Abstract
Mercuric ions accumulate preferentially in renal tubular epithelial cells and bond with intracellular thiols. Certain metal-complexing agents have been shown to promote extraction of mercuric ions via the multidrug resistance-associated protein 2 (MRP2). Following exposure to a non-toxic dose of inorganic mercury (Hg2+), in the absence of complexing agents, tubular cells are capable of exporting a small fraction of intracellular Hg2+ through one or more undetermined mechanisms. We hypothesize that MRP2 plays a role in this export. To test this hypothesis, Wistar (control) and TR− rats were injected intravenously with a non-nephrotoxic dose of HgCl2 (0.5 μmol/kg) or CH3HgCl (5 mg/kg), containing [203Hg], in the presence or absence of cysteine (Cys; 1.25 μmol/kg or 12.5 mg/kg, respectively). Animals were sacrificed 24 h after exposure to mercury and the content of [203Hg] in blood, kidneys, liver, urine and feces was determined. In addition, uptake of Cys-S-conjugates of Hg2+ and methylmercury (CH3Hg+) was measured in inside-out membrane vesicles prepared from either control Sf9 cells or Sf9 cells transfected with human MRP2. The amount of mercury in the total renal mass and liver was significantly greater in TR− rats than in controls. In contrast, the amount of mercury in urine and feces was significantly lower in TR− rats than in controls. Data from membrane vesicles indicate that Cys-S-conjugates of Hg2+ and CH3Hg+ are transportable substrates of MRP2. Collectively, these data indicate that MRP2 plays a role in the physiological handling and elimination of mercuric ions from the kidney.
Keywords: mercury, multidrug resistance-associated protein, kidney, transport
Introduction
Within biological systems, certain mercuric species have a particularly high predilection to interact with, and be transported into, epithelial cells lining the convoluted and straight segments of renal proximal tubules (Zalups, 2000). Once mercuric ions gain access to the intracellular compartment of these cells, they tend to be retained in the cells due to a complex set of bonding interactions with protein- and non-protein thiols (Zalups, 2000). This is especially true for mercuric ions bonded to protein thiols, which have a much lower probability of being eliminated from the intracellular environment than mercuric ions bonded to non-protein thiols. Despite the intracellular retention of mercuric ions, there appears to be some degree of luminal secretion of certain mercuric species, leading to the urinary excretion of mercuric ions (Zalups and Koropatnick, 2000).
It is important to note that certain small, vicinal, dithiol-containing compounds, such as 2,3-dimercaptopropane-1-sulfonic acid (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA), can effectively extract a large fraction of the cellular burden of mercuric ions from proximal tubular cells (Aposhian, 1983; Zalups et al., 1991; Aposhian et al., 1992; Zalups, 1993b; Aposhian et al., 1995; Zalups et al., 1998). At least some of this extraction appears to involve the multidrug resistance-associated protein (MRP2). MRP2 is a member of the ATP-binding cassette transporter family and is located in the apical membrane of proximal tubular epithelial cells (Schaub et al., 1997). It has been shown to mediate the transport of a broad range of substrates into the lumen of proximal tubules (Deeley et al., 2006; Aremu et al., 2008; Zhou et al., 2008). Findings from a recent set of studies from our laboratory indicate that MRP2 likely plays a significant role in the DMPS- and DMSA-mediated extraction of mercuric ions from proximal tubular cells and contributes to the subsequent urinary excretion of mercury (Hg) (Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009).
Although our previous studies provide important data implicating MRP2 in the proximal tubular accumulation and extraction of mercuric species in the presence of select metal-complexing agents, the role of MRP2 in the normal handling and disposition of mercuric species by proximal tubular cells has not been examined thoroughly. It is interesting to note that the intracellular concentration of glutathione (GSH) in renal tubular cells has been shown to increase following exposure of rats to a nontoxic dose of HgCl2 (Lash and Zalups, 1996). This increase in intracellular GSH may facilitate the formation of GSH-S-conjugates of Hg in the intracellular environment. Previous studies have shown that, in the absence of complexing agents, mercuric ions may be secreted with GSH into the lumen of proximal tubules (Tanaka- Kagawa et al., 1993). Given that MRP2 has been shown to mediate the GSH-dependent export of certain compounds (Deeley et al., 2006), and that MRP2 appears to play a role in the secretion of mercuric ions in the presence of complexing agents (Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009), we propose that this carrier may also play a role in the normal secretion of mercuric ions from proximal tubular cells. Therefore, the purpose of the current study was to assess the ability of MRP2 to mediate the extraction of mercuric ions from renal tubular cells in rats exposed to HgCl2 or CH3HgCl that were not treated with a metal-complexing agent such as DMPS or DMSA. The present study utilized Wistar (control) and TR− (MRP2-deficient) rats and provides data indicating that MRP2 does indeed play a role in the extraction of mercuric ions from kidney, as well as liver, under normal, physiological conditions.
Materials and Methods
Chemicals
Radioactive HgCl2 ([203Hg]) was produced by irradiation of mercuric oxide at the Missouri University Research Reactor facility as described previously (Belanger et al., 2001; Bridges et al., 2004). Briefly, three milligrams of mercuric oxide were sealed in quartz tubing with an acetylene torch. The encapsulated mercury was irradiated by neutron activation for 4 weeks at the Missouri University Research Reactor (MURR) facility, following which the quartz tube was crushed and rinsed with four 50-μL washes of 1 N HCl. All rinses were collected in a single polypropylene vial. The radioactivity of the solution was determined by using a PerkinElmer Wallac Wizard 3 automatic gamma counter (Gaithersburg, MD). The specific activities of the [203Hg] ranged from 6-12 mCi/mg.
Radioactive methylmercury (CH3[203Hg]) was synthesized from [203Hg] using a previously published protocol adapted from the method of Rouleau and Block (Rouleau and Block, 1997; Zalups and Bridges, 2009). Briefly, two mCi of [203Hg] were diluted in de-ionized water, to which 2 M acetate buffer and 2 mL of 1.55 mM methylcobalamin (Sigma, St. Louis, MO) were added. Methylcobalamin is used as a source of methyl groups for generating CH3Hg+. The aforementioned solution was incubated for 24 h at 25°C in a fume hood, after which 30% potassium chloride in 4% hydrochloric acid was added to the mixture. CH3[203Hg] was extracted with five washes of dichloromethane (Sigma), which was evaporated by bubbling nitrogen gas through the solution. The pure CH3[203Hg] was collected and stored at -20°C. The purity of the extracted CH3[203Hg] has been confirmed previously by thin layer chromatography (Rouleau and Block, 1997).
Animals
Male transport deficient (TR−) rats and normal Wistar (control) rats weighing 200-225 g were purchased from Harlan Laboratories (Indianapolis, IN). TR− rats are characterized by the absence of MRP2 function (Paulusma et al., 1996; Maher et al., 2005). These rats have been used in recent years to study the hepatic and renal secretion of various MRP2 substrates (de Vries et al., 1989; Masereeuw et al., 2003; Smeets et al., 2004). In addition, our laboratory and others have used these animals to study the disposition of mercuric ions (Aremu et al., 2008; Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009). There were no significant differences between body weights of the animals used for these studies. All animals were provided a commercial laboratory diet (Tekland 6% rat diet, Harlan Laboratories) and water ad libitum throughout all aspects of experimentation. Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Design
Four separate experiments were carried out. Each experiment utilized four control and four TR− rats. For the first experiment, rats were injected intravenously (i.v.) with HgCl2 (0.5 μmol/kg in 2 mL/kg normal saline). For the second experiment, rats were injected i.v. with 0.5 μmol/kg HgCl2 in the presence of 1.25 μmol/kg cysteine (Cys; in 2 mL/kg saline). The third experiment involved i.v. injection of rats with CH3HgCl (5 μmol/kg in 2 mL/kg normal saline). Similarly, rats in the fourth experiment were injected i.v. with 5 mg/kg CH3HgCl in the presence of 12.5 mg/kg cysteine (in 2 mL/kg saline). Each rat also received approximately 1 μCi of [203Hg] or CH3[203Hg] as a means to trace the disposition of mercuric ions.
