicellulares, lateraliter visae allantoidea (2.5–)3–4(–4.5) × 1–1.5 μm, a fronte visae cylindricae. Anamorphae Pesotum macronematalis et Hyalorhinocladiella micronematalis adsunt. Mycelium odore saponis, hyalinum, in agaro superficiale. Crescit optime in 30–35 °C. Crescere potest in 40 °C.
Etymology. The epithet saponiodorum refers to the soapy aroma of the colonies.
Ascomata developing after 30 d on oatmeal agar with sterilised spruce twigs when two mating types are paired: superficial or partly embedded on media (Fig. 10a). Bases dark, globose, (110–)155–211(–263) μm diam; ornamented with brown hyphal hairs (27–)45–94(–119) μm long, 1–1.5(–2) μm wide at apex, 1.5–2(–3) μm wide at base. Ascomatal necks black, straight or slightly curved, (171–)315–449(–527) μm long, (28–)32–40(–46) μm wide at base, (9–)11–16(–20) μm wide at the apex. In culture, necks sometimes 2 or 3 per ascoma. Ostiolar hyphae absent (Fig. 10b). Asci not observed. Ascospores hyaline, 1-celled, allantoid in side view, (2.5–)3–4(–4.5) × 1–1.5 μm, cylindrical in frontal view, (2.5–)3–4(–4.5) × 1–1.5 μm, globose in end view (Fig. 10b–c).
Pesotum-like macronematal anamorph predominant (Fig. 10e–g). Synnemata hyaline, (118–)188–297(–370) μm long including capitulum, (15–)20–43(–78) μm wide at base; conidiogenous cells (8–)17–24(–28) × 1–1.5(–2) μm (Fig. 10e); conidia hyaline, 1-celled, smooth, oblong, (3–)3.5–4(–5) × (0.5–)1–1.5 μm, aggregating into a cream-white mucilaginous spore drop (Fig. 10f–g). Synnematal anamorph usually plentiful on oat meal agar. Hyalorhinocladiella-like micronematal anamorph present (Fig. 10h). Conidiogenous cells arising directly from hyphae, (9–)14–24(–35) × 1–2(–2.5) μm; conidia hyaline, smooth, obovoid to bacilliform, (3–)4–6(–7) × 1–1.5(–2) μm.
Culture characteristics — Mycelium hyaline, white aerial mycelium present (Fig. 10d). Colonies rather fast growing, reaching 72 mm diam in 8 d at 25 °C, mean radial growth rate 8 mm (± 0.5) mm/d. Capable growing at high temperatures (40 °C). No growth observed at 5 °C and 45 °C. Optimal temperature for growth 30–35 °C.
Sexuality — Heterothallic.
Host range — Associated with Ips typographus and Pityogenes chalcographus on Picea abies.
Distribution — Presently known from Pyhäselkä and Jouhterinen, Finland; Roikonkoski, Russia.
Specimens examined. Finland, North Karelia, Pyhäselkä, on Ips typographus infesting Picea abies, Aug. 2005, Z.W. de Beer, holotype KUO 021882 (dried culture obtained from cross between CBS 127078 = CMW 34945 × CBS 128125 = CMW 30883, Herbarium of Kuopio Museum of Natural History, Finland), cultures ex-type CBS 127078 = CMW 34945; on Ips typographus infesting Picea abies, Aug. 2005, Z.W. de Beer, paratype KUO 021886 (dried culture obtained from CBS 128125 = CMW 30883); on Pityogenes chalcographus infesting Picea abies, Aug. 2005, Z.W. de Beer, paratype KUO 021887 (dried culture obtained from CBS 127079 = CMW 34944).
DISCUSSION
In this study, 717 fungal isolates resembling Ophiostoma were found in association with 11 different bark beetle species and their galleries, infesting Picea abies and Pinus sylvestris in the Karelia region of Finland and Russia. These fungi included seven known species: Ophiostoma ainoae, O. bicolor, O. brunneo-ciliatum, O. canum, O. floccosum, O. karelicum, and the European lineage of O. minus. Six new Ophiostoma spp. were discovered, five of which were described here as O. fuscum, O. pallidulum, O. rachisporum, O. saponiodorum and O. tapionis. The remaining novel taxon emerging from the analyses was not described formally because it was represented by only a single isolate. The identity of two species, one closely related to O. canum and the other close to O. abietinum could not be resolved with certainty and these require further investigation. Ophiostoma canum is reported for the first time from Finland, and O. brunneo-ciliatum, O. canum and O. floccosum for the first time from Russia. The presence of all the other species previously known to be present in these countries is confirmed for the first time based on DNA sequence comparisons.
The O. piceae-complex and similar species
Phylogenetic inference based on DNA sequence comparisons as well as morphological features made it possible to assign isolates obtained in this study to one of four species complexes in Ophiostoma. These included O. piceae-, O. minus- O. ips- and S. schenckii-O. stenoceras-complexes. The majority of the isolates resided in the O. piceae-complex. This complex was defined by Harrington et al. (2001) as a monophyletic lineage accommodating species having unsheathed, allantoid ascospores and both synnematous Pesotum and mononematous Sporothrix anamorphs. DNA sequence comparisons for additional species and gene regions have revealed that the O. piceae-complex includes at least two major lineages, respectively representing species isolated from hardwoods (Linnakoski et al. 2008, 2009, Nkuekam et al. 2008, Grobbelaar et al. 2009, 2010) and coniferous hosts (Uzunovic et al. 2000, Chung et al. 2006). In all species of the O. piceae-complex for which β-tubulin sequences are available, this gene region includes intron 4 and lacks intron 5 (Zipfel et al. 2006, Grobbelaar et al. 2009).
