Abstract
3D tissue imaging methods are expected to improve surgical management of cancer. In this study, we examined the feasibility of two 3D imaging technologies, optical coherence tomography (OCT) and optical coherence microscopy (OCM), to view human breast specimens based on intrinsic optical contrast. Specifically, we imaged 44 ex vivo breast specimens including 34 benign and 10 malignant lesions with an integrated OCT and OCM system developed in our laboratory. The system enabled 4 μm axial resolution (OCT and OCM) with 14 μm (OCT) and 2 μm (OCM) transverse resolution, respectively. OCT and OCM images were compared with corresponding histologic sections to identify characteristic features from benign and malignant breast lesions at multiple resolution scales. OCT and OCM provide complimentary information about tissue microstructure, demonstrating distinctive patterns for adipose tissue, fibrous stroma, breast lobules and ducts, cysts and microcysts, as well as in situ and invasive carcinomas. The 3D imaging capability of OCT and OCM provided complementary information to individual 2D images, allowing tracking features from different levels to identify low contrast structures that were difficult to appreciate from single images alone. Our results lay the foundation for future in vivo optical evaluation of breast tissues using OCT and OCM, which has the potential to guide core needle biopsies, assess surgical margins and evaluate nodal involvement in breast cancer.
Keywords: Optical coherence tomography (OCT), Optical coherence microscopy (OCM), Breast, Pathology, Cancer
Introduction
Excluding skin cancers, breast cancer has the highest incidence in women, with an estimated 207,090 new cases of invasive breast carcinoma and 54,010 new cases of in situ carcinoma expected to occur in the United States (U.S.) in 2010 (1). Even though breast cancer is the second leading cause of cancer death in women, with an estimated 39,840 occurring in 2010, breast cancer mortality has declined in the last decade. This decrease in mortality is largely attributed to the increased use of screening mammography and improved effectiveness of treatments (1). Image-guided core biopsy and surgical excisional biopsy are most often used in the diagnostic workup of breast lesions. Compared to open surgical biopsy, core biopsy is less invasive and is therefore considered a gold standard for the diagnosis of breast cancer (2). However, core biopsy has the disadvantage of false-negative rates that result from limitations in tissue sampling (3-6). The false-negative rate for palpation-guided and stereotactic-guided core biopsy ranges from 0-13% (5, 7) and 0.2%-8.9% (5, 8), respectively. Ultrasound-guided core biopsy has been shown to reduce the false negative rate to 0%-3.6% (5, 6, 9, 10). Imaging techniques providing more accurate sampling capabilities in real-time are desirable for improving the diagnostic performance of core biopsies. The emergence of breast conserving surgeries, such as partial mastectomy, allows preservation of the breast without compromising survival. The surgical margin status is considered a strong predictor for local recurrence following partial mastectomy (11-13). In clinical practice, the combination of intra-operative gross examination and post-operative histologic examination are employed to evaluate the surgical margins. However, as many as ~40% of patients require a second surgical procedure due to positive or close margins (14, 15). In addition, evaluation of sentinel and axillary nodal involvement is critical for staging breast cancers (16, 17). Post-operative histologic examination remains the gold standard in assessing the status of lymph nodes. However, sentinel and axillary lymph nodes resections may lead to potential complications such as lymphedema (18). Frozen section analysis has been used to evaluate surgical margins and nodes status intra-operatively (19, 20). However, the analysis is time-consuming and the sampling area is limited. Therefore, a real-time, non-destructive imaging method that has a large sampling field, micron-scale resolution and depth-resolved imaging capability are desirable for intra-operative evaluation of breast cancer.
Optical coherence tomography (OCT) is a promising technique for real-time, high resolution imaging of tissue morphology (21) and has the potential to be used for these important clinical applications. OCT images are formed by detecting back-reflected light, instead of sound, from tissues and therefore enable an order of magnitude in improved imaging resolution compared to ultrasound. Recently, OCT has been used to evaluate breast lesions in animal models (22) and in humans (23). Pilot studies have also investigated OCT for evaluating lymph nodes (24) and intraoperative surgical margin status (25) in breast cancer. To maintain a large imaging depth in OCT, the transverse resolution is usually limited to 10-20 μm, which is not sufficient to image cellular features. Optical coherence microscopy (OCM) is a combination of OCT and confocal microscopy that achieves cellular imaging resolution in both transverse and axial planes (26-28). By enhancing the filtering of multiply-scattered light, better image contrast and greater imaging depth are achieved with OCM (29) with lower numerical aperture compared with confocal microscopy.
