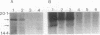Abstract
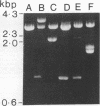
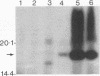
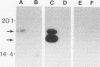
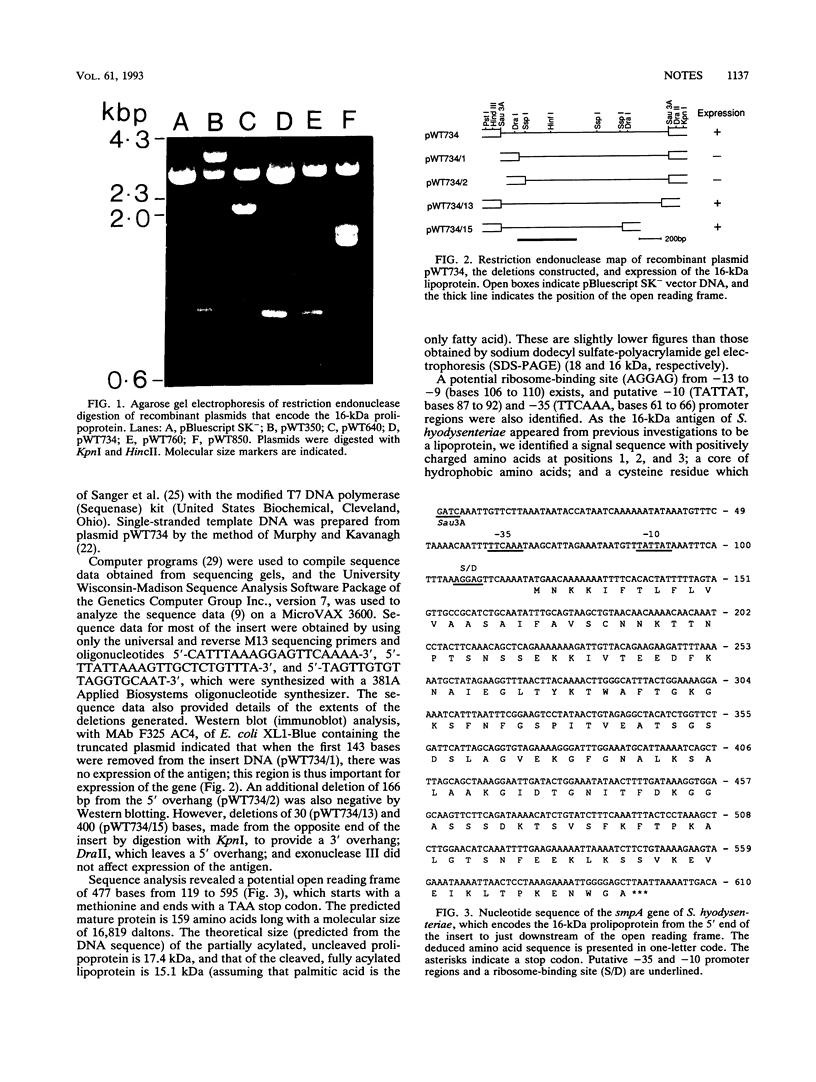
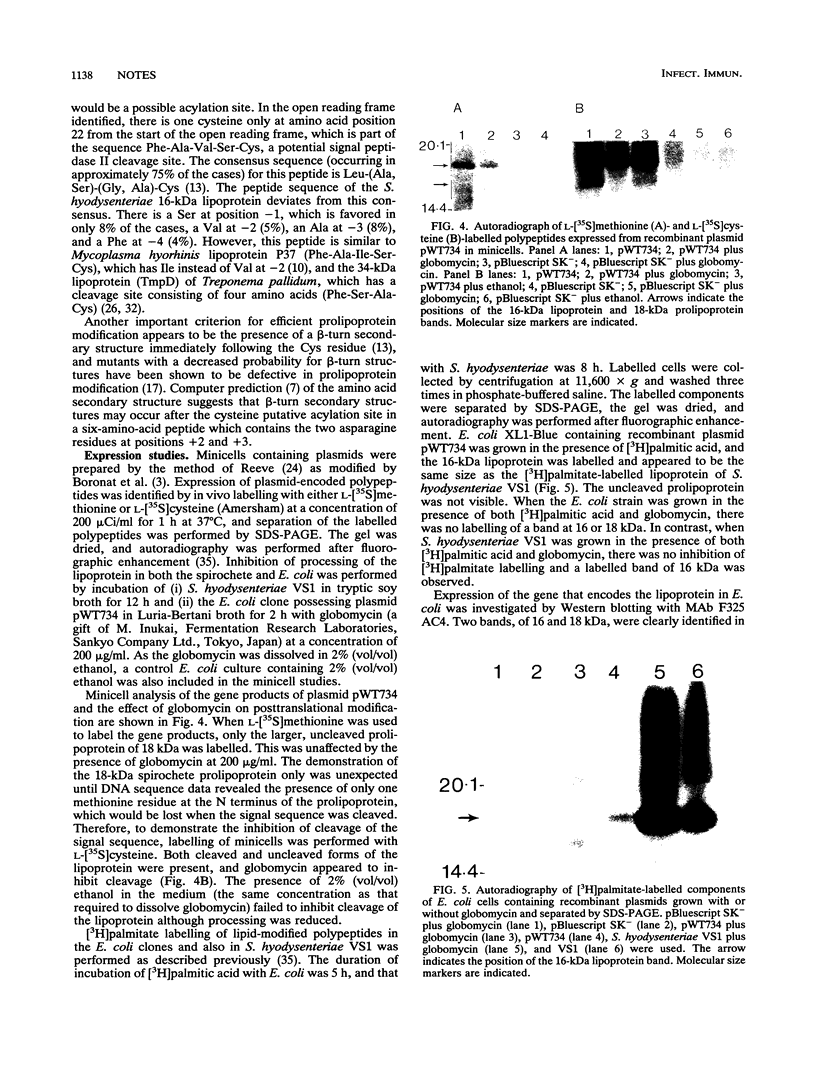
The gene (smpA) that encodes the 16-kDa outer membrane lipoprotein of Serpulina hyodysenteriae was cloned in Escherichia coli, and its primary structure was determined by nucleotide sequencing. The putative open reading frame encodes a prolipoprotein of 16.8 kDa which in its fully acylated and cleaved form is 15.1 kDa. Analysis of the N-terminal amino acid sequence derived from the DNA sequence revealed the presence of a signal sequence and a putative acylation and signal peptidase II cleavage site (Phe-Ala-Val-Ser-Cys). In E. coli, processing of the prolipoprotein was less efficient than that observed in S. hyodysenteriae, and globomycin, an inhibitor of signal peptidase II, inhibited cleavage of the lipoprotein expressed in E. coli but did not inhibit cleavage in S. hyodysenteriae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Molecular biology of antigenic variation in Lyme borreliosis and relapsing fever: a comparative analysis. Scand J Infect Dis Suppl. 1991;77:88–93. [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat A., Britton P., Jones-Mortimer M. C., Kornberg H. L., Lee L. G., Murfitt D., Parra F. Location on the Escherichia coli genome of a gene specifying O-acetylserine (thiol)-lyase. J Gen Microbiol. 1984 Mar;130(3):673–685. doi: 10.1099/00221287-130-3-673. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., DeOgny L., Slaughter C., Radolf J. D., Norgard M. V. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989 Sep;57(9):2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cox D. L., Chang P., McDowall A. W., Radolf J. D. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992 Mar;60(3):1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R., Schmidhauser C., Parish R. W., Wettenhall R. E., Schmidt T. A mycoplasma high-affinity transport system and the in vitro invasiveness of mouse sarcoma cells. EMBO J. 1988 Dec 1;7(12):3963–3970. doi: 10.1002/j.1460-2075.1988.