It has been shown that mercuric ions form linear II coordinate covalent complexes with thiol-containing molecules (Fuhr and Rabenstein, 1973). Based on this finding, we assume that HgCl2 and Cys, mixed in a 1:2.5 ratio, forms the linear conjugate, Cys-S-Hg-S-Cys. Similarly, a mixture of CH3HgCl and Cys (1:1.25 ratio) forms the linear conjugate, Cys-S-CH3Hg. For simplicity, co-exposure of rats to HgCl2 and Cys or CH3HgCl and Cys, will hereafter be referred to exposure to Cys-S-Hg-S-Cys or Cys-S-CH3Hg, respectively.
Intravenous Injections
Control and TR− rats were injected i.v. with a non-nephrotoxic dose of HgCl2 or CH3HgCl as described previously (Zalups, 1993b; Bridges et al., 2008a; Bridges et al., 2008b). Briefly, each animal was anesthetized and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. HgCl2 or CH3HgCl (with or without Cys) was administered into the vein and then the wound was closed using two 9-mm stainless steel wound clips. After injection, each animal was placed in individual plastic metabolic cages, in which water and food were provided ad libitum.
Collection of Tissues, Organs, Urine and Feces
Twenty-four h after the initial injection of HgCl2 or CH3HgCl, rats were anesthetized with an i.p. injection of ketamine/xylazine (70/15 mg/kg in 2 mL/kg saline). Two 1-mL samples of blood were obtained from the inferior vena cava. One of the samples was placed in a polystyrene tube for determination of total content of Hg, while the other sample was placed in a Microtainer tube (Becton Dickenson and Co., Franklin Lakes, NJ), which was centrifuged at 21,000 × g for 90 seconds to separate the cellular and plasma fractions. Each fraction was placed in a separate tube for estimation of Hg content. Total blood volume was estimated to be 6% of body weight.
Subsequently, the right and left kidneys were removed from each animal, weighed, and cut in half along a transverse plain. A 3-mm transverse slice of the left kidney was utilized for dissection of cortex, outer stripe of outer medulla, inner stripe of outer medulla and inner medulla. Each zone of the kidney was weighed and placed in a polystyrene tube for estimation of Hg content. In addition, the liver was excised carefully, weighed, and a 1-g section was removed for determination of Hg content.
Urine and feces were collected throughout the duration of the experiment. Urine from each animal was mixed and a 1-mL sample was weighed and placed in a polystyrene tube for estimation of Hg content. All of the feces excreted by each animal were counted to determine accurately the total fecal excretion of Hg. The amount of Hg in each sample was determined by counting in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Boston, MA).
Membrane Vesicle Transport Assays
Membrane vesicle transport assays were performed using a rapid filtration method as described previously (Chancy et al., 2000; Bridges et al., 2008b). Control and MRP2-expressing, inside-out membrane vesicles were purchased from Xenotech (Lenexa, KS), a distributor for Solvo Biotechnology (Budapest, Hungary). Control vesicles were prepared from normal Sf9 cells while MRP2 vesicles were prepared from Sf9 cells transfected stably with human MRP2. Prior to use, vesicles were centrifuged at 100,000 × g for 40 min at 4°C to remove the storage buffer. Vesicles were resuspended in ice-cold incubation buffer (250 mM sucrose, 10 mM Tris/HCl, 10 mM MgCl2, pH 7.4) by passing the suspension through a 27-gauge needle 25 times. Mercuric conjugates were formed by mixing 5 μM HgCl2 containing [203Hg] or CH3HgCl containing CH3[203Hg] with 12.5 μM Cys in incubation buffer supplemented with 10 mM creatine phosphate and 100 μg/ml creatine phosphokinase in the presence or absence of 5 mM ATP. [3H]-Leukotriene C4 (LTC4), a known substrate for MRP2 (Zhou et al., 2008), was purchased from PerkinElmer. At the time of the experiment, membrane vesicles and mercuric conjugates were warmed to 37°C. Transport was initiated by the addition of 40 μL of membrane vesicle solution (20 μg protein) to 160 μL of radiolabeled substrate. Transport was allowed to proceed for a selected period of time, after which, vesicles were collected on pre-wet Tuffryn filter discs (pore size, 0.4 μm; Pall, Ann Arbor, MI). Filters were washed with two changes (8 mL each) of ice-cold incubation buffer containing 1 mM DMPS. Filters from experiments utilizing mercury were placed in scintillation vials and the radioactivity present was determined by counting in a Wallac gamma counter. Filters from experiments utilizing LTC4 were placed in scintillation vials containing 1% SDS in 0.1 N NaOH and allowed to incubate for a minimum of 10 minutes before addition of scintillation liquid. Content of LTC4 was estimated by liquid scintillation spectroscopy and standard isotopic computational methods.
Data Analyses
Data for each experiment were analyzed first with the Kolmogorov-Smirnov test for normality and then with Levene's test for homogeneity of variances. Data were then analyzed using a two-tailed student's t-test or a two-way analysis of variance (ANOVA) to assess differences among the means. When statistically significant F-values were obtained with ANOVA, the data were analyzed using Tukey's post hoc multiple comparison test. A p-value of < 0.05 was considered statistically significant.
Results
Burden of Hg in the Total Renal Mass
In control rats, the renal burden of Hg 24 h after exposure to HgCl2 was approximately 50% of the administered dose (Figure 1A). In TR− rats exposed to HgCl2, the renal burden of Hg was 60% of the administered dose, and was significantly different than that of corresponding control (Wistar) rats. When rats were exposed to Cys-S-Hg-S-Cys, the renal burden of Hg in both, control and TR− rats was significantly greater than that of corresponding rats treated with HgCl2 (Figure 1A).
Figure 1.
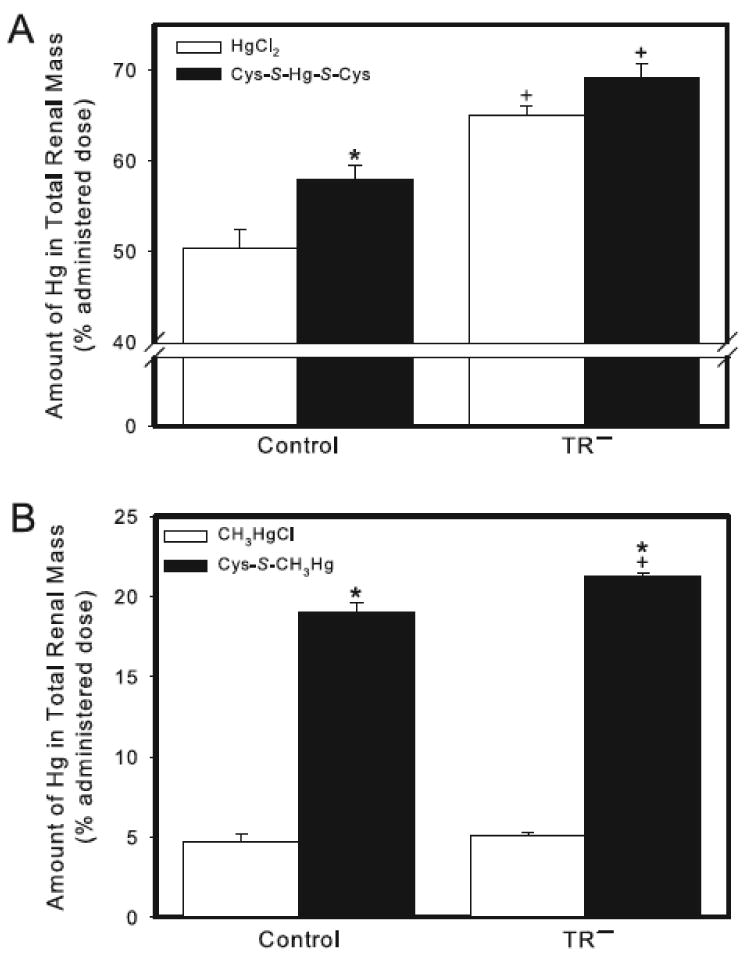
Content of Hg in the total renal mass of control and TR− rats. (A) Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). (B) Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Rats were sacrificed 24 h following injection with Hg and kidneys were harvested for estimation of Hg content. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2 or CH3HgCl. + Significantly different (p < 0.05) from the corresponding mean for control rats.
