Abstract
BACKGROUND
Incidence studies of psoriasis are rare, mainly due to lack of established epidemiological criteria and the variable disease course. The objective of this study is to determine time trends in incidence and survival of psoriasis patients over three decades.
METHODS
We identified a population-based incidence cohort of 1633 subjects aged ≥18 first diagnosed with psoriasis between 1/1/1970 and 1/1/2000. The complete medical records for each potential psoriasis subject were reviewed and diagnosis was validated by either a confirmatory diagnosis in the medical record by a dermatologist or medical record review by a dermatologist. Age- and sex-specific incidence rates were calculated and were age- and sex-adjusted to 2000 U.S. white population.
RESULTS
The overall age and sex adjusted annual incidence of psoriasis was 78.9 per 100,000 (95% confidence interval [CI]: 75.0, 82.9). When psoriasis diagnosis was restricted to dermatologist confirmed subjects, the incidence was 62.3 per 100,000 (95% CI: 58.8, 65.8). Incidence of psoriasis increased significantly over time from 50.8 in 1970–74 to reach 100.5 per 100,000 in 1995–1999 time period (p=0.001). Although the overall incidence was higher in males than females (p=0.003), incidence in females was highest in the sixth decade of life (90.7 per 100,000). Survival was similar to the general population (p=0.36).
LIMITATIONS
The study population was mostly white and limited to adult psoriasis patients.
CONCLUSION
The annual incidence of psoriasis almost doubled between the 1970s and 2000. The reasons for this increase in incidence are currently unknown, but could include a variety of factors, including a true change in incidence or changes in the diagnosing patterns over time.
Keywords: Epidemiology, Psoriasis, Population-based study
INTRODUCTION
Psoriasis is a common systemic inflammatory disorder affecting primarily the skin, nails and occasionally the joints. The prevalence of psoriasis in different populations vary between 0 and 12%1, with estimates as high as 2.8% in western populations2, 3. Apart from differences in study populations, the wide variation in prevalence estimates may be due to the remitting relapsing course of psoriasis, wide spectrum in clinical presentation and severity, and most importantly, lack of standardized classification criteria4.
Studies on the incidence of psoriasis are rare, mostly due to the same limiting factors. Two incidence studies covering different time frames were reported from Olmsted County, Minnesota. Bell et al5 reported an incidence of 60.4 (95% confidence interval [CI]: 49.5, 70.3) per 100,000 person years between 1980 and 1983. Shbeeb et al6 screened all psoriasis cases between 1982 and 1991 and reported an incidence of 107.7 (95% CI: 101.2, 114.2) per 100,000. More recently, in a large database study from the U.K., the incidence of psoriasis in 1996–1997 was estimated as 140 per 100,000 patient years7. Intriguingly, the results of these three reports from different time periods suggest an increase in the incidence of psoriasis over time. Yet, no studies to date examined trends in incidence of psoriasis.
The objective of this population based study was to describe time trends in incidence, characteristics and survival of psoriasis patients in Olmsted County, Minnesota between 1970 and 2000.
METHODS
Study Setting
This population based retrospective study was carried out in Olmsted County, Minnesota, which has a population of 124,277 according to 2000 census. We used the resources of the Rochester Epidemiology Project8, a medical records-linkage system containing complete inpatient and outpatient records from all healthcare providers in Olmsted County, including the Mayo Clinic, Olmsted Medical Group, local nursing homes and the few private practitioners. All medical, surgical, and histological diagnoses and other key information are abstracted and entered into computerized indices to facilitate case identification. The Rochester Epidemiology Project allows ready access to the complete (inpatient and outpatient, labs) medical records for each subject from different healthcare providers, including both paper and electronic medical records. Therefore, this population-based data resource ensures virtually complete ascertainment and follow-up of all clinically diagnosed cases of psoriasis in a geographically-defined community with the unique ability to access original medical records for case validation.
Study population and data collection
Using the resources of Rochester Epidemiology Project, we identified all subjects who were residents of Olmsted County aged ≥18 years with diagnostic codes consistent with psoriasis (ICD9 696.1, 696.2, 696.8, 696.5, 696.3) between January 1, 1970 and January 1, 2000. The complete medical records of all subjects were reviewed and psoriasis was validated by either a confirmatory diagnosis in the medical record by a dermatologist, or a physician's description of the lesions in the medical record or a skin biopsy, whenever available. In case of a doubtful diagnosis, the medical record was reviewed by the dermatologist co-investigator (MTM). Incidence date was defined as the physician diagnosis date. Prevalent psoriasis subjects, subjects with missing medical records and those who denied research authorization were excluded.