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie D. S., Ripley P. H., Walker P. D. Swine dysentery: protection against experimental challenge following single dose parenteral immunisation with inactivated Treponema hyodysenteriae. Res Vet Sci. 1983 Sep;35(2):217–221. [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Hubbard C. L., Gherardini F. C., Bassford P. J., Jr, Stamm L. V. Molecular cloning and characterization of a 35.5-kilodalton lipoprotein of Treponema pallidum. Infect Immun. 1991 Apr;59(4):1521–1528. doi: 10.1128/iai.59.4.1521-1528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J., Prescott J. F. Swine dysentery: protection of pigs by oral and parenteral immunisation with attenuated Treponema hyodysenteriae. Res Vet Sci. 1976 Nov;21(3):366–367. [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J., Wellstead P. D. Swine dysentery: failure of an attenuated strain of spirochaete, given orally, to protect pigs against subsequent challenge. Br Vet J. 1974 Mar-Apr;130(0):xxxvii–xl. doi: 10.1016/s0007-1935(17)35958-4. [DOI] [PubMed] [Google Scholar]
- Inouye S., Duffaud G., Inouye M. Structural requirement at the cleavage site for efficient processing of the lipoprotein secretory precursor of Escherichia coli. J Biol Chem. 1986 Aug 25;261(24):10970–10975. [PubMed] [Google Scholar]
- Joens L. A., DeYoung D. W., Glock R. D., Mapother M. E., Cramer J. D., Wilcox H. E., 3rd Passive protection of segmented swine colonic loops against swine dysentery. Am J Vet Res. 1985 Nov;46(11):2369–2371. [PubMed] [Google Scholar]