Following administration of CH3HgCl, the renal burden of Hg in both strains of rats was approximately 5% of the administered dose (Figure 1B). No significant differences were detected between control and TR− rats exposed to CH3HgCl. The renal burden of Hg in control and TR− rats exposed to Cys-S-CH3Hg was four-fold greater than that of rats exposed to CH3HgCl alone. The renal burden of Hg was slightly, yet significantly, greater in TR− rats exposed to Cys-S-CH3Hg than that in the corresponding group of control rats (Figure 1B).
Concentration of Hg in Renal Zones
The majority of renal Hg was localized in the cortex and outer stripe of the outer medulla (Figures 2 and 3). Amounts of Hg detected in the inner stripe of the outer medulla and the inner medulla represented less than 1% of the administered dose (data not shown).
Figure 2.
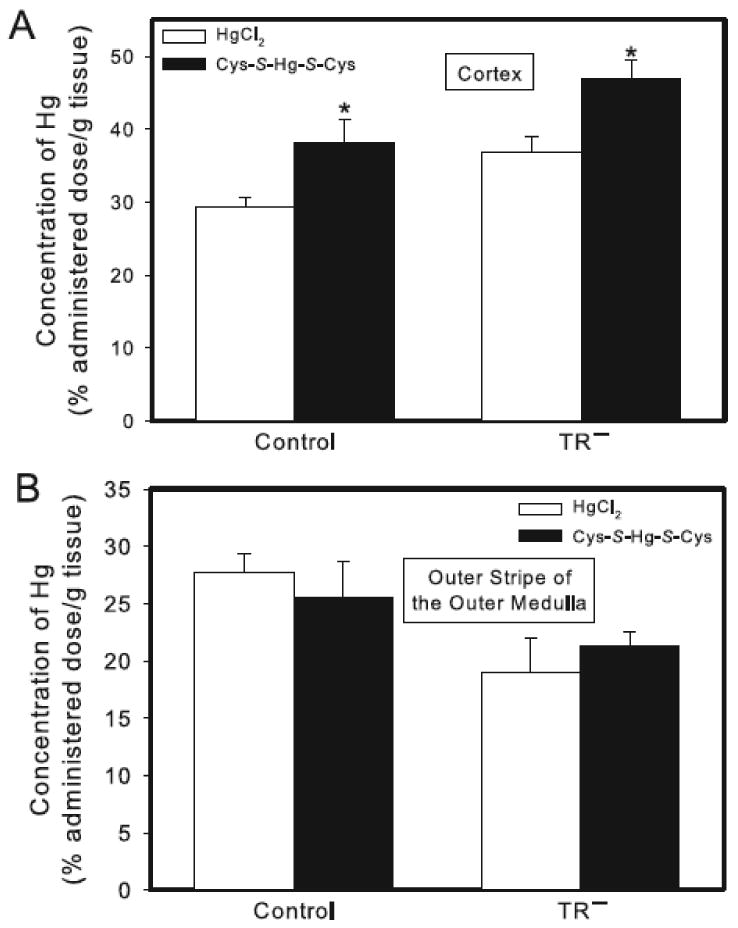
Concentration of Hg in the renal cortex and outer stripe of the outer medulla of control and TR− rats. Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). Panel A shows the cortical concentration of Hg. Panel B shows the concentration of Hg in the outer stripe of the outer medulla. Rats were sacrificed 24 h following injection with Hg and kidneys were harvested for estimation of Hg content. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2.
Figure 3.
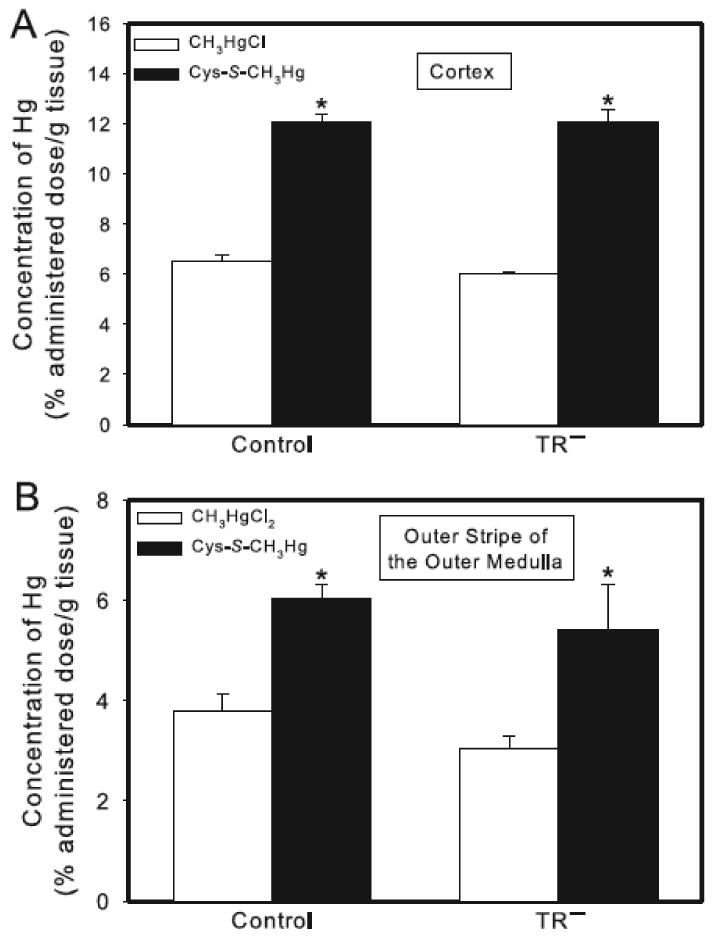
Concentration of Hg in the renal cortex and outer stripe of the outer medulla of control and TR− rats. Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Panel A shows the cortical concentration of Hg. Panel B shows the concentration of Hg in the outer stripe of the outer medulla. Rats were sacrificed 24 h following injection with Hg and kidneys were harvested for estimation of Hg content. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to CH3HgCl.
In the cortex, the concentration of Hg tended to be greater in TR− rats exposed to HgCl2 or Cys-S-Hg-S-Cys than in corresponding groups of control rats (although differences were not significant statistically) (Figure 2A). Moreover, exposure of rats to Cys-S-Hg-S-Cys resulted in a significant increase in the cortical concentration of Hg in both strains of rats (Figure 2A).
The concentration of Hg in the outer stripe of the outer medulla was slightly greater (albeit not significantly) in control rats exposed to HgCl2 or Cys-S-Hg-S-Cys than that in the corresponding groups of TR− rats (Figure 2B). The concentration of Hg in the outer stripe of the outer medulla of control or TR− rats (Figure 2B) following administration of Cys-S-Hg-S-Cys was not significantly different from that of the corresponding group of rats exposed to HgCl2.
When rats were exposed to CH3HgCl, the cortical concentration of Hg was similar in control and TR− rats (Figure 3A). Following exposure of rats to Cys-S-CH3Hg, the cortical concentration of Hg was significantly greater in both, control and TR− rats than in corresponding rats exposed to CH3HgCl. There was no significant difference in the cortical concentration of Hg between control and TR− rats exposed to Cys-S-CH3Hg (Figure 3A).