In the current study, we employ an integrated OCT and OCM system developed in our laboratory to study freshly excised human breast tissue with various pathologic diagnoses ex vivo based on intrinsic optical contrast. Integrated OCT and OCM has the additional advantage of enabling investigation of tissue structure at the architectural and cellular scale, in a way similar to traditional light microscopy. Few studies using integrated OCT and OCM imaging have been performed to date (30, 31), largely due to the lack of advanced OCM instrumentation. The objective of our study was to establish certain imaging features observed in OCT and OCM and compare these with histology to identify characteristic features of breast lesions that can be visualized with OCT and OCM. The results provide a basis for the interpretation of future OCT and OCM breast tissue images and lay the foundation for future in vivo optical evaluation of breast lesions.
Materials and Methods
Instrumentation
A portable integrated time-domain 3D-OCT and OCM system was employed for the study. A detail description of the system can be found in (32). Briefly, a compact, spectrally broadened, femtosecond Nd:Glass laser was used as the light source to provide >200 nm bandwidth centered at 1060 nm. This enables a <4 μm axial resolution for the OCT and OCM subsystems, providing optical image slices thinner than traditional histological sections. The transverse resolution was ~14 μm and ~2 μm for the OCT and OCM subsystems, respectively. Objectives for OCT and OCM were turret mounted on the sample arm to allow seamlessly switching between high (OCM) and low (OCT) magnifications. The ability to visualize tissue morphology at multiple scales is important for pathologists to differentiate clinically relevant features. Cross-sectional OCT images with 1344 × 1000 (X × Z) pixels were acquired at 1 frame/second. A 3D-OCT data set was constructed with 640 cross-sectional scans covering a volume of 1.5 × 3 × 1.3 mm3 (Y × X × Z). OCM images were obtained with rapid en face raster scanning over a 400 × 400 μm2 field (500 × 750 pixels) at 2 frames/second. 3D-OCM data sets were obtained on selected samples by translating the sample stage in the axial direction at 5 μm/s. A detection sensitivity of -98 dB was achieved with ~10 mW of incident power on the samples.
Study protocol and imaging procedures
The study protocol was approved by the institutional review boards at Beth Israel Deaconess Medical Center (BIDMC) and Massachusetts Institute of Technology (MIT). The protocol was given exempted status as discarded human tissue was collected without interfering with routine pathologic examination. Freshly excised breast specimens were selected based on the presence of pathologic findings on gross examination. Fresh tissue (typically measuring 1 × 1 × 0.5 cm3) from surgical specimens was collected for imaging and placed in RPMI medium 1640 (Invitrogen, Carlsbad, CA) within 1 hour following surgical excision. Imaging was performed within ~2-6 hours of tissue procurement. In total, 44 breast specimens were imaged from 22 patients (median age, 51 years; range 20-90). Thirty-four benign breast specimens were imaged. The specimens with benign diagnosis include fibroadenoma (n = 4), benign fibrocystic disease (n = 13), fat necrosis (n = 3), usual ductal hyperplasia (UDH, n = 4) and normal breast parenchyma (n = 14). Ten specimens were diagnosed as breast carcinoma including invasive ductal carcinoma (n = 5), invasive lobular carcinoma (n = 4), mucinous (colloid) carcinoma (n = 1), ductal carcinoma in situ (DCIS, n = 5) and lobular carcinoma in situ (LCIS, n = 2). On average, 1.4 3D-OCT data sets (range, 1-5) and 1011 en face OCM images (range, 449-2566) were obtained from each specimen. Specimens were classified based upon histologic diagnosis by experienced pathologists.
Before imaging, a cover slip was gently placed on the specimen to create a flat surface and reduce optical aberration. The light pressure applied to the specimen is presumed not to influence tissue morphology and histological comparison with OCT/OCM imaging. 3D-OCT and en face OCM images were acquired within the same sample area to ensure good co-registration. After imaging, a gross photo was taken before the specimen was marked with black and red ink on the imaging surface to indicate specimen orientation. The specimen was then submitted in formalin for standard histologic processing. Five-micron sections were cut from formalin-fixed paraffin-embedded tissue. Slides were stained with hematoxylin and eosin (H&E).
Image analysis
En face slices of OCT images (3 × 1.5 mm2) were reconstructed from the 3D data sets by averaging over 10 μm intervals in the axial direction to reduce speckle noise (31). En face visualization of OCT data has the advantage that the entire image plane is within constant focus and has similar intensity levels. The en face OCT and OCM images were contrast adjusted and displayed with an inverse grayscale color-map, where black represents increased reflectivity. In this retrospective study, the entire en face OCT and OCM database and corresponding histology slides were reviewed to identify matching features that were seen in both OCT/OCM and histologic sections. The generation of volumetric OCT and OCM data provides more comprehensive image information than individual 2D images alone and facilitates the interpretation of OCT and OCM imaging features. Representative benign and malignant specimens were selected for presentation. Photomicrographs of matching histologic sections were taken, to further provide comparison with the en face OCT and OCM images. Photomicrographs were digitally acquired using a standard microscope (Olympus BX40).