In the present study, the most commonly encountered species were O. canum and a closely related putatively new species, which we have referred to as O. canum-like. These species were isolated from all locations in association with several different bark beetle species infesting both pine and spruce. The two species could not be distinguished based on ITS and β-tubulin sequences, but they are morphologically distinct. Ophiostoma canum produces globose conidia, while the O. canum-like species produces obovoid conidia, which are more similar to O. piceae and other species in the O. piceae-complex (Harrington et al. 2001). We were not able to amplify the EF 1-α gene region for these species, but additional gene regions should be explored further to distinguish between these taxa.
Ophiostoma canum was originally described from sapstained pine in Germany (Münch 1907). It has since been reported from Tomicus piniperda and T. minor in Sweden (Mathiesen 1950, Rennerfelt 1950) and Japan (Masuya et al. 1999), although identification of isolates in the latter study was based only on morphology. Results of the present study are first to suggest that the species is vectored by other beetle species such as Ips typographus, Hylastes brunneus, Hylurgops palliatus, Pityogenes chalcographus and Trypodendron lineatum.
The unidentified O. canum-like species that was frequently isolated in this study is morphologically similar to O. piceae. Surprisingly, despite the relatively large number of different bark beetle species and locations sampled in this study, no isolates of O. piceae (sensu Morelet 1992, Brasier & Kirk 1993, Harrington et al. 2001) were obtained. Ophiostoma piceae had been reported from Finland and Russia in several earlier studies (Potlajczuk & Schekunova 1985, Viiri 1997, Pashenova et al. 2007), as well as from similar beetles and hosts in neighbouring Scandinavian countries (Lagerberg et al. 1927, Mathiesen 1950, Rennerfelt 1950, Roll-Hansen & Roll-Hansen 1980, Solheim 1986). The O. canum-like species emerging from this study might be the same as isolates from Scandinavia previously identified as O. piceae based on morphology only. Beta-tubulin sequences of these O. canum-like isolates differ substantially (Fig. 2, 3) from those of authentic O. piceae-isolates from other studies (Jacobs & Kirisits 2003, Kim et al. 2005, Bommer et al. 2009, Grobbelaar et al. 2009). DNA sequence comparisons must thus be used to confirm the identification of the so-called O. piceae isolates from previous studies in Scandinavia and Russia.
The third species belonging to O. piceae-complex found emerging from the present study is also related to O. canum and the O. canum-like species, but could be distinguished from these taxa in ITS and β-tubulin sequences. This species was described as O. rachisporum, which produces both Pesotum and Sporothrix anamorphs and allantoid ascospores. The species was mainly isolated from Trypodendron lineatum on both pine and spruce. It is consequently well placed in the conifer lineage of the O. piceae-complex based on phylogeny, morphology and ecology.
It was surprising to find O. karelicum, a representative of the hardwood clade in O. piceae-complex, in the present study. This fungus has consistently been isolated in association with the birch bark beetle, Scolytus ratzeburgi and has been found in Finland, Russia and Norway (Linnakoski et al. 2008, 2009). In the present study, six isolates were found in association with Ips typographus and Tomicus minor from pine and spruce. These results suggest that O. karelicum can be vectored by bark beetle species other than S. ratzeburgi, possibly together with predators and mites associated with these insects.
Another species previously associated with the O. piceae-complex, O. floccosum (Harrington et al. 2001), was found in association with several bark beetle species infesting both pine and spruce in Russia. This species groups only peripherally to the hardwood and conifer clades of the O. piceae-complex (Zipfel et al. 2006, Grobbelaar et al. 2009), but based on morphology, resides within the complex (Harrington et al. 2001). Ophiostoma floccosum is widely distributed, but usually reported in low numbers associated with bark beetles primarily on pine trees (Mathiesen 1951, Harrington et al. 2001, de Beer et al. 2003b, Jacobs et al. 2003, Kim et al. 2007, Lu et al. 2009, Yamaoka et al. 2009).
The O. minus-complex
The O. minus-complex presently includes O. minus, O. pseudotsugae, and O. kryptum (Jacobs & Kirisits 2003, Jacobs et al. 2003, Gorton et al. 2004). These species are distinguished from those in the O. piceae-complex by ascomata with short necks, elongated ascospores and Hyalorhinocladiella anamorphs (Hedgcock 1906, Upadhyay 1981, Jacobs & Kirisits 2003, Jacobs et al. 2003). The β-tubulin intron composition corresponds with that of the O. piceae-complex, which includes intron 4 and lacks intron 5 (Gorton et al. 2004). Although O. tetropii groups among species of the O. minus complex based on our β-tubulin analysis and that of Jacobs & Kirisits (2003), it groups closer to the hardwood clade of the O. piceae complex based on ITS (Jacobs & Kirisits 2003, Jacobs et al. 2003). Its ascomata can also be distinguished from those of the other three species in the complex by its much longer necks (Jacobs et al. 2003). We thus do not treat O. tetropii as part of the O. minus complex until more data proves otherwise.
Several phylogenetic studies have shown a distinction between European isolates of O. minus and North American isolates that represent the type of O. minus (Hedgcock 1906, Gorton & Webber 2000, Gorton et al. 2004, Lu et al. 2009). The isolates collected in this study from Russia and Finland grouped with the European clade of O. minus, which probably corresponds with O. pini. Ophiostoma pini was described from pine by Münch (1907), but since 1956 has been considered a synonym of O. minus (Hunt 1956, Olchowecki & Reid 1974, Upadhyay 1981). Further studies including an inspection of the type material, additional cultures of both species, and comparisons of sequences for gene regions additional to those previously considered, will be needed to clarify the taxonomy of this group.