Similarly, the concentration of Hg in the outer stripe of the outer medulla of control rats exposed to CH3HgCl or Cys-S-CH3Hg was not significantly different from that of corresponding TR− rats (Figure 3B). The concentration of Hg in the outer stripe of the outer medulla following administration of Cys-S-CH3Hg was significantly greater than that following administration of CH3HgCl in both strains of rats. No significant differences were detected between corresponding groups of control and TR− rats.
Amount of Hg in Liver and Blood
The hepatic burden of Hg 24 h after exposure to HgCl2 was approximately 3.5% of the administered dose in controls and 6.5% of the dose in TR− rats (Figure 4A). When control rats were exposed to Cys-S-Hg-S-Cys, the hepatic burden of Hg increased significantly to nearly 5% of the administered dose. When TR− rats were exposed to Cys-S-Hg-S-Cys, the hepatic burden of Hg was not significantly different from that of TR− rats exposed to HgCl2, but was significantly greater than that of control rats exposed to either HgCl2 or Cys-S-Hg-S-Cys (Figure 4A).
Figure 4.
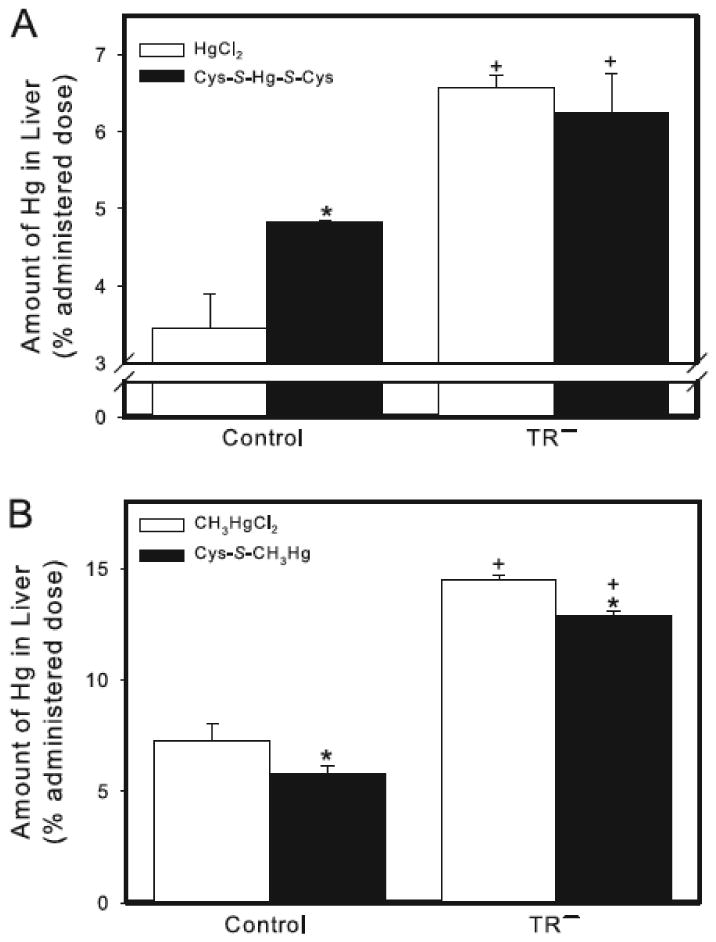
Content of Hg in the liver of control and TR− rats. (A) Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). (B) Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Rats were sacrificed 24 h following injection with Hg and livers were harvested for estimation of Hg content. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2 or CH3HgCl. + Significantly different (p < 0.05) from the corresponding mean for control rats.
Following exposure of control rats to CH3HgCl, the hepatic burden of Hg was approximately 7% (Figure 4B). This burden was two-fold greater in corresponding TR− rats (about 14% of the dose), which was significantly different from that in control animals. In control and TR− rats exposed to Cys-S-CH3Hg, the hepatic burden of Hg was significantly greater than that in corresponding rats exposed to CH3HgCl alone (Figure 4B).
In blood, the amount of Hg following exposure to HgCl2 was significantly greater in TR− rats than in corresponding controls (Figure 5A). When rats were exposed to Cys-S-Hg-S-Cys, the hematological burden of Hg was slightly, but not significantly, greater in TR− rats than in the corresponding group of controls (Figure 5A). In TR− rats exposed to Cys-S-Hg-S-Cys, the amount of Hg in blood was significantly lower than that of TR− rats exposed to HgCl2 only (Figure 5A). In each group of rats, the burden of Hg was distributed evenly between plasma and cellular components (data not shown).
Figure 5.
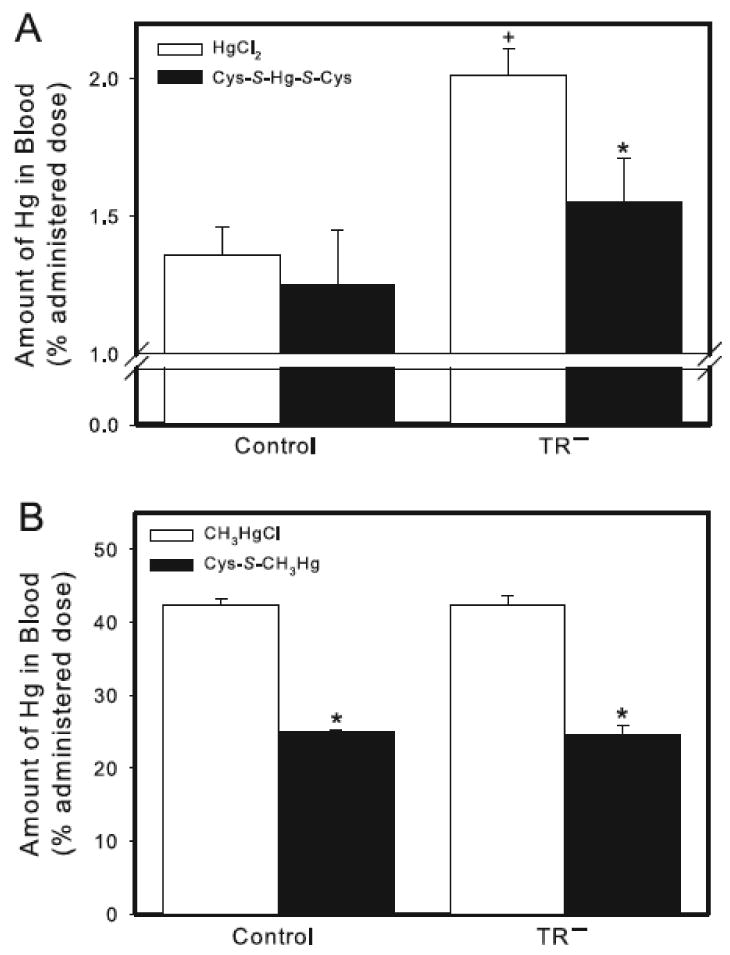
Amount of Hg in blood of control and TR− rats. (A) Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). (B) Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Rats were sacrificed 24 h following injection with Hg and blood was removed for estimation of Hg content. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2 or CH3HgCl. + Significantly different (p < 0.05) from the corresponding mean for control rats.
Exposure of control and TR− rats to Cys-S-CH3Hg resulted in a hematological burden of Hg that was significantly lower than that of corresponding groups of rats exposed to CH3HgCl alone (Figure 5B). The form of Hg to which rats were exposed did not significantly alter the content of Hg in blood between corresponding groups of control and TR− rats. In both, control and TR− rats, approximately 99% of the hematological burden of Hg was associated with cellular components of blood, with the remaining 1% present in plasma (data not shown).
Amount of Hg in Urine and Feces
When control rats were exposed to HgCl2, approximately 4% of the administered dose was detected in urine in 24 h (Figure 6A). In contrast, only about 1.5% of the administered dose of HgCl2 was excreted in urine of TR− rats. The content of Hg excreted by control rats exposed to HgCl2 was not significantly different from that of control rats exposed to Cys-S-Hg-S-Cys (Figure 6A). Similarly, urinary excretion of Hg of TR− rats exposed to HgCl2 was not significantly different from that of TR− rats exposed to Cys-S-Hg-S-Cys.