Results
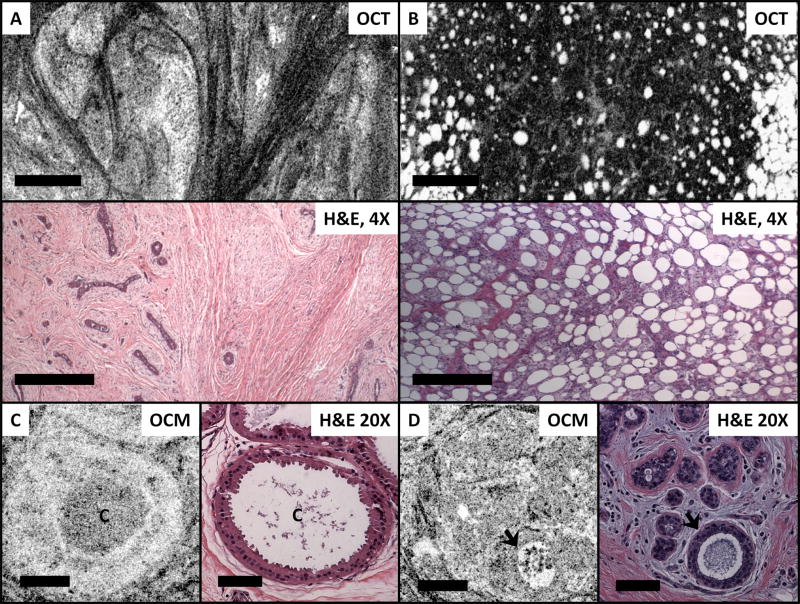
Figures 1 and 2 demonstrate characteristic features observed in normal breast tissue. Adipose tissue is apparent on OCT and OCM images, and is visible as round hyposcattering monomorphous circular structures. Large blood vessels and/or interlobular ducts can also be easily identified as long hyposcattering tubes with hyperscattering walls. Most normal breast tissue consists of fibrous stroma, which has high collagen content and is birefringent. Therefore, loose fibrous stroma appears in the OCT/OCM images as angulated black-and-white short linear reticulation with high contrast. Compared to fibrous stroma, epithelial cell nuclei have a lower backscattering. As a results, normal breast terminal duct lobular units (TDLUs) appear as pale areas that are relatively homogeneous in the OCT images. Under high magnification with OCM, individual TDLUs can be clearly identified with hyposcattering lumina.
Figure 1.
Normal breast tissues. (A) En face OCT image (~90 μm beneath the tissue surface) and corresponding H&E histologic section demonstrate features like breast terminal duct lobular units (TDLUs), fibrous stroma (FS), adipose tissue (A), and blood vessels (V) in normal breast tissue. Scale bars, 500 μm. (B) OCM (~30 μm beneath the tissue surface) and corresponding histologic section demonstrate TDLUs at a higher magnification. Glandular lumina can be clearly seen as hyposcattering circles (arrow). Scale bars, 100 μm.
Figure 2.
OCM images (~50 μm beneath the tissue surface) and corresponding H&E histologic section of normal breast tissue. (A, B) Fibrous stroma; (C) TDLUs; and (D) A single interlobular duct (D). Scale bars, 100 μm. A 3D OCM reconstruction of normal breast lobules can be found in the supplementary materials (Video S1).
Figure 3 shows several representative features observed in benign breast lesions. Figure 3A is an example of a fibroadenoma, in which breast ducts are compressed by surrounding stroma. The OCT image demonstrates the well-circumscribed nodule on low magnification; the interface consists of a smooth border separating hyperscattering dense fibrous tissue. Figure 3B shows characteristic features of fat necrosis, in which necrotic (hyposcattering) adipocytes of different sizes can be clearly identified in the OCT image. Furthermore, OCT and OCM images can also identify cysts and microcysts. Figure 3C shows an example of cysts with apocrine metaplasia, in which cysts (~400 μm in diameter) with a prominent epithelial lining are clearly seen. Figure 3D is another example where a dilated gland with secretions (arrow) can be clearly identified.
Figure 3.
Benign breast lesions. (A) OCT (120 μm beneath the tissue surface) and corresponding H&E histologic section of a fibroadenoma, scale bars, 500 μm; (B) OCT (60 μm beneath the tissue surface) and corresponding H&E histologic section of fat necrosis, scale bars, 500 μm; and (C, D) OCM (~150 μm and ~50 μm beneath the tissue surface, respectively) and corresponding H&E histologic section of small cysts (C) with apocrine metaplasia and a dilated/cystic gland (arrow). Scale bars, 100 μm. 3D OCM reconstruction of the cyst with apocrine metaplasia can be found in the supplementary materials (Video S2).