Data were collected on demographics (date of birth, sex, race and ethnicity); duration of psoriasis symptoms (lesions) before the physician diagnosis; type of lesions (chronic plaque, guttate, erythrodermic, pustular or sebo-psoriasis); site of lesions (palms and/or soles, elbows and/or knees, trunk, face, scalp, axilla, groin, inframammary, intergluteal/perianal or genital); and presence of nail involvement at the time of diagnosis and presence of psoriatic arthritis. Severity information as described by the physician was recorded, where available. Dates and results of skin biopsies and confirmation of diagnosis by a dermatologist or rheumatologist in subjects with psoriatic arthritis at any time during the course of the disease were also retrieved. Data on phototherapy, PUVA and systemic drug therapy (methotrexate, oral retinoids, azathioprine, cyclosporine, hydroxyurea, sulphasalazine and biologics) were collected in a random subset of 420 (26%) subjects.
Statistical analysis
Age and sex-specific incidence rates for psoriasis were calculated for individuals aged ≥18 years. Age- and sex-specific incidence rates were calculated by using the number of incident psoriasis cases as the numerator and population estimates based on decennial census counts as the denominator, with linear interpolation between census years, as described previously9. Only subjects who were residents of Olmsted County at the onset of disease and who fulfilled the study criteria for psoriasis were included in the incidence calculations. Overall incidence rates were age- and sex-adjusted to the 2000 white population of the United States. Ninety-five percent confidence intervals (95% CIs) for the incidence rates were constructed using the assumption that the number of incident cases per year follows a Poisson distribution. Incidence trends were examined using Poisson regression models with smoothing splines for age and calendar year. Predictions from these models were used to illustrate trends in Figures 2 and 3. Survival was estimated using the Kaplan-Meier method both for the overall cohort and the random sample of subjects with detailed information on phototherapy and systemic therapy. Expected survival was based on the age and sex distribution of the study population and death rates derived from the Minnesota (white population) life tables. Observed and expected survival was compared using a one-sample log-rank test.
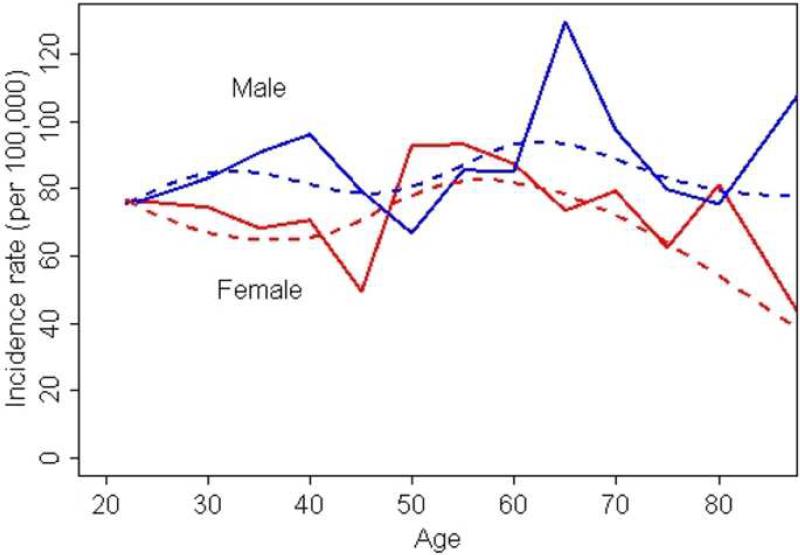
Figure 2.
Incidence of psoriasis by age groups for males and females.
Bold lines represent the overall (crude) incidence curves and dotted lines represent incidence curves derived using smoothing splines.
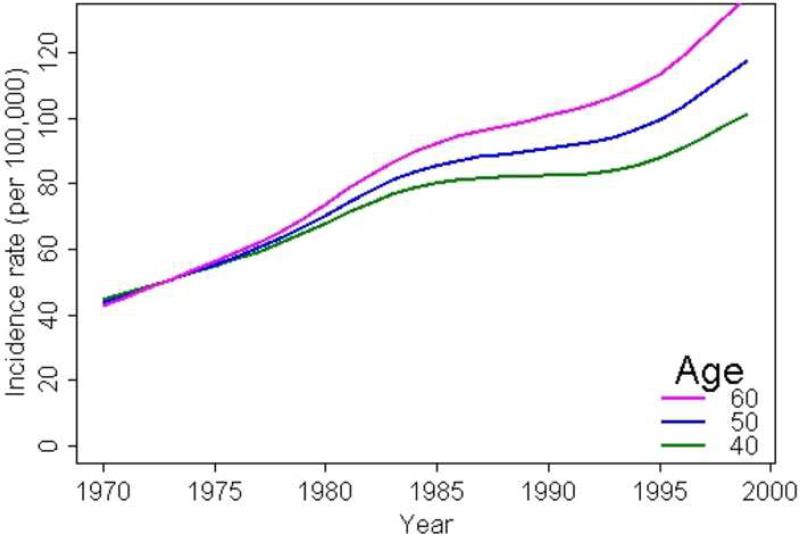
Figure 3.