Figure 6.
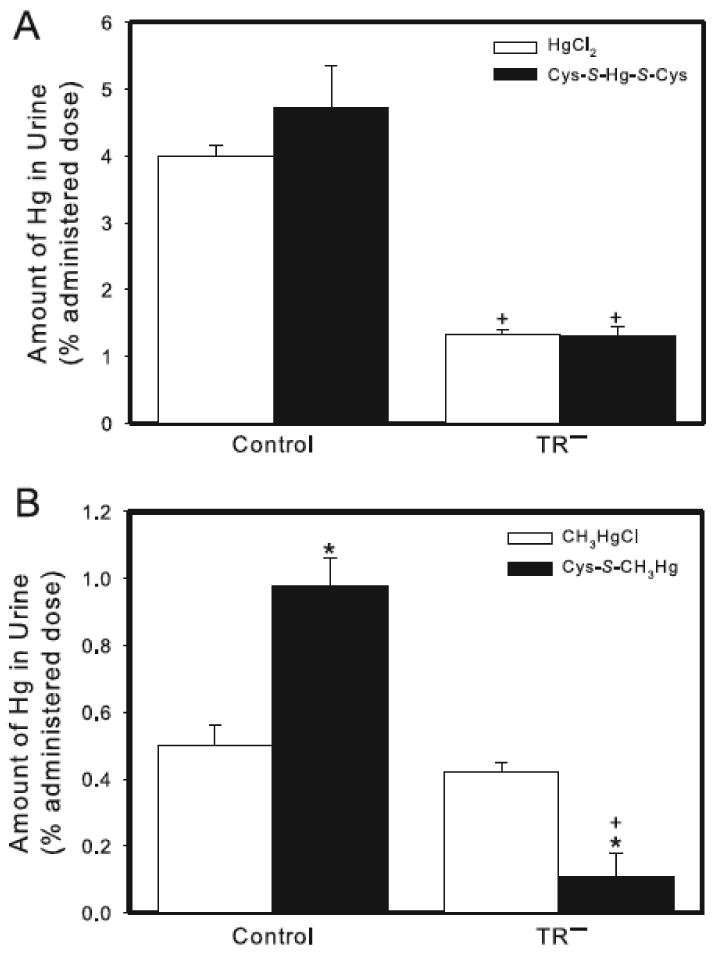
Content of Hg in urine of control and TR− rats. (A) Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). (B) Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Rats were sacrificed 24 h following injection with Hg. Data represent the total amount of Hg excreted in urine throughout the 24-h experiment. Data represent mean ± SE of four rats. * Significantly different (< 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2 or CH3HgCl. + Significantly different (p < 0.05) from the corresponding mean for control rats.
Twenty-four h after control rats were exposed to CH3HgCl, the urinary excretion of Hg was approximately 0.5% of the administered dose (Figure 6B). This excretion was not significantly different from that of corresponding TR− rats. When control rats were exposed to Cys-S-CH3Hg, the urinary excretion of Hg was twofold greater than that of control rats exposed to CH3HgCl alone. In contrast, TR− rats exposed to Cys-S-CH3Hg excreted less Hg in the urine than TR− rats exposed to CH3HgCl (Figure 6B).
The fecal excretion of Hg after 24 h in control rats exposed to HgCl2 accounted for approximately 3% of the administered dose (Figure 7A). This excretion was significantly greater than that of the corresponding group of TR− rats, in which fecal excretion of Hg was approximately 1.5% of the administered dose. Following exposure of control rats to Cys-S-Hg-S-Cys, the fecal excretion of Hg was significantly greater than that of corresponding rats exposed to HgCl2 alone. This excretion was also greater than that of TR− rats exposed to Cys-S-Hg-S-Cys. In contrast, the fecal excretion of Hg in TR− rats following exposure to Cys-S-Hg-S-Cys was not significantly different from that of TR− rats exposed to HgCl2 (Figure 7A).
Figure 7.
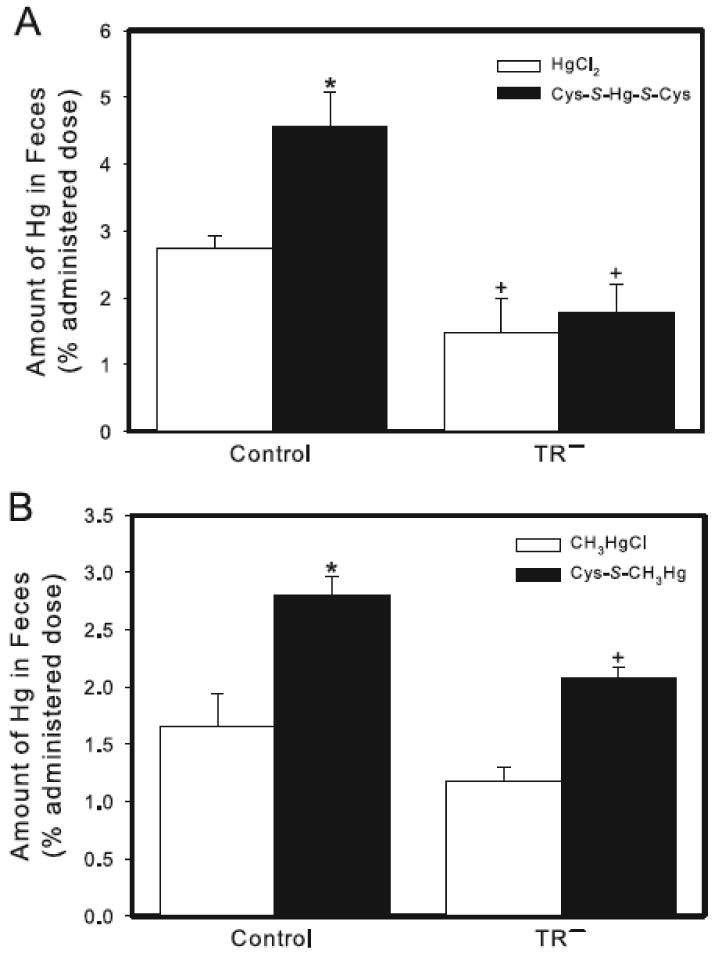
Content of Hg in feces of control and TR− rats. (A) Rats were injected (i.v.) with either HgCl2 (0.5 μmol/kg) or Cys-S-Hg-S-Cys (0.5 μmol/kg HgCl2 + 1.25 μmol/kg Cys). (B) Rats were injected (i.v.) with either CH3HgCl (5 μmol/kg) or Cys-S-CH3Hg (5 μmol/kg CH3HgCl + 12.5 μmol/kg Cys). Rats were sacrificed 24 h following injection with Hg. Data represent the total amount of Hg excreted in feces throughout the 24-h experiment. Data represent mean ± SE of four rats. * Significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to HgCl2 or CH3HgCl. + Significantly different (p < 0.05) from the corresponding mean for control rats.
When rats were exposed to CH3HgCl, the fecal excretion of Hg in TR− rats was slightly, albeit not significantly, lower than that of corresponding control rats (Figure 7B). Fecal excretion of Hg in control rats increased significantly following administration of Cys-S-CH3Hg. This excretion was significantly greater than that of corresponding TR− rats (Figure 7B).
Uptake of LTC4 and Hg by MRP2-expressing Membrane Vesicles
Uptake of [3H]-LTC4 was measured in inside-out vesicles prepared from either control Sf9 cells (control vesicles) or Sf9 cells transfected stably with human MRP2 (MRP2 vesicles) (Figure 8). The uptake of LTC4 was significantly greater in MRP2 vesicles than in control vesicles.
Figure 8.