Ophiostoma minus is one of the most important agents of blue stain in Europe and North America (Solheim & Långström 1991, Seifert 1993). The species is frequently isolated from coniferous trees, mainly from pine species attacked by Tomicus piniperda in Europe (Mathiesen 1950, Mathiesen-Käärik 1953, Lieutier et al. 1989, Solheim & Långström 1991), but it is also occasionally found in association with other beetle species in the region (Käärik 1980, Upadhyay 1981, Kirisits 2004). A single isolate with sequence similarity to the European lineage was recently reported from China (Lu et al. 2009). In the present study, O. minus was most commonly isolated from pine attacked by T. minor, but also in association with several other beetle species.
The O. ips-complex
Species in Ophiostoma ips-complex (s.str.) are characterised by cylindrical or allantoid ascospores with pillow-shaped sheaths, and they produce a continuum of anamorph forms ranging from primarily Hyalorhinocladiella-type structures to more rarely Pesotum-like synnematous structures (Zipfel et al. 2006). Five species have been shown to reside in the O. ips-complex s.str. based on phylogenetic inference. These include O. adjuncti, O. bicolor, O. ips, O. montium and O. pulvinisporum (Kim et al. 2003, Zhou et al. 2004, 2006, Massoumi Alamouti et al. 2007). Kim et al. (2003) showed that the β-tubulin gene of O. montium lacked introns 4 and 5 and consequently, this species was excluded from our analyses of the β-tubulin dataset, in which all the other species had intron 4, and no intron 5. However, based on ITS sequences and morphology, O. montium remains best placed in the O. ips-complex s.str., at least until sequence data for other gene regions can resolve its status.
Massoumi Alamouti et al. (2007) included an undescribed Hyalorhinocladiella sp. (1) in the O. ips-complex. Analyses in the present study and the results of Massoumi Alamouti et al. (2007) showed that several species group close to, but not as part of the monophyletic O. ips-complex s.str. (Fig. 2, 4). For the purpose of this discussion, we treat this larger lineage as the O. ips-complex s.l. The larger lineage includes an undescribed Hyalorhinocladiella sp. (2) and two undescribed Ambrosiella spp. from the study of Massoumi Alamouti et al. (2007). Data from the present study also suggest a similar placement for O. ainoae and O. brunneo-ciliatum, somewhere between the O. ips- and O. piceae-complexes, but closer to the O. ips-complex s.str. The sheathed, pillow-shaped ascospores and Pesotum-like anamorphs of O. ainoae (Solheim 1986) and O. brunneo-ciliatum (Mathiesen-Käärik 1953) correspond well with those of species in the O. ips-complex s.str.
From the bark beetle-associated isolates collected in Finland and Russia, seven species could be placed in the O. ips-complex s.l. as defined above. The first of these was O. brunneo-ciliatum, previously isolated from pines and larch infested by Ips spp. in Europe and Japan (Mathiesen-Käärik 1953, Lieutier et al. 1989, Yamaoka et al. 1998). Isolates in the present study came from Russia and mainly from I. typographus on spruce, but also from Pityogenes chalcographus, Tomicus minor and T. piniperda on pine. The fungus seems to be a constant associate on larch of I. cembrae in Europe and I. subelongatus in Japan (Yamaoka et al. 2009) and was shown to be only mildly pathogenic to its host trees (Lieutier et al. 1989, Yamaoka et al. 1998).
Another species found in the present study was O. ainoae, normally associated with Ips typographus infesting spruce in Europe (Solheim 1986, Jankowiak 2005). All our O. ainoae isolates were collected from Russian sites, mainly in association with I. typographus, but also with Dryocoetes autographus and Hylurgops palliatus infesting spruce, and T. minor infesting pine. This species has also been reported from I. typographus f. japonicus from Japan (Yamaoka et al. 1997), but an isolate from the original Japanese collection groups in Grosmannia, close to G. cucullata, based on ribosomal DNA sequences (Okada et al. 1998, Gebhardt et al. 2005). The appropriate placement of the Japanese species thus needs to be verified using DNA sequence comparisons and including additional cultures.
The third known species from the present study belonging to the O. ips-complex was O. bicolor, originally described by Davidson (1955). It is a common species in the Northern Hemisphere, occurring on spruce infested by Ips spp. (Davidson et al. 1967, Käärik 1975, Solheim 1986, Yamaoka et al. 1997, Jankowiak 2005, Massoumi Alamouti et al. 2007). In our study, isolates of O. bicolor were collected from Russia in an area with extensive I. typographus damage on spruce. These ecological characteristics are consistent with the previously reported fungus-vector relationships for the species.
DNA sequence comparisons for two gene regions of the new taxa described here as O. tapionis, O. fuscum and O. pallidulum confirmed that these species form discrete, well-supported lineages within the broader O. ips-complex. None of the new species produced teleomorphs in culture, but all three produced a Hyalorhinocladiella anamorph, typical of species in the complex. One species, O. fuscum, also had a Pesotum-like synnematous anamorph, similar to those produced by O. ips (Benade et al. 1995, Okada et al. 1998) and O. pulvinisporum (Zhou et al. 2004). In the ITS tree (Fig. 2), O. pallidulum grouped most closely to O. nigricarpum and O. saponiodorum, which are outliers of the S. schenckii-O. stenoceras complex. However, the Hyalorhinocladiella anamorph and β-tubulin intron 4 of O. pallidulum resemble those of species in the larger O. ips-complex (s.l.), in which we have treated it (Fig. 2, 4). Ophiostoma fuscum, O. pallidulum and O. tapionis were isolated from various beetle species on pine and spruce. Ophiostoma pallidulum was found only in Finland.
The species that remains undescribed, Ophiostoma sp. I, was isolated only once from Dryocoetes autographus on spruce in Finland. This isolate also produces a Hyalorhinocladiella anamorph and a Pesotum-like synanamorph, which supports its phylogenetic placement in the O. ips-complex close to other undescribed Hyalorhinocladiella species (Fig. 2, 4) from Canada (Massoumi Alamouti et al. 2007). Additional isolates are needed to justify the description of Ophiostoma sp. I as new.