Trends in Incidence of psoriasis by calendar year for subjects aged 40, 50, and 60 years
RESULTS
We identified a total of 3,105 potential subjects with diagnostic codes consistent with psoriasis between January 1, 1970 and January 1, 2000. After screening the complete medical records from all healthcare providers, 1,633 (52.6%) psoriasis subjects fulfilled criteria for inclusion in the incidence cohort. Of the 1,472 excluded subjects, 64.8% had diagnoses other than psoriasis (e.g., parapsoriasis, pityriasis rosea, psoriasiform dermatitis) and 35.2% were prevalent subjects (i.e. psoriasis prior to study time frame, diagnosed before 18 years of age or moved to Olmsted County within the study time frame with pre-existing psoriasis).
The mean age at diagnosis (± standard deviation) of psoriasis was 43.2 ± 17.0 years and 828 (51.0%) subjects were male (Table 1). Average age at incidence increased across the three decades from 39.8 years in 1970–1979, to 41.9 years in 1980–1989 and 45.5 years in 1990–1999 (p<0.001). The psoriasis diagnosis was confirmed by a dermatologist in 1,305 (80%) subjects, of whom 1,171 received a dermatologist confirmation at incidence (i.e., at initial diagnosis). Percentage of subjects confirmed by a dermatologist did not change substantially over time. The majority of subjects (1,292; 79%) in the incidence cohort had chronic plaque psoriasis, followed by guttate psoriasis in 133 (8%) and sebo-psoriasis in 87 (5.3%) subjects. Percentage of incident psoriasis subjects with sebo-psoriasis increased slightly over time from 2.6% in 1970–1979 to 7.6% in 1990–1999. Psoriasis lesions most commonly involved the scalp (685; 42%), the arms and legs (564; 35%) and elbows and/or knees (564; 35%). Nearly half of the subjects (762, 46.7%) had lesions at more than one anatomic site. Skin biopsy was performed in only 304 (19%) of subjects. Nail involvement at incidence was present in 236 (14%) subjects (Table 1).
Table I.
Characteristics of 1633 incident psoriasis subjects between 1/1/1970 and 1/1/2000 in Olmsted County, Minnesota.
| Time period | ||||
|---|---|---|---|---|
| 1970–1979 | 1980–1989 | 1990–1999 | Total | |
| Total no. of subjects | 311 | 554 | 768 | 1,633 |
| Male | 155 (50%) | 278 (50%) | 395 (51%) | 828 (51%) |
| Female | 156 (50%) | 275 (50%) | 373 (49%) | 805 (49%) |
| Age at incidence, mean (± SD) | 39.8 (± 16.8) | 41.9 (± 17.0) | 45.5 (± 16.9) | 43.2 (± 17.0) |
| Male | 41.3 (± 16.3) | 41.1 (± 15.8) | 45.0 (± 16.7) | 43.0 (± 16.4) |
| Female | 38.3 (± 17.2) | 42.7 (± 18.0) | 46.0 (± 17.2) | 43.4 (± 17.7) |
| Dermatologist confirmation, no.(%) | 234 (75%) | 475 (86%) | 596 (78%) | 1,305 (80%) |
| Skin biopsy, no. (%) | 83 (27%) | 118 (21%) | 103 (13%) | 304 (19%) |
| Psoriasis type, no. (%) | ||||
| Chronic plaque psoriasis | 241 (77%) | 450 (81%) | 601 (78%) | 1292 (79%) |
| Guttate psoriasis | 40 (13%) | 49 (9%) | 44 (6%) | 133 (8%) |
| Sebo-psoriasis | 8 (2.6%) | 21 (3.8%) | 58 (7.6%) | 87 (5.3%) |
| Pustular psoriasis | 14 (4.5%) | 15 (2.7%) | 24 (3.1%) | 53 (3.3%) |