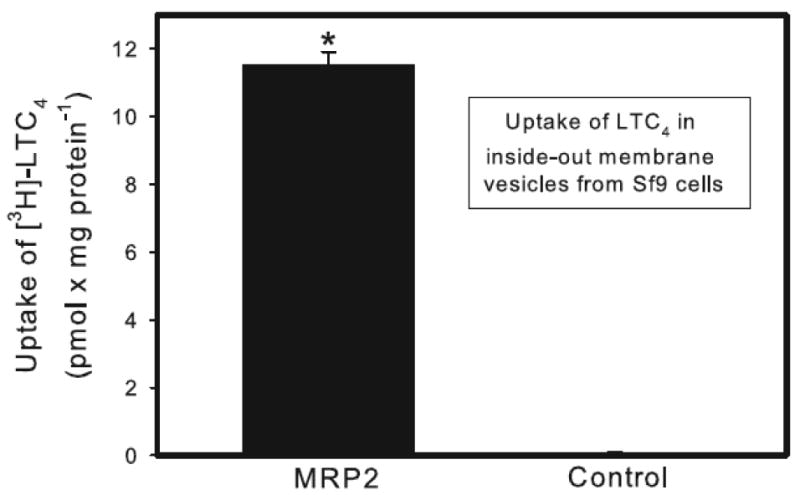
Uptake of leukotriene C4 (LTC4) into inside-out membrane vesicles from control and MRP2-expressing Sf9 cells. Control and MRP2-containing vesicles were exposed to 50 nM LTC4 at 37°C for 2.5 min. Data represent two experiments performed in triplicate. * Significantly different (p < 0.05) from the corresponding mean for control vesicles.
Uptake of Cys-S-Hg-S-Cys (Figure 9A) and Cys-S-CH3Hg (Figure 9B) was measured in control vesicles and MRP2 vesicles. Significant differences in the uptake of Cys-S-Hg-S-Cys between control and MRP2 vesicles were observed only after measuring transport for 5 minutes. The uptake of Cys-S-Hg-S-Cys was significantly greater in MRP2 vesicles than in control vesicles. Similarly, the uptake of Cys-S-CH3Hg was significantly greater in MRP2 vesicles than in control vesicles. Significant differences in the uptake of Cys-S-CH3Hg between control and MRP2-containing membrane vesicles were detected when samples were incubated for 2.5 and 5 minutes.
Figure 9.
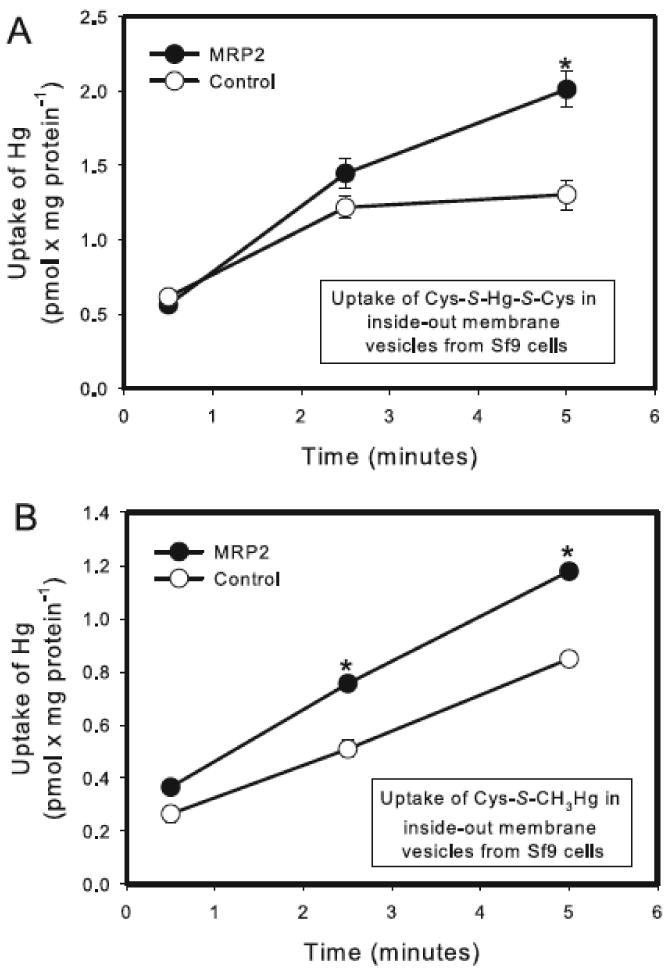
Uptake of Hg into inside-out membrane vesicles from control and MRP2-expressing Sf9 cells. Control and MRP2-containing vesicles were exposed to 5 μM Cys-S-Hg-S-Cys (A) or Cys-S-CH3Hg (B) at 37°C for times specified above. Data represent two experiments performed in triplicate. * Significantly different (p < 0.05) from the corresponding mean for control vesicles.
Discussion
Following exposure to Hg-containing compounds, mercuric ions accumulate readily in target tissues and organs, particularly the kidneys. Previous dispositional studies have shown that the kidney is the primary site of Hg accumulation following exposure to inorganic forms of Hg (Zalups, 2000). Indeed, in as little as one hour, 50% of an administered dose of inorganic mercury is present in the kidney (Zalups, 1993a). Within the kidney, mercuric ions accumulate preferentially in proximal tubular epithelial cells (Zalups, 2000). Mercuric species appear to gain access to the intracellular compartments of these cells via specific mechanisms on the luminal and basolateral plasma membranes. At the luminal membrane, the sodium-independent amino acid transporter, system b0,+, has been shown to transport at least a fraction of the Cys-S-Hg-S-Cys present within the tubular lumen into cells (Bridges et al., 2004). At the basolateral membrane, Cys-S-Hg-S-Hcy present in blood appears to gain access to proximal tubular cells via the organic anion transporter (OAT1) and/or OAT3 (Aslamkhan et al., 2003; Zalups et al., 2004).
The primary site where the toxic effects of organic forms of mercury, specifically methylmercury (CH3Hg+), are manifested is the brain. However, the kidney is one of the major sites for accumulation of CH3Hg+. Like Cys-S-Hg-S-Cys, Cys-S-conjugates of CH3Hg+ (Cys-S-CH3Hg) have been shown to be taken up into proximal tubular cells by OAT1 in the basolateral membrane (Zalups and Ahmad, 2005). On the luminal plasma membrane, the sodium-dependent amino acid transporter, system B0,+, appears to play a role in the proximal tubular uptake of Cys-S-CH3Hg (Bridges and Zalups, 2006). It should be noted that, within biological systems, methylmercuric ions may be oxidized to inorganic species of Hg (Gage, 1964; Norseth and Clarkson, 1970b; Norseth and Clarkson, 1970a; Omata et al., 1980).
Regardless of the form of Hg to which one is exposed, once mercuric ions enter the intracellular compartment of proximal tubular cells, they form strong bonds with protein and nonprotein thiols. This bonding significantly decreases the cellular elimination of mercuric ions. Numerous studies have shown that metal-complexing agents such as DMPS and DMSA can effectively extract mercuric ions from within proximal tubular cells (Aposhian, 1983; Aposhian et al., 1992; Zalups, 1993b; Aposhian et al., 1995; Bridges et al., 2008a; Bridges et al., 2008b). These compounds are water-soluble and are used throughout the world as antidotes for mercury poisoning. Until recently, the cellular mechanisms by which DMPS and DMSA extracted mercuric ions were unknown. Our recent studies in control and TR− rats indicate that MRP2 is a significant component in at least one of the cellular pathways utilized by DMPS and DMSA to extract mercuric ions from proximal tubular cells (Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009).
Despite our recent findings implicating MRP2 in the DMPS- and DMSA-mediated extraction of Hg, little is known about the renal handling and elimination of mercuric ions under normal, homeostatic conditions. A thorough understanding of the way in which Hg is handled under these conditions is important since many humans are exposed, either occupationally or environmentally, to low levels of mercury on a daily basis. The exposure received by these individuals rarely leads to clinical intervention and chelation therapy. Rather, mercuric ions accumulating in tissues and organs are eliminated slowly over time via mechanisms that are unclear currently. Since MRP2 plays a role in the DMPS- and DMSA-mediated elimination of mercuric ions, we hypothesized that it may also participate in the elimination of mercuric ions in the absence of these compounds. Indeed, our current data indicate that MRP2 is involved in the normal handling and elimination of mercuric ions. In addition, we show that the disposition of mercuric ions is different depending on whether rats were exposed to HgCl2, Cys-S-Hg-S-Cys, CH3HgCl or Cys-S-CH3Hg. These data are novel in that they were obtained from experiments that examined the handling and disposition of mercuric ions in the absence of metal-complexing agents.