The S. schenckii-O. stenoceras-complex
The S. schenckii-O. stenoceras-complex is characterized by reniform ascospores without a sheath, a Sporothrix anamorph (de Beer et al. 2003a), and the absence of intron 4 and presence of intron 5 in the β-tubulin gene (Zipfel et al. 2006). The complex accommodates several Sporothrix species associated with human disease (Marimon et al. 2007), soil, wood (Aghayeva et al. 2004, de Meyer et al. 2008), or Protea infructescences with their suite of insects and mites (Roets et al. 2008). It is interesting that most species in the S. schenckii-O. stenoceras-complex does not have bark beetle associates, which are typical for the all other genera and species complexes in the Ophiostomatales.
Isolates obtained in the present study included one new taxon, O. saponiodorum, that grouped in a lineage with O. coronatum, O. nigricarpum, O. rostrocoronatum and O. tenellum, peripheral to the major lineage of the S. schenckii-O. stenoceras-complex (Fig. 2, 5). These species are all of North American origin and most have been collected from stained conifer wood (Davidson 1958, 1966, Olchowecki & Reid 1974). The exception is O. rostrocoronatum that was isolated from birch pulpwood (Eslyn & Davidson 1976). Like these species, O. saponiodorum also produced unsheathed, allantoid ascospores. However, O. saponiodorum differs from other species in the complex with which it groups that have Sporothrix anamorphs. The micronematous anamorph of O. saponiodorum lacks clear denticles characteristic of Sporothrix species (de Hoog 1974), and thus looks more Hyalorhinocladiella-like (Upadhyay & Kendrick 1975). In addition, it has a hyaline Pesotum-like synanamorph. Olchowecki & Reid (1974) described the anamorph of O. coronatum as ‘micronematous or semimacronematous’, which might suggest a similar type of structure, although this cannot be seen in their illustrations. Hyaline synnemata, referred to by some authors as so-called Hyalopesotum anamorphs (Upadhyay & Kendrick 1975), are not a unique character and have been reported for several other species from different lineages in the Ophiostomatales. These are now considered to be a reduced form of the more common Pesotum-like synnemata (Wingfield et al. 1991, Okada et al. 1998), which often occur together with Sporothrix or Hyalorhinocladiella anamorphs.
The identity of lineage N, consisting only of a single isolate from pine in Russia, could not be resolved. The isolate is closely related to O. abietinum, O. fusiforme and O. lunatum. Ophiostoma abietinum was originally described from galleries of a Pseudohylesinus sp. on Abies vejari in Mexico (Marmolejo & Butin 1990). It has subsequently often been reported erroneously as O. nigricarpum-like and occurs in low numbers associated with pine bark beetles in various countries (de Beer et al. 2003a, Aghayeva et al. 2004, Thwaites et al. 2005, Zhao et al. 2005, Zhou et al. 2006, Lu et al. 2009). Ophiostoma fusiforme and O. lunatum have been found only on hardwoods in Europe (Aghayeva et al. 2004). Considering its host, it seems possible that the Russian isolate represents a new haplotype of O. abietinum, but additional isolates and sequences for an additional number of gene regions will be necessary to resolve its identity.
Conclusions
The aim of this study was to characterize Ophiostoma species associated with pine- and spruce-infesting bark beetles in Finland and neighbouring Russia. From a superficial assessment of our results, the diversity of Ophiostoma species is higher in Russia than in Finland, with 10 species found in Russia, and only seven from Finland.
Results of the present study identified some of the fungal associates of Hylastes brunneus. These include Ophiostoma canum, an O. canum-like species, O. pallidulum and O. tapionis. To the best of our knowledge, there have been no previous reports regarding the fungal associates of H. brunneus.
A surprising outcome of the present study is that there are many more Ophiostoma spp. occurring on pine and spruce in the boreal forests of Finland and Russia, than has previously been recognized. The number of new taxa encountered, covering a relatively small geographical area considered in this survey, emphasizes the importance of developing a clear understanding of the possible threats of moving timber and wood products, without knowledge of the micro-organisms that might also be moved. It will, therefore, be important to expand these surveys to wider geographical areas of Finland and Russia, and also to consider the pathogenicity of the species involved.
Acknowledgments
We are grateful to St. Petersburg State Technical University, Russia, for their help in fieldwork in Russia. Thanks are also due to Dr Henri Vanhanen for assistance with fieldwork and identification of the bark beetle species collected. We thank Prof. Heikki Roininen for the collection of Ips typographus from the outbreak area in Ohtama, Russia, and Dr Kaija Keinonen for her advice regarding laboratory work. We also thank Duong Anh Tuan for three reference sequences, and the Finnish IT Center for Science (CSC) for providing computational resources. We are grateful to Dr Hugh Glen for the Latin diagnoses and Markku A. Huttunen for his assistance in naming the novel species. Our laboratory assistants are thanked for their invaluable help with the fungal cultures. The study was supported financially by the Graduate School in Forest Sciences (GSForest), the Finnish Forest Industries Federation, Finnish Forest Research Institute (Metla), Finnish Food Safety Authority (Evira), and North Karelia University of Applied Sciences, Finland; Saint Petersburg State Forest Technical University, Russia; the members of the Tree Protection Co-operative Programme (TPCP) and the THRIP initiative of the Department of Trade and Industry, South Africa.