| No documentation | 20 (6.4%) | 31 (5.6%) | 50 (6.5%) | 101 (6.2%) |
| Location of lesions, no. (%) | ||||
| Scalp | 111 (36.0%) | 228 (41.0%) | 346 (45.0%) | 685 (42.0%) |
| Elbows and/or knees | 99 (32.0%) | 201 (36.0%) | 264 (34.0%) | 564 (35.0%) |
| Limbs arms and/or legs | 128 (41.0%) | 190 (34.0%) | 246 (32.0%) | 564 (35.0%) |
| Palms and/or soles | 19 (6.1%) | 401 (7.2%) | 59 (7.7%) | 118 (7.2%) |
| Trunk | 73 (23.0%) | 123 (22.0%) | 122 (16.0%) | 318 (19.0%) |
| Face | 27 (8.9%) | 73 (13.2%) | 76 (9.9%) | 176 (10.8%) |
| Axilla | 1 (0.3%) | 8 (1.4%) | 12 (1.6%) | 21 (1.3%) |
| Inframammary | 3 (1.0%) | 2 (0.4%) | 5 (0.6%) | 10 (0.6%) |
| Intragluteal/perianal | 34 (11.0%) | 62 (11.0%) | 74 (10.0%) | 170 (10.0%) |
| Genital | 22 (7.1%) | 39 (7.0%) | 49 (6.4%) | 110 (6.7%) |
| Groin | 9 (2.9%) | 17 (3.1%) | 24 (3.1%) | 50 (3.1%) |
| Multiple locations involved | 145 (46.6%) | 271 (48.9%) | 345 (45.1%) | 762 (46.7%) |
| Nail involvement | 36 (12.0%) | 97 (18.0%) | 103 (13.0%) | 236 (14.0%) |
Incidence of psoriasis
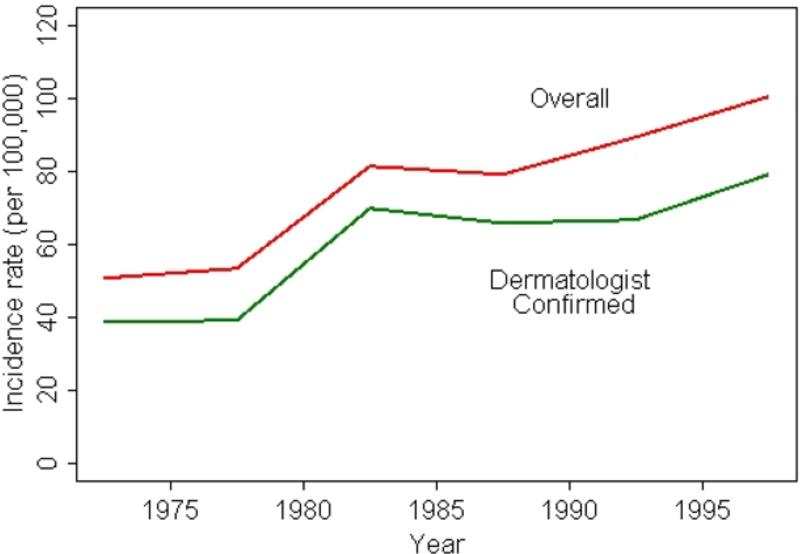
Figure 1 and Table 2 illustrate the overall annual incidence of psoriasis over the 30 years time period between 1970 and 2000. The overall annual age and sex adjusted incidence of psoriasis between 1970 and 2000 was 78.9 (95% CI: 75.0, 82.9) per 100,000. Overall annual incidence gradually increased from 50.8 (95% CI: 41.9, 59.6) per 100,000 in 1970–1974 to 80.9 (95% CI: 70.8, 91.1) per 100,000 in 1980–1984 and 88.7 (95% CI: 79.1, 98.3) per 100,000 in 1990–1994. This increasing trend continued throughout the 1990s reaching 100.5 (95% CI: 90.8, 110.2) per 100,000 in 1995–1999 (p for trend=0.001). When the incidence cohort was restricted to dermatologist confirmed subjects, the overall incidence was 62.3 per 100,000 (95% CI: 58.8, 65.8) and the increasing trend was similarly observed for dermatologist confirmed psoriasis subjects over the study period (Figure 1).
Figure 1.
Trends in incidence of psoriasis between 1970 and 2000
Table II.
Overall annual incidence of psoriasis over time (per 100,000)
| Incidence rate (per 100,000)* and 95% confidence interval | ||
|---|---|---|
| Time period | Dermatology confirmed | Overall |
| 1970–1974 | 38.5 (30.8, 46.1) | 50.8 (41.9, 59.6) |
| 1975–1979 | 38.9 (31.8, 46.1) | 53.2 (44.8, 61.6) |
| 1980–1984 | 69.2 (59.9, 78.5) | 80.9 (70.8, 91.1) |
| 1985–1989 | 65.6 (57.1, 74.2) | 78.9 (69.5, 88.4) |
| 1990–1994 | 66.2 (58.0, 74.4) | 88.7 (79.1, 98.3) |
| 1995–1999 | 79.1 (70.6, 87.7) | 100.5 (90.8, 110.2) |
| Total | 62.3 (58.8, 65.8) | 78.9 (75.0, 82.9) |
Age- and sex-adjusted to the U.S. white 2000 population.
Figure 2 and Table 3 illustrate the age- and sex-specific annual incidence of psoriasis. Overall age-adjusted incidence in males (85.5 per 100,000; 95% CI: 79.5, 91.6) was higher than in females (73.2 per 100,000; 95% CI: 68.0, 78.4; p=0.003). Age- and sex-specific annual incidence in females was highest in the sixth decade of life (90.7 per 100,000) whereas among men, we observed a peak in incidence in the seventh decade of life (115.3 per 100,000).