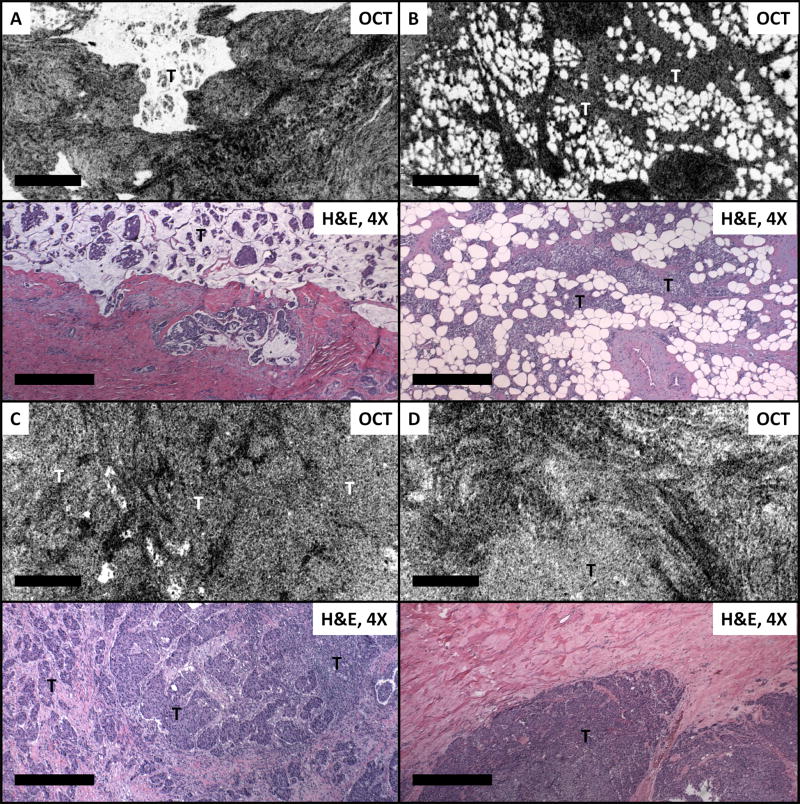
Figures 4-6 demonstrate OCT and OCM images with features observed in malignant breast lesions. Figure 4A shows an example of classic mucinous carcinoma, which is an uncommon type of invasive carcinoma that accounts for ~2-3% of all cases. The characteristic dissecting mucin with “floating” tumor cells are clearly reflected in the OCT image. Figure 4B is a case of invasive ductal carcinoma, in which (pale) tumor cells infiltrating the surrounding adipose tissue are seen. Figure 4C shows a high grade invasive ductal carcinoma. In this case, sheets of (pale) invading cells with irregular borders infiltrate the surrounding hyperscattering (dark) fibrous stroma are clearly visible. In another case of high grade invasive ductal carcinoma (Figure 4D) the infiltrating tumor is completely solid with a pushing border that is clearly seen on OCT imaging.
Figure 4.
OCT images and corresponding H&E histologic sections of invasive carcinoma. (A) Mucinous (colloid) carcinoma (~100 μm beneath the tissue surface); (B-D) Invasive ductal carcinoma (~50 μm, ~80 μm and ~150 μm beneath the tissue surface, respectively). T: tumor. Scale bars, 500 μm.
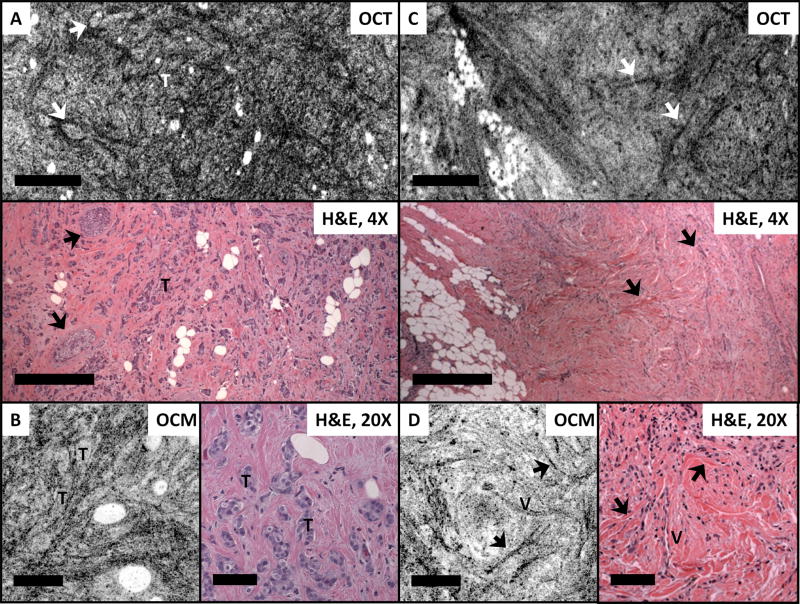
Figure 6.