REFERENCES
- Aghayeva DN, Wingfield MJ, Beer ZW de, Kirisits T.2004. Two new Ophiostoma species with Sporothrix anamorphs from Austria and Azerbaijan. Mycologia 96: 866 – 878 [DOI] [PubMed] [Google Scholar]
- Beer ZW de, Harrington TC, Vismer HF, Wingfield BD, Wingfield MJ.2003a. Phylogeny of the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia 95: 434 – 441 [PubMed] [Google Scholar]
- Beer ZW de, Wingfield BD, Wingfield MJ.2003b. The Ophiostoma piceae complex in the Southern Hemisphere: a phylogenetic study. Mycological Research 107: 469 – 476 [DOI] [PubMed] [Google Scholar]
- Benade E, Wingfield MJ, Wyk PS van.1995. Conidium development in the Hyalorhinocladiella anamorph of Ophiostoma ips. Mycologia 87: 298 – 303 [Google Scholar]
- Bommer M, Hutter M-L, Stilgenbauer S, Hoog GS de, Beer ZW de, Wellinghausen N.2009. Fatal Ophiostoma piceae infection in a patient with acute lymphoblastic leukaemia. Journal of Medical Microbiology 58: 381 – 385 [DOI] [PubMed] [Google Scholar]
- Brasier CM.1990. China and the origins of Dutch elm disease: an appraisal. Plant Pathology 39: 5 – 16 [Google Scholar]
- Brasier CM.1991. Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia 115: 151 – 161 [Google Scholar]
- Brasier CM, Kirk SA.1993. Sibling species within Ophiostoma piceae. Mycological Research 97: 811 – 816 [Google Scholar]
- Chung W-H, Kim J-J, Yamaoka Y, Uzunovic A, Masuya H, Breuil C.2006. Ophiostoma breviusculum sp. nov. (Ophiostomatales, Ascomycota) is a new species in the Ophiostoma piceae complex associated with bark beetles infesting larch in Japan. Mycologia 98: 801 – 814 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Davidson RW.1955. Wood-staining fungi associated with bark beetles in Engelman spruce in Colorado. Mycologia 47: 58 – 67 [Google Scholar]
- Davidson RW.1958. Additional species of Ophiostomataceae from Colorado. Mycologia 50: 661 – 670 [Google Scholar]
- Davidson RW.1966. New species of Ceratocystis from conifers. Mycopathologia 28: 273 – 286 [Google Scholar]
- Davidson RW, Francke-Grosmann H, Käärik A.1967. A restudy of Ceratocystis penicillata and report of two American species of this genus from Europe. Mycologia 59: 928 – 932 [Google Scholar]
- Dudina VS.1936. Dutch elm disease. Publishing of Central Quarantine Laboratory, Moscow: [Google Scholar]
- Dudina VS.1938. Dutch elm disease. Selhozgiz, Moscow-Leningrad: [Google Scholar]
- Eslyn WE, Davidson RW.1976. Some wood-staining fungi from pulpwood chips. Memoirs of the New York Botanical Garden 28: 50 – 57 [Google Scholar]
- Felsenstein J.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783 – 791 [DOI] [PubMed] [Google Scholar]
- Finnish Forest Industries Federation 2010 (22.03.2010). The forest cluster – a network of expertise and business activities. http://www.forestindustries.fi/infokortit/Forest%20cluster_basicinfo/Pages/default.aspx [Google Scholar]
- Gardes M, Bruns TD.1993. ITS primers with enhanced specificity for Basidiomycetes – application to the identification of mycorrhiza and rusts. Molecular Ecology 2: 113 – 118 [DOI] [PubMed] [Google Scholar]
- Gebhardt H, Weiss M, Oberwinkler F.2005. Dryadomyces amasae: a nutritional fungus associated with ambrosia beetles of the genus Amasa (Coleoptera: Curculionidae, Scolytinae). Mycological Research 109: 687 – 696 [DOI] [PubMed] [Google Scholar]
- Gibbs JN.1978. Intercontinental epidemiology of Dutch elm disease. Annual Review of Phytopathology 16: 287 – 307 [Google Scholar]
- Glass NL, Donaldson GC.1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323 – 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloboff PA, Farris JS, Nixon K.2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774 – 786 [Google Scholar]
- Gorton C, Kim SH, Henricot B, Webber J, Breuil C.2004. Phylogenetic analysis of the bluestain fungus Ophiostoma minus based on partial ITS rDNA and β-tubulin gene sequences. Mycological Research 108: 759 – 765 [DOI] [PubMed] [Google Scholar]
- Gorton C, Webber JF.2000. Reevaluation of the status of the bluestain fungus and bark beetle associate Ophiostoma minus. Mycologia 92: 1071 – 1079 [Google Scholar]
- Grobbelaar J, Aghayeva D, Beer ZW de, Bloomer P, Wingfield M, Wingfield B.2009. Delimitation of Ophiostoma quercus and its synonyms using multiple gene phylogenies. Mycological Progress 8: 221 – 236 [Google Scholar]
- Grobbelaar J, Beer ZW de, Bloomer P, Wingfield M, Wingfield B.2010. Ophiostoma tsotsi sp. nov., a wound-infesting fungus of hardwood trees in Africa. Mycopathologia 169: 413 – 423 [DOI] [PubMed] [Google Scholar]
- Harrington TC, McNew D, Steimel J, Hofstra D, Farrell R.2001. Phylogeny and taxonomy of the Ophiostoma piceae-complex and the Dutch elm disease. Mycologia 93: 111 – 136 [Google Scholar]
- Hedgcock GG.1906. Studies upon some chromogenic fungi which discolor wood. Missouri Botanical Garden Annual Report 17: 59 – 114 [Google Scholar]
- Hoog GS de.1974. The genera Blastobotrys, Sporothrix, Calcarisporium and Calcarisporiella gen. nov. Studies in Mycology 7: 1 – 84 [Google Scholar]
- Hunt J.1956. Taxonomy of the genus Ceratocystis. Lloydia 19: 1 – 58 [Google Scholar]