Table III.
Age- and sex-specific annual incidence of psoriasis (per 100,000)
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Age group | No | Rate | No | Rate | No | Rate |
| 18–29 years | 210 | 79.4 | 234 | 75.6 | 444 | 77.4 |
| 30–39 years | 222 | 93.3 | 169 | 69.2 | 391 | 81.1 |
| 40–49 years | 132 | 73.6 | 128 | 69.0 | 260 | 71.3 |
| 50–59 years | 109 | 85.2 | 121 | 90.7 | 230 | 88.0 |
| 60–69 years | 98 | 115.3 | 76 | 76.2 | 174 | 94.2 |
| 70–79 years | 39 | 77.9 | 55 | 71.2 | 94 | 73.8 |
| ≥80 years | 18 | 80.0 | 22 | 39.8 | 40 | 51.4 |
| Total (95% CI) | 828 | 85.5 (79.5, 91.6)* | 805 | 73.2 (68.0, 78.4)* | 1,633 | 78.9 (75.0, 82.9)† |
Age-adjusted to the U.S. white 2000 population.
Age- and sex-adjusted to the U.S. white 2000 population.
We observed a significant interaction between calendar year and age (p<0.001), indicating that incidence rates increased more rapidly in the older ages. The incidence rates at ages 40, 50 and 60 years are displayed in Figure 3. As illustrated with the slope of the lines, increase in psoriasis incidence was highest in the 60 year olds.
Survival
Survival in the overall psoriasis cohort was similar to that expected in the Minnesota white population (standardized mortality ratio [SMR]: 0.95, 95% CI: 0.84, 1.07, p=0.36) over the entire time period. For the subset of 111 subjects who received phototherapy and/or systemic drug therapy, survival was also similar to the referent group (SMR: 0.73, 95% CI: 0.40, 1.22, p= 0.23).
DISCUSSION
In this population based study, we describe trends in incidence of psoriasis over time and demonstrate, for the first time, that the incidence of psoriasis increased significantly over the last three decades between 1970 and 2000. The reasons for this increase in incidence are unknown, but could include a variety of factors, including a true change in incidence or changes in the diagnosing patterns in this population. Our findings also indicate that the age and sex specific incidence of psoriasis is in general higher in males than in females, except for the sixth decade of life, suggesting the potential role of sex hormones in the etiology of psoriasis. These intriguing findings underscore the importance of time trends and natural history studies in generating important etiological clues. With a prevalence of at least 2%, psoriasis is the most common systemic inflammatory disease and yet, relatively little is known regarding risk factors, disease burden and long term outcomes. Further research is urgently needed to replicate these findings in other populations as well as potential causes of increase in psoriasis incidence.
Although the characteristic lesions help to diagnose psoriasis easily, epidemiologic studies on psoriasis are limited due to a number of reasons. First, there is no universally accepted and validated criteria for the diagnosis and classification of psoriasis4. In addition to clarifying the status of the indeterminate cases, a classification criterion which utilizes such parameters as the type of lesions, age of onset, pattern, and extent of distribution would be very helpful in identifying homogenous subject groups to carry on further investigations2, 10, 11. The second reason is the heterogeneous disease course and severity in individual patients12. Not all patients seek medical care immediately after the onset of symptoms11 and some patients with early remissions or response to topical treatments with OTC drugs may not require medical care at all, leading to underestimation of the true incidence and prevalence of psoriasis. Still, many survey based studies in different populations reported the prevalence of psoriasis, with values ranging between 0.1 and 2.8%3. To date, only three studies, two from Olmsted County and one from the U.K., reported on the incidence of psoriasis.
When compared to the previous incidence reports, our annual estimates for the earlier time periods 1980–1983 (77.4 [95%CI: 66.2, 88.6] per 100,000) and 1982–1991 (85.3 [95%CI: 78.3, 92.3] per 100,000) are slightly higher than the incidence estimates previously reported for the same population by Bell et al5 (60.4 [95% CI: 49.5, 70.3] per 100,000 for 1980–1983) but lower than the estimates of Shbeeb et al6 (107.7 [95% CI: 101.2, 114.2] per 100,000 for 1982–1991). The recent overall incidence estimate from the U.K. of 140 per 100,0007, which is based on psoriasis codes in an electronic database, is approximately 50% above our estimate for the same time period. This difference possibly reflects the different study populations, or more likely, different methods used in case definition and ascertainment. Indeed, we were able to validate psoriasis diagnosis in 68.7% of subjects with ICD code 69613 which was possibly used in the U.K. study. As part of the validation in the U.K. study, the investigators could confirm psoriasis diagnosis in 82% of the 564 (14%) reviewed records, despite the fact that the definition of psoriasis included presence of more than 1 code7.