OCM images and corresponding H&E histologic section of LCIS (A - B), DCIS (C), and high grade invasive ductal carcinoma (D). T: tumor. Scale bars, 100 μm. OCM images in (A-D) were within 50 μm beneath the tissue surface. 3D OCM reconstruction of LCIS in A and B can be found in the supplementary materials (Video S3 and S4).
Figure 5A shows an invasive ductal carcinoma with co-existing DCIS (arrows) observed in the OCT image. The area of DCIS is clearly delineated by a basement membrane within a background of hyperscattering stromal tissue. In addition, the OCM image in Figure 5B also demonstrates small clusters of infiltrating carcinoma. In Figure 5C, and D, a linear infiltration pattern characteristic of invasive lobular carcinoma can be observed on OCT and OCM imaging. Although individual invasive carcinoma cells are not directly seen in the OCT and OCM images, the stromal changes that surround the characteristic cellular infiltration show up prominently in OCT and OCM images as individual straight hyperscattering lines (arrows), mirroring the histological features of invasive lobular carcinoma. A micro vessel (V) is also observed from the OCM image as a double-line (Figure 5D) and correlates with the vessel seen from the histologic section.
Figure 5.
OCT, OCM images and corresponding H&E histologic sections of invasive / in situ ductal carcinoma (A, B) and invasive lobular carcinoma (C, D). Arrows in A point to the DCIS involvements in invasive carcinoma. Arrows in C and D indicate stromal changes surrounding the characteristic cellular infiltration pattern of lobular carcinoma. T: tumor; V: micro vessels. Scale bars, 500 μm for OCT images and corresponding histology and 100 μm for OCM images and corresponding histology. OCT images in A and C were reconstructed from ~70 μm and ~180 μm beneath the tissue surface, respectively. OCM images in B and D were within 50 μm beneath the tissue surface.
OCM images shown in Figure 6 compare features observed in in situ and invasive breast carcinoma. Figure 6A-B show two examples of LCIS. and Figure 6C shows an example of DCIS. As can be seen from the OCM images and corresponding videos provided in the supplementary materials (Video 3 and 4), in situ carcinoma appears well-circumscribed with a regular contour and is clearly confined within the stroma. In situ carcinoma can have a similar OCT/OCM appearance as benign breast lobules (as seen in Figures 1 and 2), however, proliferating tumor cells in general may occlude the lumina of TDLUs and result in tightly packed cell clusters with no fine substructure observed under OCT or OCM. In the high grade invasive ductal carcinoma case shown in Figure 6D, no normal tissue architecture is observed.
Discussion
The OCT and OCM technologies presented in this study may be a useful complementary tool for real time evaluation of breast lesions. Resolutions of OCT and OCM are 10-100 times finer than the state-of-the-art ultrasound technology. In our study, OCT and OCM were shown to successfully visualize characteristic features found in benign and malignant lesions of human breast at multiple resolution scales without exogenous contrast agents or histologic processing. Distinctive OCT and OCM patterns were observed in adipose tissue, fibrous stroma, benign lobules and ducts, cysts and in situ and invasive carcinoma. OCT and OCM provide complimentary information about tissue microstructure. For example, architectural features associated with fibroadenoma, fat necrosis, and invasive carcinoma can be identified on low magnification OCT images, while fine structures such as lumina within normal terminal ducts and lobules, microcysts and clusters of tumor cells can be clearly observed on the high magnification OCM images. The images shown in the fat necrosis example (Figure 3B) may not be easily differentiated from invasive carcinoma into the fat, such as the case in Figure 4B. However calcification usually accompanies fat necrosis and is readily seen, which may be used as an imaging indicator distinguishing fat necrosis from invasive carcinoma, in conjunction with other clinical information to help with the differential diagnosis.
The capability of 3D imaging in OCT and OCM enables volumetric rendering of the tissue, which can be important for identifying characteristic features in various breast pathologies. Three-dimensional imaging provides complementary information to individual 2D OCT or OCM images, and allows tracking features from different levels to identify 3D architecture and low contrast structures that are difficult to appreciate from single images.
Consistent with previous findings (23), we have observed reduced scattering in cell-rich regions, such as normal lobules or tumor, compared to collagen-rich fibrous stroma. A previous study has suggested that occlusion of lumina together with distortion of surrounding architecture could potentially be used to characterize ductal hyperplasia or carcinoma in situ (23). This is consistent with most of our current observations. We encountered cases where distinguishing carcinoma in situ from normal breast lobules (Figure S1), which also have a high cellular density, was quite challenging. This is partly attributed to the limited optical contrast (tissue back-scattering contrast) available in standard OCT and OCM technologies. The development of polarization sensitive OCT (PS-OCT) (33-36) provides contrast based on tissue birefringence properties and can be a useful addition to OCT structural imaging. Moreover, novel methods have been developed to utilize contrast agents, such as gold nanoparticles (37-40), for OCT to enable molecular targeted imaging for early cancer detection.
