Abstract
By using stage-specific monoclonal antibodies, an in vitro model has been developed to analyze the kinetics of expression of stage-specific antigens during the conversion process between tachyzoites and bradyzoites of Toxoplasma gondii. Following infection of murine macrophages with bradyzoites, the expression of bradyzoite-specific antigens declined, whereas the expression of tachyzoite-specific antigens increased during the first 72 h postinfection. Conversely, in gamma interferon-treated murine macrophages infected with tachyzoites, the inhibitory effect of gamma interferon on replication of parasites was accompanied by the induction of bradyzoite-specific antigens.
Full text
PDF
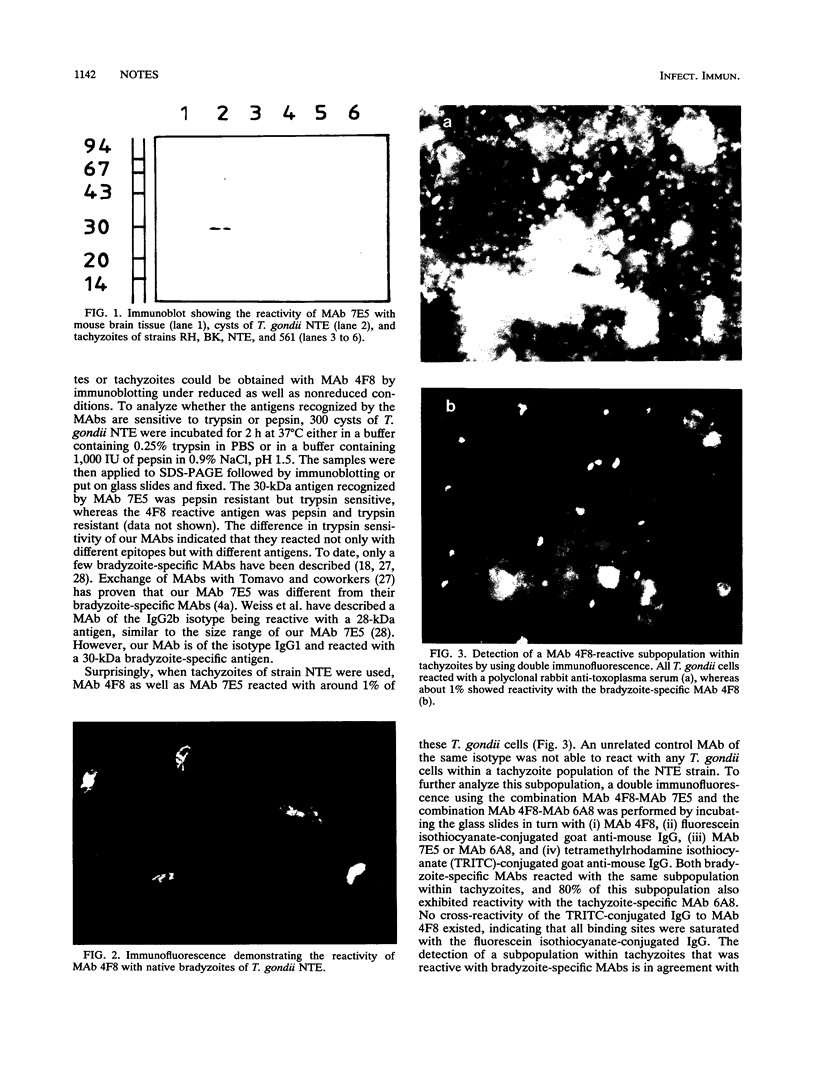

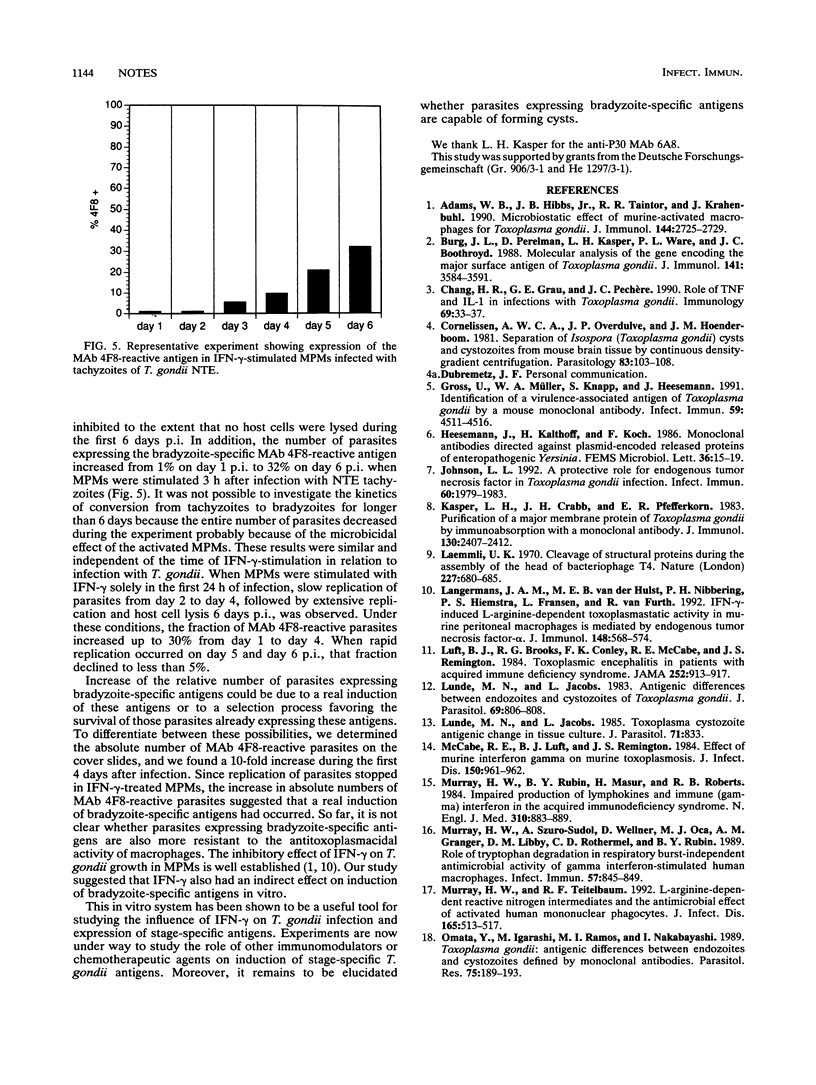

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Burg J. L., Perelman D., Kasper L. H., Ware P. L., Boothroyd J. C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988 Nov 15;141(10):3584–3591. [PubMed] [Google Scholar]
- Chang H. R., Grau G. E., Pechère J. C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990 Jan;69(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- Cornelissen A. W., Overdulve J. P., Hoenderboom J. M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981 Aug;83(Pt 1):103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- Gross U., Müller W. A., Knapp S., Heesemann J. Identification of a virulence-associated antigen of Toxoplasma gondii by use of a mouse monoclonal antibody. Infect Immun. 1991 Dec;59(12):4511–4516. doi: 10.1128/iai.59.12.4511-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992 May;60(5):1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L. H., Crabb J. H., Pfefferkorn E. R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983 May;130(5):2407–2412. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Lunde M. N., Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol. 1983 Oct;69(5):806–808. [PubMed] [Google Scholar]