There are several potential explanations for the increase in incidence of psoriasis over time. First, it is conceivable that psoriasis patients seek medical care for psoriatic lesions not at the time of first eruption but later in the course of the disease, leading to an accumulation of cases later in time and a false increase in incidence over time. If this is the case, the observed incidence in a population with a stable psoriasis incidence should not change over time since a similar proportion of all new patients would be reflected in the pool of cases at a later date. Another possibility is improvements in access to medical care over time, resulting in inclusion of patients with milder disease in incidence estimates. However, this is unlikely in Olmsted County because health care needs of the community have been well met for over a hundred years. The high and consistent proportion (80%) of psoriasis patients whose diagnosis was validated by a dermatologist clearly exemplifies that access to specialty care is not a concern in this setting. Furthermore, previous incidence studies in this community for a variety of diseases, some of which a lot more challenging to diagnose, indicate that access to health care does not influence incidence trends14. Third, increased incidence may be due to increased awareness or recognition of psoriasis over time. For example, promotional bias, described as “newer, better treatments and the promotional awareness campaigns associated with them”, may lead to a false increase in incidence over the recent years12. Although we have no robust evidence whether recognition of psoriasis changed over time, this is unlikely for psoriasis which has characteristic lesions, especially in a setting where Goeckerman therapy was first described back in 1925. Therefore, our findings possibly reflect a true increase in the incidence of psoriasis over time. Unfortunately, the causes for this increase in incidence are currently unknown. In addition to genetic susceptibility, various environmental risk factors, including trauma to the skin (including sunburns)15, infections (streptococcal, viral, HIV)11, 16, obesity17, smoking18, alcohol use19, emotional stress18 and various drugs (lithium salts, antimalarials, interferon, beta blockers)20–22 have been associated with psoriasis. However, it is controversial which of these factors are accountable for disease onset, relapse or both. Intriguingly, our findings indicate that the increase in incidence is mainly in older age groups (Figure 3), suggesting that this trend may result from environmental factors, the frequency of which have also increased during the same time period. Indeed, the prevalence of obesity23, 24 and beta blocker use in patients with myocardial infarction25 and hypertension26 increased significantly during the same period. Similar trends have been observed for both obesity and beta-blocker use in subjects with cardiovascular disease in Olmsted County27, 28. Further research is needed to better understand the potential impact of obesity and beta blocker use on time trends in incidence of psoriasis.
The bimodal pattern of incidence age in psoriasis has been recognized for a long time29, 30. As shown in figure 2, incidence in males displayed a slightly bimodal pattern but this was not significant. In the absence of incidence patterns in children, it is not feasible to investigate the bimodal pattern in this population. Quite unexpectedly, among female subjects, we observed a prominent increase in incidence in the sixth decade of life which corresponds to the postmenopausal period. A similar pattern was also observed in the incidence of psoriasis in the U.K. study7 and of psoriatic arthritis in Olmsted County31. Menopause, a state of estrogen deprivation, has been previously implicated in onset32 and exacerbation of psoriasis33. In a survey conducted among 63 women with psoriasis, half of them reported exacerbation of psoriasis with menopause33. In contrast to menopause, pregnancy is a state of natural immunomodulation associated with alleviation or exacerbations in the course of various inflammatory diseases, including psoriasis34, 35. Although the increase in female incidence in the sixth decade of life is consistent with previous observations regarding disease course during pregnancy and menopause, the potential role of hormonal factors in etiology of psoriasis in men is currently unknown.
Majority of studies to date suggest an increased risk of death in patients with severe psoriasis, defined by a history of hospitalization or use of systemic therapy, including phototherapy36–39. We did not observe an increase in mortality in our overall psoriasis cohort compared to the general population. A supplementary analysis of a random sample of 111 subjects who received phototherapy or systemic therapy did not also detect an increase in mortality. There are at least 3 possible explanations why our findings differ from these earlier observations. Our study population includes all psoriasis subjects, regardless of disease severity and therefore, subjects with severe psoriasis are under-represented. Indeed, in previous studies, excess mortality was limited to patients with severe psoriasis who received systemic therapy, phototherapy or PUVA39. Similar to our study, there was no excess mortality in a large PUVA cohort followed up for ten years40. Second, our cohort is not large enough to detect a small excess in mortality. Indeed, our confidence intervals for SMR among patients who received systemic therapy indicate that, if there is a small increase in risk of death, we cannot exclude a risk less than 22%. Finally, comparison with expected death rates derived from general population statistics may result in underestimation of the true mortality risk41.