Following exposure of rats to HgCl2, Cys-S-Hg-S-Cys, and Cys-S-CH3Hg, more Hg was detected in kidneys of TR− rats than in corresponding control rats. This finding is likely due to the absence of MRP2 activity in the proximal tubular epithelial cells of TR− rats. We proposed previously that MRP2 mediates the transport of mercuric ions from the intracellular compartment of proximal tubular cells into the tubular fluid for elimination in the urine (Bridges et al., 2008b; Zalups and Bridges, 2009). When this carrier is absent or non-functional, as is the case in TR− rats, mercuric ions that would normally be exported via MRP2 are retained within tubular epithelial cells. Considering this, the present data indicate that MRP2 plays a role in the normal handling and export of mercuric ions by renal tubules.
When rats were exposed to Hg as Cys-S-Hg-S-Cys or Cys-S-CH3Hg, we found that the renal burden of Hg was greater than that of corresponding rats exposed to HgCl2 or CH3HgCl. Similarly, previous studies from our laboratory using normal Sprague-Dawley rats indicate that co-administration of HgCl2 with Cys enhances the renal accumulation of Hg in as little as one hour (Zalups and Barfuss, 1995b; Zalups and Barfuss, 1995a; Zalups and Barfuss, 1996). More recently, we have used Wistar (control) and TR− rats to assess the disposition of Hg 48 h following injection of HgCl2 or Cys-S-Hg-S-Cys (Bridges et al., 2008a). Exposure of rats to Cys-S-Hg-S-Cys resulted in a renal burden of Hg that was greater than that observed when rats were exposed to HgCl2 alone. It was not surprising to find that the renal burden of Hg was slightly lower 48h after exposure to HgCl2 or Cys-S-Hg-S-Cys than it was 24 h after exposure to these compounds. The increase in renal burden of Hg following exposure to Cys-S-Hg-S-Cys may be related directly to the increased availability of transportable forms of Hg (i.e., Cys-S-Hg-S-Cys and Cys-S-CH3Hg).
Within the kidney, the majority of mercuric ions were detected in the cortex and outer stripe of the outer medulla. This finding was expected considering that the proximal tubule, which spans these two renal zones, is the primary site of accumulation of mercuric ions (Zalups, 2000). In addition, the proximal tubule is also the location of MRP2 (Schaub et al., 1997). Given this localization, it was not surprising to find that the cortical concentration of Hg, following exposure to HgCl2 or Cys-S-Hg-S-Cys, was greater in TR− rats than in control rats. As mentioned previously, this difference is likely due to the lack of MRP2 activity in TR− rats. Interestingly, when rats were exposed to CH3HgCl or Cys-S-CH3Hg, the concentration of Hg in the cortex and outer stripe of the outer medulla of control rats was not different from that of TR− rats. It is unclear why this pattern of disposition was observed, especially considering that significant differences in Hg disposition were observed at the level of the total renal mass between control and TR− rats.
Urinary excretion of Hg was related inversely to the renal burden of Hg. The content of Hg in urine of control rats was greater than that of corresponding TR− rats. This pattern was similar in rats sacrificed 48 h after exposure to mercuric compounds (Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009). In the absence of MRP2 activity, one would expect to find an increase in the accumulation of mercuric ions in renal tissue and a corresponding decrease in urinary elimination. Based on the current findings, we suggest that MRP2 plays a role in the proximal tubular elimination of Hg. It should be noted that the form of Hg (with or without Cys) to which TR− rats were exposed did not alter the content of Hg in the urine of these rats. Although exposure to a readily transportable form of Hg (i.e., Cys-S-Hg-S-Cys) enhanced renal accumulation of this metal, the urinary elimination of mercuric ions was not increased. This finding suggests that intracellular aspects of the excretory pathway for mercuric ions may significantly affect the ability of cells to export mercuric species. Our data in membrane vesicles from Sf9 cells suggest that Cys-S-Hg-S-Cys is capable of being exported from proximal tubular cells via MRP2; therefore, we suggest that interactions of mercuric ions with intracellular components may reduce the ability of proximal tubular cells to export mercuric species into the tubular lumen for elimination in the urine.
Based on the current data, methylmercury and inorganic mercury appear to be handled differently by the kidney. One major difference is in the renal accumulation of mercuric ions. When rats are exposed to HgCl2 or Cys-S-Hg-S-Cys, the renal burden of Hg is approximately 50 and 70% of the dose, respectively, while the renal burden of Hg in rats exposed to CH3HgCl or Cys-S-CH3Hg is only about 5 and 20% of the administered dose, respectively. In addition, the pattern of urinary excretion of Hg was different between rats exposed to HgCl2 or Cys-S-Hg-S-Cys and those exposed to CH3HgCl or Cys-S-CH3Hg. In control rats exposed to Cys-S-CH3Hg, the amount of Hg in urine was significantly greater than that of rats exposed to CH3HgCl. This finding may be due simply to the increased burden of Hg in renal tubules. In addition, the chemical properties of methylmercury may facilitate its transport across plasma membranes and subsequent elimination in urine. Significant differences were not observed in the urinary elimination of mercuric ions between control and TR− rats exposed to CH3HgCl, but the total renal burden of Hg was not different between these two groups of rats. One possible explanation is that a large fraction of the mercuric ions excreted by control and TR− rats exposed to CH3HgCl are derived from the glomerular filtrate rather than from within proximal tubular cells and that this difference masks differences in the proximal tubular elimination of mercuric ions between control and TR− rats. This idea is supported by the fact that over 40% of the administered dose of CH3HgCl is present in blood (compared with 1-2% of an HgCl2 dose). In TR− rats exposed to Cys-S-CH3Hg, the urinary elimination of mercuric ions was lower than that of rats exposed to CH3HgCl. This lower elimination may be due to the observed retention of mercuric ions within renal tissue of TR− rats.
In liver, MRP2 is localized in the canalicular membrane and participates in the hepatobiliary export of numerous substances (Zhou et al., 2008). It has also been suggested that MRP2 participates in the export of mercuric ions from hepatocytes following exposure to HgCl2 (Sugawara et al., 1998). Therefore, it was not surprising to find that the hepatic burden of Hg was significantly greater in all groups of TR− rats than that of the corresponding group of control rats. These data correspond well with the observed fecal elimination of Hg. The amount of Hg eliminated in the feces was greater in both groups of control rats than in corresponding groups of TR− rats. These findings suggest that mercuric ions are being sequestered within hepatocytes of TR− rats because of the inability of these cells to export mercuric conjugates into bile via MRP2. It is important to note that some fecal elimination of Hg was observed. This excretion may be due to intestinal secretion of mercuric ions (Zalups, 1998) and/or export from hepatocytes via one or more mechanisms other than MRP2. It should be noted that the hepatic burden of Hg was significantly greater in control rats exposed to Cys-S-Hg-S-Cys than in corresponding rats exposed to HgCl2. As discussed previously, this finding is likely related to the availability of a transportable form of Hg. The fecal elimination of control rats exposed to Cys-S-Hg-S-Cys was slightly greater than that of control rats exposed to CH3HgCl and is likely a direct result of the increased hepatic accumulation of mercuric ions in following exposure to Cys-S-Hg-S-Cys.