Each year, about one million core biopsies are performed for the diagnosis of breast cancer in the U.S. (10). During each core biopsy procedure, from six to twenty-four individual tissue cores are sampled from suspicious sites. There have been several efforts in developing miniature needle probes for OCT (41-43), which can potentially be integrated with a biopsy needle to guide tissue sampling. A preliminary assessment at the needle tip can therefore be made based on the imaging findings. A positive optical reading would indicate sampling from the lesion site. A negative reading would guide repositioning of the needle in order to minimize sampling from adjacent benign tissue. This approach would have the potential to improve the diagnostic accuracy of the procedure.
In breast tissues, en face OCT and OCM images with good contrast and resolution can be obtained from depths up to 300-400 μm and 100-200 μm beneath the tissue surface, respectively. This limitation is mainly due to multiple scattering of the tissues and optical aberration as the imaging depth increases. Optical aberrations can be partially corrected by adaptive optics techniques, which may improve imaging depths (44, 45). In addition, imaging at a longer wavelength where the tissue scattering is reduced, e.g. 1300 nm, would help to improve the imaging depth. However, for in vivo evaluation of microstructures several millimeters to centimeters deep inside the tissue, OCT and OCM technologies need to be combined with needle based imaging probes.
One limitation in the current imaging system is the slow imaging speed, which is not sufficient for in vivo applications. Advances in the development of Fourier domain OCT techniques significantly improve (50 - 100x) the imaging speed and sensitivity (46-49). Recent results demonstrate that swept source OCT can achieve 20M axial scans per second (50), enabling a whole 3D-OCT data set presented in the current study to be obtained in a fraction of a second. These technological advances enable imaging speeds sufficient for future in vivo clinical applications.
The current study is a preliminary investigation and the limited specimen number prevents us from performing an accurate statistical analysis of sensitivity and specificity for diagnosing breast cancers using OCT and/or OCM. Prospective studies with a larger sample cohort and blinded evaluation are necessary to evaluate the detection accuracy associated with these new imaging technologies.
In summary, we demonstrate that OCT and OCM are capable of identifying characteristic imaging features in benign and malignant breast lesions based on intrinsic optical contrast. OCT and OCM provide complimentary information about tissue microstructure at multiple resolution scales, demonstrating distinctive patterns for adipose tissue, fibrous stroma, normal breast lobules and ducts, cysts and and in situ and invasive carcinomas. The 3D imaging capability allows tracking features from different levels to identify three dimensional architecture and low contrast structures that are difficult to appreciate from single images. These results lay the foundation for future in vivo optical evaluations of breast lesions based on OCT and OCM technologies.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants R01-CA75289-13 (JGF and JLC), Air Force Office of Scientific Research contract FA9550-07-1-0014 (JGF), Medical Free Electron Laser Program contract FA9550-07-1-0101 (JGF) and the MIT/CIMIT Medical Engineering Fellowship and Taiwan Merit Scholarship from the National Science Council of Taiwan (THT).
Footnotes
Conflict of Interest: J.G.F. receives royalties from intellectual property owned by Massachusetts Institute of Technology and licensed to Lightlab Imaging, Inc., Westford, Mass, and Carl Zeiss Meditec, Dublin, Calif.