- Ivanchenko JI.1957. The causes of oak wilt in the Lipetsky garden in the Saval’sky forest. Trudy Vsesojuzniy Institut Zashchity Rasteniy (Leningrad) 8: 221 – 225 [Google Scholar]
- Jacobs K, Kirisits T.2003. Ophiostoma kryptum sp. nov. from Larix decidua and Picea abies in Europe, similar to O. minus. Mycological Research 107: 1231 – 1242 [DOI] [PubMed] [Google Scholar]
- Jacobs K, Seifert KA, Harrison KJ, Kirisits T.2003. Identity and phylogenetic relationships of ophiostomatoid fungi associated with invasive and native Tetropium species (Coleoptera: Cerambycidae) in Atlantic Canada. Canadian Journal of Botany 81: 316 – 329 [Google Scholar]
- Jankowiak R.2005. Fungi associated with Ips typographus on Picea abies in southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. Forest Pathology 35: 37 – 55 [Google Scholar]
- Käärik A.1975. Succession of micro-organisms during wood decay. In: Liese W. (ed), Biological transformation of wood by micro-organisms: 39–51. Proceedings of the Sessions of Wood Products Pathology, September 10–12, 1973, Minneapolis, USA: [Google Scholar]
- Käärik A.1980. Fungi causing sap stain in wood. Swedish University of Agricultural Sciences, Department of Forest Products Report R 114, Sweden: [Google Scholar]
- Kamgan Nkuekam G, Solheim H, de Beer ZW, Grobbelaar JW, Jacobs K, Wingfield MJ, Roux J.2010. Ophiostoma species, including Ophiostoma borealis sp. nov., infecting wounds of native broad-leaved trees in Norway. Cryptogamie Mycologie 31: 285 – 303 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059 – 3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G-H, Kim JJ, Breuil C.2007. Sap-staining fungi from logs and boards of two commercially important pines in Korea. Holzforschung 61: 333 – 336 [Google Scholar]
- Kim G-H, Kim JJ, Lim YW, Breuil C.2005. Ophiostomatoid fungi isolated from Pinus radiata logs imported from New Zealand to Korea. Canadian Journal of Botany 83: 272 – 278 [Google Scholar]
- Kim JJ, Kim SH, Lee S, Breuil C.2003. Distinguishing Ophiostoma ips and Ophiostoma montium, two bark beetle-associated sapstain fungi. FEMS Microbiology Letters 222: 187 – 192 [DOI] [PubMed] [Google Scholar]
- Kirisits T.2004. Fungal associates of European bark beetles with emphasis on the Ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans H. (eds), Bark and wood boring insects in living trees in Europe, a synthesis: 181–235. Kluwer Academic Publishers, Dordrecht: [Google Scholar]
- Kryukova EA, Plotnikova TS.1979a. Biological features of fungus from genus Ophiostoma – a vascular mycosis agent on oak in south-east of European part of RSFSR. Mikologia i Fitopatologia 13: 146 – 152 [Google Scholar]
- Kryukova EA, Plotnikova TS.1979b. Vascular mycosis of oak in south-east of European part of RSFSR. Lesnoe Khozyaistvo 1: 69 – 73 [Google Scholar]
- Kryukova EA, Terehova NA.1974. Proceedings of Seventh Conference of All-Union Entomological Society, 2, Leningrad: [Google Scholar]
- Kumar S, Tamura K, Nei M.2004. MEGA3: Integrated Software for Molecular Evolutionary Genetics Analysis and Sequence Alignment. Briefing in Bioinformatics 5: 150 – 163 [DOI] [PubMed] [Google Scholar]
- Lagerberg T, Lundberg G, Melin E.1927. Biological and practical researches into blueing in pine and spruce. Svenska Skogsvårdsföreningens Tidskrift 25: 145 – 272 [Google Scholar]
- Lieutier F, Yart A, Garcia J, Ham MC, Morelet M, Levieux J.1989. Champignons phytopathogènes associès á deux colèoptères scolytidae du pin sylvastre (Pinus sylvestris L) et étude préliminaire de leur agressivité envers l’ ôhote. Annales des Sciences Forestières 46: 201 – 216 [Google Scholar]
- Linnakoski R, Beer ZW de, Rousi M, Niemelä P, Pappinen A, Wingfield MJ.2008. Fungi, including Ophiostoma karelicum sp. nov., associated with Scolytus ratzeburgi infesting birch in Finland and Russia. Mycological Research 112: 1475 – 1488 [DOI] [PubMed] [Google Scholar]
- Linnakoski R, Beer ZW de, Rousi M, Solheim H, Wingfield MJ.2009. Ophiostoma denticiliatum sp. nov. and other Ophiostoma species associated with the birch bark beetle in southern Norway. Persoonia 23: 9 – 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liubarski LV.1937. Fungal diseases of forest trees in the Zeya and Rukhlovo districts of the Russian Far East. Review of Applied Mycology 16: 357 – 358 [Google Scholar]
- Lu M, Zhou XD, Beer ZW de, Wingfield MJ, Sun J-H.2009. Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Diversity 38: 133 – 145 [Google Scholar]
- Malloch DW, Blackwell M.1993. Dispersal biology of the Ophiostomatoid fungi. In: Wingfield MJ, Seifert KA, Webber J. (eds), Ceratocystis and Ophiostoma: Taxonomy, ecology and pathogenicity: 195–206. APS Press, Minnesota: [Google Scholar]
- Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J.2007. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. Journal of Clinical Microbiology 45: 3198 – 3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmolejo JG, Butin H.1990. New conifer-inhabiting species of Ophiostoma and Ceratocystiopsis (Ascomycetes, Microascales) from Mexico. Sydowia 42: 193 – 199 [Google Scholar]