- Joens L. A., Whipp S. C., Glock R. D., Neussen M. E. Serotype-specific protection against Treponema hyodysenteriae infection in ligated colonic loops of pigs recovered from swine dysentery. Infect Immun. 1983 Jan;39(1):460–462. doi: 10.1128/iai.39.1.460-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. A., Sellwood R., Lemcke R. M., Burrows M. R., Lysons R. J. Analysis of the axial filaments of Treponema hyodysenteriae by SDS-PAGE and immunoblotting. J Gen Microbiol. 1989 Jun;135(6):1625–1632. doi: 10.1099/00221287-135-6-1625. [DOI] [PubMed] [Google Scholar]
- Murphy G., Kavanagh T. Speeding-up the sequencing of double-stranded DNA. Nucleic Acids Res. 1988 Jun 10;16(11):5198–5198. doi: 10.1093/nar/16.11.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. Use of minicells for bacteriophage-directed polypeptide synthesis. Methods Enzymol. 1979;68:493–503. doi: 10.1016/0076-6879(79)68038-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls L. M., Mout R., Dekker J., van Embden J. D. Characterization of lipid-modified immunogenic proteins of Treponema pallidum expressed in Escherichia coli. Microb Pathog. 1989 Sep;7(3):175–188. doi: 10.1016/0882-4010(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Kent K. A., Burrows M. R., Lysons R. J., Bland A. P. Antibodies to a common outer envelope antigen of Treponema hyodysenteriae with antibacterial activity. J Gen Microbiol. 1989 Aug;135(8):2249–2257. doi: 10.1099/00221287-135-8-2249. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Radolf J. D., Norgard M. V. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990 Feb;58(2):384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Riley B. S., Radolf J. D., Norgard M. V. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3314–3323. doi: 10.1128/iai.57.11.3314-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W., Sellwood R., Lysons R. J. A 16-kilodalton lipoprotein of the outer membrane of Serpulina (Treponema) hyodysenteriae. Infect Immun. 1992 Aug;60(8):3111–3116. doi: 10.1128/iai.60.8.3111-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W., Sellwood R. Monoclonal antibodies to a 16-kDa antigen of Serpulina (Treponema) hyodysenteriae. J Med Microbiol. 1992 Sep;37(3):214–220. doi: 10.1099/00222615-37-3-214. [DOI] [PubMed] [Google Scholar]