The disposition of mercuric ions in blood was dependent upon the form of mercury to which rats were exposed. When control and TR− rats were exposed to Cys-S-CH3Hg, the hematological burden of Hg was half that of corresponding rats exposed to CH3HgCl. Of the dose of mercury in blood, 99% was associated with cellular components. It is possible that following exposure to CH3HgCl, a large fraction of CH3Hg+ is taken up into erythrocytes and is subsequently sequestered there. Consequently, fewer mercuric ions are available for transport into cells and therefore, absorption into target cells (e.g. renal and hepatic) is reduced. When mercury is administered as Cys-S-CH3Hg, it is possible that the bonding of Cys with CH3Hg+ inhibits the movement of mercuric ions into erythrocytes long enough for Cys-S-CH3Hg to be taken up by target organs. This phenomenon would explain why the burden of Hg in blood is twofold greater in rats exposed to CH3HgCl than corresponding rats exposed to Cys-S-CH3Hg. A similar pattern of mercury disposition was observed in rats exposed to Cys-S-Hg-S-Cys and HgCl2. The hematological burden of mercury was greater in rats exposed to HgCl2 than in corresponding rats exposed to Cys-S-Hg-S-Cys. This difference may be due, in part, to sequestration of a fraction of mercuric ions in erythrocytes and/or to the presence of a transportable species of mercury in circulating blood. Interestingly, the mercuric ions detected in blood following exposure to Cys-S-Hg-S-Cys or HgCl2 were distributed evenly between plasma and cellular components. This distribution was different than that of Cys-S-CH3Hg and CH3Hg+, and is likely due to differences in the way in which Hg2+ and CH3Hg+ are handled by cells.
The interpretation of our current data is limited somewhat due to the single time point utilized in this study. Nonetheless, these data appear to support a role for MRP2 in the physiological handling (i.e., in the absence of complexing agents) of mercuric ions following exposure to HgCl2, Cys-S-Hg-S-Cys, CH3HgCl, or Cys-S-CH3Hg. In addition, the present study shows that co-administration of Hg with Cys enhanced accumulation of mercuric ions in both, control and TR− rats. Regardless of the form of Hg to which rats are exposed, MRP2 appears to be involved in the renal export of mercuric ions. Collectively, our data indicate that there is movement and elimination of mercuric ions under normal, physiological conditions and that MRP2 is probably involved in this elimination pathway.
Acknowledgments
This research was supported by awards from the National Institutes of Health/National Institute of Environmental Health Sciences to R.K.Z. (ES015157, ES05980, and ES11288) and C.C.B. (ES015511).
Abbreviations
- MRP2
multidrug resistance-associated protein 2
- HgCl2
mercuric chloride
- Cys-S-Hg-S-Cys
Cysteine-S-conjugate of HgCl2
- CH3HgCl
methylmercuric chloride
- Cys-S-CH3Hg
Cysteine-S-conjugate of CH3HgCl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aposhian HV. DMSA and DMPS--water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, Dart RC, Aposhian MM. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995;97:23–38. doi: 10.1016/0300-483x(95)02965-b. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Rivera M, Bruce DC, Dart RC, Hurlbut KM, Levine DJ, Zheng W, Fernando Q, Carter D, et al. Human studies with the chelating agents, DMPS and DMSA. J Toxicol Clin Toxicol. 1992;30:505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116:26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol. 2003;63:590–596. doi: 10.1124/mol.63.3.590. [DOI] [PubMed] [Google Scholar]
- Belanger M, Westin A, Barfuss DW. Some health physics aspects of working with 203Hg in university research. Health Phys. 2001;80:S28–30. [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, Zalups RK. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol. 2004;15:663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA- mediated elimination of mercury in TR(-) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008a;105:211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008b;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. System B0,+ and the transport of thiol-s-conjugates of methylmercury. J Pharmacol Exp Ther. 2006;319:948–956. doi: 10.1124/jpet.106.109371. [DOI] [PubMed] [Google Scholar]
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- de Vries MH, Redegeld FA, Koster AS, Noordhoek J, de Haan JG, Oude Elferink RP, Jansen PL. Hepatic, intestinal and renal transport of 1-naphthol-beta- D-glucuronide in mutant rats with hereditary-conjugated hyperbilirubinemia. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:588–592. doi: 10.1007/BF00260615. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Fuhr BJ, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc. 1973;95:6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- Gage JC. Distribution and Excretion of Methyl and Phenyl Mercury Salts. Br J Ind Med. 1964;21:197–202. doi: 10.1136/oem.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Zalups RK. Alterations in renal cellular glutathione metabolism after in vivo administration of a subtoxic dose of mercuric chloride. J Biochem Toxicol. 1996;11:1–9. doi: 10.1002/(SICI)1522-7146(1996)11:1<1::AID-JBT1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- Masereeuw R, Notenboom S, Smeets PH, Wouterse AC, Russel FG. Impaired renal secretion of substrates for the multidrug resistance protein 2 in mutant transport-deficient (TR-) rats. J Am Soc Nephrol. 2003;14:2741–2749. doi: 10.1097/01.asn.0000094083.82845.fa. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem Pharmacol. 1970a;19:2775–2783. doi: 10.1016/0006-2952(70)90104-8. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health. 1970b;21:717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- Omata S, Sato M, Sakimura K, Sugano H. Time-dependent accumulation of inorganic mercury in subcellular fractions of kidney, liver, and brain of rats exposed to methylmercury. Arch Toxicol. 1980;44:231–241. doi: 10.1007/BF00278031. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- Rouleau C, Block M. Fast and high yield synthesisi of radioactive CH3203Hg(II) Appl Organomet Chem. 1997;11:751–753. [Google Scholar]
- Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- Smeets PH, van Aubel RA, Wouterse AC, van den Heuvel JJ, Russel FG. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol. 2004;15:2828–2835. doi: 10.1097/01.ASN.0000143473.64430.AC. [DOI] [PubMed] [Google Scholar]
- Sugawara N, Lai YR, Sugaware C, Arizono K. Decreased hepatobiliary secretion of inorganic mercury, its deposition and toxicity in the Eisai hyperbilirubinemic rat with no hepatic canalicular organic anion transporter. Toxicology. 1998;126:23–31. doi: 10.1016/s0300-483x(97)00170-4. [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T, Naganuma A, Imura N. Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther. 1993;264:776–782. [PubMed] [Google Scholar]
- Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology. 1993a;79:215–228. doi: 10.1016/0300-483x(93)90213-c. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3- dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Ther. 1993b;267:791–800. [PubMed] [Google Scholar]
- Zalups RK. Intestinal handling of mercury in the rat: implications of intestinal secretion of inorganic mercury following biliary ligation or cannulation. J Toxicol Environ Health A. 1998;53:615–636. doi: 10.1080/009841098159079. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int. 2005;68:1684–1699. doi: 10.1111/j.1523-1755.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Aslamkhan AG, Ahmad S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int. 2004;66:251–261. doi: 10.1111/j.1523-1755.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Accumulation and handling of inorganic mercury in the kidney after coadministration with glutathione. J Toxicol Environ Health. 1995a;44:385–399. doi: 10.1080/15287399509531968. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Renal disposition of mercury in rats after intravenous injection of inorganic mercury and cysteine. J Toxicol Environ Health. 1995b;44:401–413. doi: 10.1080/15287399509531969. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Nephrotoxicity of inorganic mercury co-administrated with L-cysteine. Toxicology. 1996;109:15–29. doi: 10.1016/0300-483x(95)03297-s. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235:10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Gelein RM, Cernichiari E. DMPS as a rescue agent for the nephropathy induced by mercuric chloride. J Pharmacol Exp Ther. 1991;256:1–10. [PubMed] [Google Scholar]
- Zalups RK, Koropatnick J. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther. 2000;295:74–82. [PubMed] [Google Scholar]
- Zalups RK, Parks LD, Cannon VT, Barfuss DW. Mechanisms of action of 2,3-dimercaptopropane-1-sulfonate and the transport, disposition, and toxicity of inorganic mercury in isolated perfused segments of rabbit proximal tubules. Mol Pharmacol. 1998;54:353–363. doi: 10.1124/mol.54.2.353. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]