Our results should be interpreted in light of some potential limitations. Psoriasis subjects included in this study are limited to clinically recognized patients. Subjects with mild psoriasis may never seek medical care, leading to an underestimation of the true incidence of psoriasis. We assume that most patients will be diagnosed, albeit with a delay, during the 30 year study period. It is also difficult to define an accurate incidence date for psoriasis due to the lead time between onset of lesions and seeking medical care. The lack of a consistent and reliable severity instrument was another limitation that precluded comparison of psoriasis severity over time and effect of severity on mortality. Although we made an attempt to record PASI scores and physician's assessment of severity in the medical records, this information was not consistently documented. Our ongoing data collection includes information on psoriasis treatment and we plan to address the potential role of disease severity in explaining trends in incidence. This incidence study is limited to psoriasis patients aged ≥18 years at incidence. Previous studies suggest that psoriasis lesions appear by 16 years of age in one third of the psoriatic patients42. Therefore, there is a need to investigate incidence trends in the pediatric population. Finally, the population of Olmsted County, Minnesota, is primarily white (90%), limiting the generalizability of our incidence estimates to racially more diverse populations. Nevertheless, strengths of our study include the unique medical records-linkage system provided by the Rochester Epidemiology Project that allows for near complete ascertainment and validation of all clinically-recognized cases of psoriasis in a well-defined population. In addition, this is the first study that examines time trends in incidence of psoriasis over an extended period.
In conclusion, this population based study indicates that the incidence of psoriasis almost doubled between 1970 and 2000. The reasons for this increase in incidence are currently unknown, but could include a variety of factors, including a true change in risk factors and incidence of psoriasis or changes in the diagnosing patterns in this population. Further research is needed to replicate these findings in other populations as well as potential causes of increase in psoriasis incidence.
ACKNOWLEDGEMENTS
We would like to thank Rita Black, Deborah Olson and Diane Carlson for data abstraction, Justin Carlin for database management, David Tines for computer programming and Mitch Baias for record retrieval.
Conflict of interest: Funded by an unrestricted research grant from Amgen Inc.
This study was made possible by the Rochester Epidemiology Project (Grant R01 AG034676 from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005 May 5;352(18):1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004 Jun;3(2):121–128. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- 3.Farber E, Nall L. Epidemiology: Natural History and Genetics. In: Roenigk HH Jr, Maibach H, editors. Psoriasis. Marcel Dekker Inc.; New York: 1998. pp. 107–157. [Google Scholar]
- 4.Rzany B, Naldi L, Schafer T, Stern R, Williams H. The diagnosis of psoriasis: diagnostic criteria. British Journal of Dermatology. 1998;138(5):917. doi: 10.1046/j.1365-2133.1998.02245.x. comment. [DOI] [PubMed] [Google Scholar]
- 5.Bell LM, Sedlack R, Beard CM, Perry HO, Michet CJ, Kurland LT. Incidence of psoriasis in Rochester, Minn, 1980–1983. Archives of Dermatology. 1991;127(8):1184–1187. [PubMed] [Google Scholar]
- 6.Shbeeb M, Sunku J, Hunder G, Gibson L, O'Fallon W, Gabriel S. Incidence of psoriasis and psoriatic arthritis, a population-based study. Arthritis Rheum. 1995 [Google Scholar]
- 7.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007 Dec;143(12):1559–1565. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 8.Melton L. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder D, Offord K. A SAS macro which utilizes local and reference population counts appropriate for incidence, prevalence, and mortality rate calculations in Rochester and Olmsted County, Minnesota. Technical Report Series. Rochester, MN: Section of Medical Research Statistics, Mayo Clinic. 1982 Report No: 20. [Google Scholar]
- 10.Plunkett A, Marks R. A review of the epidemiology of psoriasis vulgaris in the community. Australasian Journal of Dermatology. 1998;39(4):225–232. doi: 10.1111/j.1440-0960.1998.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 11.Naldi L. Psoriasis. Dermatologic Clinics. 1995;13(3):635–647. [PubMed] [Google Scholar]
- 12.Griffiths CE. Psoriasis: future research needs and goals for the twenty-first century. Dermatol Clin. 2004 Oct;22(4):493–499. doi: 10.1016/j.det.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Icen M, Crowson C, McEvoy M, Gabriel S, Maradit-Kremers H. Potential misclassification of psoriasis patients in electronic databases. J Am Acad Dermatol. 2008 doi: 10.1016/j.jaad.2008.08.034. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis & Rheumatism. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 15.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007 Nov–Dec;25(6):535–546. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Mallon E, Young D, Bunce M, et al. HLA-Cw*0602 and HIV-associated psoriasis. Br J Dermatol. 1998 Sep;139(3):527–533. doi: 10.1046/j.1365-2133.1998.02495.x. [DOI] [PubMed] [Google Scholar]