- Lunde M. N., Jacobs L. Toxoplasma cystozoite antigenic change in tissue culture. J Parasitol. 1985 Dec;71(6):833–833. [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Szuro-Sudol A., Wellner D., Oca M. J., Granger A. M., Libby D. M., Rothermel C. D., Rubin B. Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989 Mar;57(3):845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Omata Y., Igarashi M., Ramos M. I., Nakabayashi T. Toxoplasma gondii: antigenic differences between endozoites and cystozoites defined by monoclonal antibodies. Parasitol Res. 1989;75(3):189–193. doi: 10.1007/BF00931274. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Eckel M., Rebhun S. Interferon-gamma suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986 Sep;20(3):215–224. doi: 10.1016/0166-6851(86)90101-5. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Subauste C. S., Remington J. S. Role of gamma interferon in Toxoplasma gondii infection. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):58–67. doi: 10.1007/BF01964408. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]
- Tomavo S., Fortier B., Soete M., Ansel C., Camus D., Dubremetz J. F. Characterization of bradyzoite-specific antigens of Toxoplasma gondii. Infect Immun. 1991 Oct;59(10):3750–3753. doi: 10.1128/iai.59.10.3750-3753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., LaPlace D., Tanowitz H. B., Wittner M. Identification of Toxoplasma gondii bradyzoite-specific monoclonal antibodies. J Infect Dis. 1992 Jul;166(1):213–215. doi: 10.1093/infdis/166.1.213. [DOI] [PubMed] [Google Scholar]
- Wong B., Gold J. W., Brown A. E., Lange M., Fried R., Grieco M., Mildvan D., Giron J., Tapper M. L., Lerner C. W. Central-nervous-system toxoplasmosis in homosexual men and parenteral drug abusers. Ann Intern Med. 1984 Jan;100(1):36–42. doi: 10.7326/0003-4819-100-1-36. [DOI] [PubMed] [Google Scholar]
- Woodman J. P., Dimier I. H., Bout D. T. Human endothelial cells are activated by IFN-gamma to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991 Sep 15;147(6):2019–2023. [PubMed] [Google Scholar]