- Massoumi Alamouti S, Kim J-J, Humble L, Uzunovic A, Breuil C.2007. Ophiostomatoid fungi associated with the northern spruce engraver, Ips perturbatus, in western Canada. Antonie van Leeuwenhoek 91: 19 – 34 [DOI] [PubMed] [Google Scholar]
- Masuya H, Kaneko S, Yamaoka Y, Osawa M.1999. Comparisons of Ophiostomatoid fungi associated with Tomicus piniperda and T. minor in Japanese red pine. Journal of Forest Research 4: 131 – 135 [Google Scholar]
- Mathiesen A.1950. Über einige mit Borkenkäfern assoziierte Bläuepilze in Schweden. Oikos 2: 275 – 308 [Google Scholar]
- Mathiesen A.1951. Einige neue Ophiostoma-arten in Schweden. Svensk Botanisk Tidskrift 45: 203 – 232 [Google Scholar]
- Mathiesen-Käärik A.1953. Eine übersicht uber die gewöhnlichsten mit Borkenkäfern assoziierten Bläupilze in Schweden und einige für Sweden neue Bläuepilze. Meddelanden Statens Skogsforskning institut Stockholm 43: 1 – 74 [Google Scholar]
- Meyer E.1946. Stimulatory action of staining fungi on the development of house fungi. Review of Applied Mycology 26: 369 – 370 [Google Scholar]
- Meyer EM de, Beer ZW de, Summerbell RC, Moharram AM, Hoog GS de, Vismer HF, Wingfield MJ.2008. Taxonomy and phylogeny of new wood- and soil-inhabiting Sporothrix species in the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia 100: 647 – 661 [DOI] [PubMed] [Google Scholar]
- Minkevich II.1965. Role of the yellow spotted long horn beetle in the spreading of the infectious drying-up of oak propagation. Forestry Journal 2: 21 – 23 [Google Scholar]
- Morelet M.1992. Ophiostoma querci sur chêne en France. Annales de la Societe des Sciences Naturelles et d’Archeologie de Toulon et du Var 44: 109 – 112 [Google Scholar]
- Münch E.1907. De Blaufäule des Nadelholzes. I-II. Naturwissenschaftliche Zeitschrift für Forst- und Landwirtschaft 5: 531 – 573 [Google Scholar]
- Nkuekam GK, Jacobs K, Beer ZW de, Wingfield MJ, Roux J.2008. Pesotum australi sp. nov. and Ophiostoma quercus associated with Acacia mearnsii trees in Australia and Uganda, respectively. Australasian Plant Pathology 37: 406 – 416 [Google Scholar]
- O’Donnel K, Cigelnik E.1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 [DOI] [PubMed] [Google Scholar]
- Odgen TH, Rosenberg MS.2007. How should gaps be treated in parsimony? A comparison of approaches using simulation. Molecular Phylogenetics and Evolution 42: 817 – 826 [DOI] [PubMed] [Google Scholar]
- Okada G, Seifert KA, Takematsu A, Yamaoka Y.1998. A molecular phylogenetic reappraisal of the Graphium complex based on 18S rDNA sequences. Canadian Journal of Botany 76: 1495 – 1506 [Google Scholar]
- Olchowecki A, Reid J.1974. Taxonomy of the genus Ceratocystis in Manitoba. Canadian Journal of Botany 52: 1675 – 1711 [Google Scholar]
- Pashenova NV, Polyakova GG, Shineleva EV.2007. Blue stain fungi of Ophiostomataceae in coniferous forests of Central Siberia. In: XV Congress of European Mycologists, Saint Petersburg, Russia, September 16–21, 2007: 91 St. Petersburg. Treeart LLC: [Google Scholar]
- Pashenova NV, Vetrova VP, Aphanasova EN, Polyakova GG, Konstantinov MYu.2004. Ophiostomatoid fungi in Middle Siberia. In: Proceedings of the IVth International Symposium of structure, properties and quality of wood: 443–446. [Google Scholar]
- Pashenova NV, Vetrova VP, Konstantinov MYu, Aphanasova EN.2001. Ophiostomataceae fungi associated with Ips typographus in coniferous forests of Central Siberia. Lesovedenie 4: 11 – 19 [Google Scholar]
- Pashenova NV, Vetrova VP, Matrenina RM, Sorokina EN.1995. Ophiostomataceae fungi in larch bark beetle galleries. Lesovedenie 6: 62 – 68 [Google Scholar]
- Potlajczuk VI.1957. On the biology of the causal agent of oak wilt. Trudy Vsesouznogo Naucno-Issledovatel’skogo Instituta Zascity Rasteniy; 8: 227 – 237 [Google Scholar]
- Potlajczuk VI, Schekunova EG.1985. The distribution of species from the genus Ceratocystis Ell. et Halst. emend. Bakshi in the USSR. Novosti Sistematiki nizsich rastienij. “Nauka” Akademia Nauk SSSR 22: 148 – 156 [Google Scholar]
- Rennerfelt E.1950. Über den Zusammenhang Zwischen dem Verblauen des Holzes und den Insekten. Oikos 2: 120 – 137 [Google Scholar]
- Riddell RW.1950. Permanent stained mycological preparations obtained by slide culture. Mycologia 42: 265 – 270 [Google Scholar]
- Rodigin MN.1947. An account of house fungi in the Bashkir Autonomous Soviet Socialistic Republic. Review of Applied Mycology 26: 572 [Google Scholar]
- Roets F, Beer ZW de, Wingfield MJ, Crous PW, Dreyer LL.2008. Ophiostoma gemellus and Sporothrix variecibatus from mites infesting Protea infructescences in South Africa. Mycologia 100: 496 – 510 [DOI] [PubMed] [Google Scholar]
- Roll-Hansen F, Roll-Hansen H.1980. Microorganisms which invade Picea abies in seasonal stem wounds. I. General aspects. Hymenomycetes. European Journal of Forest Pathology 10: 321 – 339 [Google Scholar]
- Ronquist F, Huelsenbeck JP.2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Savonmäki S.1990. Tärkeimmät kaarnakuoriaisten mäntyyn ja kuuseen levittämät sinistäjäsienilajit. Masters Thesis, Department of Plant Pathology, University of Helsinki; [Google Scholar]