- 17.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses' Health Study II. Arch Intern Med. 2007 Aug 13–27;167(15):1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 18.Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005 Jul;125(1):61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 19.Behnam SM, Behnam SE, Koo JY. Alcohol as a risk factor for plaque-type psoriasis. Cutis. 2005 Sep;76(3):181–185. [PubMed] [Google Scholar]
- 20.Citro V, Fristachi R, Tarantino G. Extensive psoriasis induced by pegylated interferon: a case report. J Med Case Reports. 2007;1:86. doi: 10.1186/1752-1947-1-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel EA, DiCicco LM, Orenberg EK, Fraki JE, Farber EM. Drugs in exacerbation of psoriasis. J Am Acad Dermatol. 1986 Nov;15(5 Pt 1):1007–1022. doi: 10.1016/s0190-9622(86)70265-x. [DOI] [PubMed] [Google Scholar]
- 22.Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Recognition and management. Am J Clin Dermatol. 2000 May–Jun;1(3):159–165. doi: 10.2165/00128071-200001030-00003. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998 Jan;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007 May;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Spencer FA, Lessard D, Yarzebski J, Gore JM, Goldberg RJ. Decade-long changes in the use of combination evidence-based medical therapy at discharge for patients surviving acute myocardial infarction. Am Heart J. 2005 Oct;150(4):838–844. doi: 10.1016/j.ahj.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Tu K, Campbell NR, Chen Z, McAlister FA. Use of beta-blockers for uncomplicated hypertension in the elderly: a cause for concern. J Hum Hypertens. 2007 Apr;21(4):271–275. doi: 10.1038/sj.jhh.1002128. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Jimenez F, Jacobsen SJ, Reeder GS, Weston SA, Meverden RA, Roger VL. Prevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community. Chest. 2004 Apr;125(4):1205–1212. doi: 10.1378/chest.125.4.1205. [DOI] [PubMed] [Google Scholar]
- 28.Perschbacher JM, Reeder GS, Jacobsen SJ. Evidence-based therapies for myocardial infarction: secular trends and determinants of practice in the community. Mayo Clin Proc. 2004 Aug;79(8):983–991. doi: 10.4065/79.8.983. [DOI] [PubMed] [Google Scholar]
- 29.Burch PR, Rowell NR. Psoriasis: aetiological aspects. Acta Derm Venereol. 1965;45(5):366–380. [PubMed] [Google Scholar]
- 30.Henseler T. Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985 Sep;13(3):450–456. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 31.Wilson F, Icen M, Crowson C, McEvoy M, Gabriel S, Maradit Kremers H. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: A population-based study. J Rheumatol. 2008 doi: 10.3899/jrheum.080691. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanbeck G, Inerot A, Martinsson T, Wahlstrom J. A population genetic study of psoriasis. Br J Dermatol. 1994 Jul;131(1):32–39. doi: 10.1111/j.1365-2133.1994.tb08454.x. [DOI] [PubMed] [Google Scholar]
- 33.Mowad CM, Margolis DJ, Halpern AC, Suri B, Synnestvedt M, Guzzo CA. Hormonal influences on women with psoriasis. Cutis. 1998 May;61(5):257–260. [PubMed] [Google Scholar]
- 34.Oumeish OY, Al-Fouzan AW. Miscellaneous diseases affected by pregnancy. Clin Dermatol. 2006 Mar–Apr;24(2):113–117. doi: 10.1016/j.clindermatol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Farber EM, Nall ML. The natural history of psoriasis in 5,600 patients. Dermatologica. 1974;148(1):1–18. doi: 10.1159/000251595. [DOI] [PubMed] [Google Scholar]
- 36.Lindegard B. Mortality and causes of death among psoriatics. Dermatologica. 1989;179(2):91–92. doi: 10.1159/000248321. [DOI] [PubMed] [Google Scholar]
- 37.Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patients with psoriasis. Archives of Dermatology. 1999;135(12):1490–1493. doi: 10.1001/archderm.135.12.1490. see comment. [DOI] [PubMed] [Google Scholar]
- 38.Mallbris L, Akre O, Granath F, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19(3):225–230. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- 39.Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007 Dec;143(12):1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 40.Stern RS, Lange R. Cardiovascular disease, cancer, and cause of death in patients with psoriasis: 10 years prospective experience in a cohort of 1,380 patients. Journal of Investigative Dermatology. 1988;91(3):197–201. doi: 10.1111/1523-1747.ep12464847. [DOI] [PubMed] [Google Scholar]
- 41.Card TR, Solaymani-Dodaran M, Hubbard R, Logan RF, West J. Is an internal comparison better than using national data when estimating mortality in longitudinal studies? J Epidemiol Community Health. 2006 Sep;60(9):819–821. doi: 10.1136/jech.2005.041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000 May–Jun;17(3):174–178. doi: 10.1046/j.1525-1470.2000.01746.x. [DOI] [PubMed] [Google Scholar]