References
- 1.Cancer Facts and Figures: American Cancer Society. 2010. [Google Scholar]
- 2.Silverstein MJ, Recht A, Lagios MD, et al. Image-detected breast cancer: state-of-the-art diagnosis and treatment. J Am Coll Surgeons. 2009;209:504–20. doi: 10.1016/j.jamcollsurg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Parker SH, Burbank F, Jackman RJ, et al. Percutaneous Large-Core Breast Biopsy - a Multiinstitutional Study. Radiology. 1994;193:359–64. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 4.Crowe JP, Rim A, Patrick RJ, et al. Does core needle breast biopsy accurately reflect breast pathology? Surgery. 2003;134:523–6. doi: 10.1016/s0039-6060(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 5.Dillon MF, Hill ADK, Quinn CM, O’Doherty A, McDermott EW, O’Higgins N. The accuracy of ultrasound, stereotactic, and clinical core biopsies in the diagnosis of breast cancer, with an analysis of false-negative cases. Ann Surg. 2005;242:701–7. doi: 10.1097/01.sla.0000186186.05971.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VI, Raju U, Chitale D, Deshpande V, Gregory N, Strand V. False-negative core needle biopsies of the breast - An analysis of clinical, radiologic, and pathologic findings in 27 consecutive cases of missed breast cancer. Cancer. 2003;97:1824–31. doi: 10.1002/cncr.11278. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal T, Patel B, Rajan P, Cunningham DA, Darzi A, Hadjiminas DJ. Core biopsy versus FNAC for palpable breast cancers. Is image guidance necessary? Eur J Cancer. 2003;39:52–6. doi: 10.1016/s0959-8049(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 8.Jackman RJ, Nowels KW, Rodriguez-Soto J, Marzoni FA, Finkelstein SI, Shepard MJ. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: False-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210:799–805. doi: 10.1148/radiology.210.3.r99mr19799. [DOI] [PubMed] [Google Scholar]
- 9.Parker SH, Jobe WE, Dennis MA, et al. Us-Guided Automated Large-Core Breast Biopsy. Radiology. 1993;187:507–11. doi: 10.1148/radiology.187.2.8475299. [DOI] [PubMed] [Google Scholar]
- 10.Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: Use and cost-effectiveness. Radiology. 1998;208:717–23. doi: 10.1148/radiology.208.3.9722851. [DOI] [PubMed] [Google Scholar]
- 11.Connolly JL, Boyages J, Nixon AJ, et al. Predictors of breast recurrence after conservative surgery and radiation therapy for invasive breast cancer. Modern Pathol. 1998;11:134–9. [PubMed] [Google Scholar]
- 12.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78:1921–8. doi: 10.1002/(sici)1097-0142(19961101)78:9<1921::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: Influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668–75. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 14.Fleming FJ, Hill ADK, Mc Dermott EW, O’Doherty A, O’Higgins NJ, Quinn CM. Intraoperative margin assessment and re-excision rate in breast conserving surgery. European Journal of Surgical Oncology. 2004;30:233–7. doi: 10.1016/j.ejso.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Swanson GP, Rynearson K, Symmonds R. Significance of margins of excision on breast cancer recurrence. Am J Clin Oncol-Canc. 2002;25:438–41. doi: 10.1097/00000421-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer - A multicenter validation study. New Engl J Med. 1998;339:941–6. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 17.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer I. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 18.Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–14. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Olson TP, Harter J, Munoz A, Mahvi DM, Breslin TM. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007;14:2953–60. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin SA, Ochoa-Frongia LM, Patil SM, Cody HS, Sclafani LM. Influence of frozen-section analysis of sentinel lymph node and lumpectomy margin status on reoperation rates in patients undergoing breast-conservation therapy. J Am Coll Surgeons. 2008;206:76–82. doi: 10.1016/j.jamcollsurg.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Huang D, Swanson EA, Lin CP, et al. Optical Coherence Tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boppart SA, Luo W, Marks DL, Singletary KW. Optical coherence tomography: feasibility for basic research and image-guided surgery of breast cancer. Breast Cancer Res Tr. 2004;84:85–97. doi: 10.1023/B:BREA.0000018401.13609.54. [DOI] [PubMed] [Google Scholar]
- 23.Hsiung PL, Phatak DR, Chen Y, Aguirre AD, Fujimoto JG, Connolly JL. Benign and malignant lesion in the human breast depicted with ultrahigh resolution and dimensional optical coherence tomography. Radiology. 2007;244:865–74. doi: 10.1148/radiol.2443061536. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin RA, Scolaro L, Robbins P, Hamza S, Saunders C, Sampson DD. Imaging of human lymph nodes using optical coherence tomography: potential for staging cancer. Cancer Res. 2010;70:2579–84. doi: 10.1158/0008-5472.CAN-09-4062. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen FT, Zysk AM, Chaney EJ, et al. Intraoperative Evaluation of Breast Tumor Margins with Optical Coherence Tomography. Cancer Res. 2009;69:8790–6. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izatt JA, Hee MR, Owen GM, Swanson EA, Fujimoto JG. Optical coherence microscopy in scattering media. Optics Letters. 1994;19:590–2. doi: 10.1364/ol.19.000590. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre AD, Hsiung P, Ko TH, Hartl I, Fujimoto JG. High-resolution optical coherence microscopy for high-speed, in vivo cellular imaging. Opt Letters. 2003;28:2064–6. doi: 10.1364/ol.28.002064. [DOI] [PubMed] [Google Scholar]