- Seifert KA.1993. Sapstain of commercial lumber by species of Ophiostoma and Ceratocystis. In: Wingfield MJ, Seifert KA, Webber J. (eds), Ceratocystis and Ophiostoma: Taxonomy, ecology and pathogenicity: 141–151. APS Press, Minnesota; [Google Scholar]
- Selochnik NN.1998a. Oak tracheomycosis. Mycology and Phytopathology 4: 63 – 74 [Google Scholar]
- Selochnik NN.1998b. New evidence on taxonomy of oak tracheomycosis pathogens (Ophiostoma/Ceratocystis complex). In: Modern problems of mycology, algology and phytopathology. Proceedings of the MSU Conference, 21–23 April 1998. Moscow, Russia: [Google Scholar]
- Sherbin-Parfenenko AL.1953. Cancerous and vascular diseases of deciduous trees. Goslesbumizdat, Moscow-Leningrad: [Google Scholar]
- Siitonen J.1990. Potential forest pest beetles conveyed to Finland on timber imported from the Soviet Union. Silva Fennica 3: 315 – 321 [Google Scholar]
- Six DL.2003. Bark beetle-fungus symbiosis. In: Bourtzis K, Miller TA. (eds), Insect symbiosis: 97–114. CRC Press, Washington, DC: [Google Scholar]
- Solheim H.1986. Species of Ophiostomataceae isolated from Picea abies infested by bark beetle Ips typographus. Nordic Journal of Botany 6: 199 – 207 [Google Scholar]
- Solheim H, Långström B.1991. Blue-stain fungi associated with Tomicus piniperda in Sweden and preliminary observations on their pathogenicity. Annales des Sciences Forestières 48: 149 – 156 [Google Scholar]
- Stamakis A, Hoover P, Rougemont J.2008. A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758 – 771 [DOI] [PubMed] [Google Scholar]
- Storozhenko VG, Krutov VI, Selochnik NN.2000. Fungal communities in forest ecosystems: Materials of coordination investigations. Karelskiy nauchniy centr RAN, Moscow and Petrozavodsk: [Google Scholar]
- Thwaites JM, Farrell RL, Duncan SM, Reay SD, Blanchette RA, Hadar E, Hadar Y, Harrington TC, McNew D.2005. Survey of potential sapstain fungi on Pinus radiata in New Zealand. New Zealand Journal of Botany 43: 653 – 663 [Google Scholar]
- Tkacz BM.2002. Pest risks associated with importing wood to the United States. Canadian Journal of Plant Pathology 24: 111 – 116 [Google Scholar]
- Upadhyay HP.1981. A monograph of Ceratocystis and Ceratocystiopsis. The University of Georgia Press, Georgia, USA: [Google Scholar]
- Upadhyay HP, Kendrick WB.1975. Prodromus for a revision of Ceratocystis (Microascales, Ascomycetes) and its conidial states. Mycologia 67: 798 – 805 [Google Scholar]
- Uzunovic A, Seifert KA, Kim SH, Breuil C.2000. Ophiostoma setosum, a common sapwood staining fungus from western North America, a new species of the Ophiostoma piceae complex. Mycological Research 104: 486 – 494 [Google Scholar]
- Viiri H.1997. Fungal associates of the spruce bark beetle Ips typographus L. (Col. Scolytidae) in relation to different trapping methods. Journal of Applied Entomology 121: 529 – 533 [Google Scholar]
- Vorontsov AI.1986. Current problems in forest pathology. Review of Plant Pathology 66: 420 [Google Scholar]
- Wegelius T.1938. Sulphite wood decay and its influence on the manufacturing process and pulp yield. Finsk Papper- och Trävarutidskrift 15a: 125 – 126, 128 – 130 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–321. Academic Press, California: [Google Scholar]
- Wingfield MJ, Kendrick B, Wyk PS van.1991. Analysis of conidium ontogeny in anamorphs of Ophiostoma: Pesotum and Phialographium are synonyms of Graphium. Mycological Research 95: 1328 – 1333 [Google Scholar]
- Wingfield MJ, Seifert KA, Webber JF. (eds). 1993. Ceratocystis and Ophiostoma: Taxonomy, ecology and pathogenicity. APS Press, St Paul, MN: [Google Scholar]
- Yamaoka Y, Chung W-H, Masuya H, Hizai M.2009. Constant association of ophiostomatoid fungi with the bark beetle Ips subelongatus invading Japanese larch logs. Mycoscience 50: 165 – 172 [Google Scholar]
- Yamaoka Y, Wingfield MJ, Ohsawa M, Kuroda Y.1998. Ophiostomatoid fungi associated with Ips cembrae in Japan and their pathogenicity of Japanese larch. Mycoscience 39: 367 – 378 [Google Scholar]
- Yamaoka Y, Wingfield MJ, Takahashi I, Solheim H.1997. Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Japan. Mycological Research 101: 1215 – 1227 [Google Scholar]
- Zhao G, Chen X, Li D.2005. [New record species of Ophiostoma in China.] In Chinese with English abstract. Forest Research 18: 726 – 729 [Google Scholar]
- Zhou X, Beer ZW de, Cibrian D, Wingfield BD, Wingfield MJ, 2004. Characterisation of Ophiostoma species associated with pine bark beetles from Mexico, including O. pulvinisporum sp. nov. Mycological Research 108: 690 – 698 [DOI] [PubMed] [Google Scholar]
- Zhou X, Beer ZW de, Wingfield MJ.2006. DNA sequence comparisons of Ophiostoma spp., including Ophiostoma aurorae sp. nov., associated with pine bark beetles in South Africa. Studies in Mycology 55: 269 – 277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel RD, Beer ZW de, Jacobs K, Wingfield BD, Wingfield MJ.2006. Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Studies in Mycology 55: 75 – 97 [DOI] [PMC free article] [PubMed] [Google Scholar]