- 28.Huang SW, Aguirre AD, Huber RA, Adler DC, Fujimoto JG. Swept source optical coherence microscopy using a Fourier domain mode-locked laser. Opt Express. 2007;15:6210–7. doi: 10.1364/oe.15.006210. [DOI] [PubMed] [Google Scholar]
- 29.Izatt JA, Kulkarni MD, Wang H-W, Kobayashi K, Sivak MV., Jr Optical coherence tomography and microscopy in gastrointestinal tissues. IEEE Journal of Selected Topics in Quantum Electronics. 1996;2:1017–28. [Google Scholar]
- 30.Aguirre AD, Chen Y, Bryan B, et al. Cellular resolution ex vivo imaging of gastrointestinal tissues with optical coherence microscopy. J Biomed Opt. 2010;15:016025. doi: 10.1117/1.3322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Wang Y, Aguirre AD, et al. Ex vivo imaging of human thyroid pathology using integrated optical coherence tomography and optical coherence microscopy. J Biomed Opt. 2010;15:016001. doi: 10.1117/1.3306696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguirre AD. Advances in Optical Coherence Tomography and Microscopy for Endoscopic Applications and Functional Neuroimaging. Cambridge: Massachusetts Institute of Technology; 2008. [Google Scholar]
- 33.Hee MR, Huang D, Swanson EA, Fujimoto JG. Polarization-Sensitive Low-Coherence Reflectometer for Birefringence Characterization and Ranging. J Opt Soc Am B. 1992;9:903–8. [Google Scholar]
- 34.deBoer JF, Milner TE, vanGemert MJC, Nelson JS. Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography. Optics Letters. 1997;22:934–6. doi: 10.1364/ol.22.000934. [DOI] [PubMed] [Google Scholar]
- 35.Everett MJ, Schoenenberger K, Colston BW, Da Silva LB. Birefringence characterization of biological tissue by use of optical coherence tomography. Optics Letters. 1998;23:228–30. doi: 10.1364/ol.23.000228. [DOI] [PubMed] [Google Scholar]
- 36.Strasswimmer J, Pierce MC, Park BH, Neel V, de Boer JF. Polarization-sensitive optical coherence tomography of invasive basal cell carcinoma. Journal of Biomedical Optics. 2004;9:292–8. doi: 10.1117/1.1644118. [DOI] [PubMed] [Google Scholar]
- 37.Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res T. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 38.Adler DC, Huang SW, Huber R, Fujimoto JG. Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography. Opt Express. 2008;16:4376–93. doi: 10.1364/oe.16.004376. [DOI] [PubMed] [Google Scholar]
- 39.Skala MC, Crow MJ, Wax A, Izatt JA. Photothermal Optical Coherence Tomography of Epidermal Growth Factor Receptor in Live Cells Using Immunotargeted Gold Nanospheres. Nano Lett. 2008;8:3461–7. doi: 10.1021/nl802351p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou C, Tsai TH, Adler DC, et al. Photothermal optical coherence tomography in ex vivo human breast tissues using gold nanoshells. Optics Letters. 2010;35:700–2. doi: 10.1364/OL.35.000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Chudoba C, Ko T, Pitris C, Fujimoto JG. Imaging needle for optical coherence tomography. Optics Letters. 2000;25:1520–2. doi: 10.1364/ol.25.001520. [DOI] [PubMed] [Google Scholar]
- 42.Zysk AM, Marks DL, Liu DY, Boppart SA. Needle-based reflection refractometry of scattering samples using coherence-gated detection. Opt Express. 2007;15:4787–94. doi: 10.1364/oe.15.004787. [DOI] [PubMed] [Google Scholar]
- 43.Iftimia NV, Mujat M, Ustun T, Ferguson RD, Danthu V, Hammer DX. Spectral-domain low coherence interferometry/optical coherence tomography system for fine needle breast biopsy guidance. Rev Sci Instrum. 2009;80:024302. doi: 10.1063/1.3076409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rueckel M, Mack-Bucher JA, Denk W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. P Natl Acad Sci USA. 2006;103:17137–42. doi: 10.1073/pnas.0604791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debarre D, Botcherby EJ, Watanabe T, Srinivas S, Booth MJ, Wilson T. Image-based adaptive optics for two-photon microscopy. Optics Letters. 2009;34:2495–7. doi: 10.1364/ol.34.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fercher AF, Hitzenberger CK, Kamp G, Elzaiat SY. Measurement of Intraocular Distances by Backscattering Spectral Interferometry. Opt Commun. 1995;117:43–8. [Google Scholar]
- 47.Choma MA, Sarunic MV, Yang CH, Izatt JA. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183–9. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 48.de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Optics Letters. 2003;28:2067–9. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 49.Leitgeb R, Hitzenberger CK, Fercher AF. Performance of fourier domain vs. time domain optical coherence tomography. Opt Express. 2003;11:889–94. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 50.Wieser W, Biedermann B, Klein T, Eigenwillig C, Huber R. Multi-Megahertz OCT: High quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second. Opt Express. 2010;18:14685–704. doi: 10.1364/OE.18.014685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.