Abstract
A 73-year-old woman was referred to the eye clinic in February 2005 with reduced vision in both eyes. On examination, her visual acuity was 20/40, N6 right eye and 20/64, N6 left eye. Bilateral unusual ‘vitelliform-like’ lesions at the macula (accumulation of yellow material in the subretinal space), which demonstrated blocked fluorescence on fluorescein angiography and a lack of increased autofluorescence signal on fundus autofluorescence imaging, were detected. The patient was followed-up until April 2007 when retinal haemorrhages were detected and blood work-up was undertaken; during this follow-up period the material present at the macula progressively disappeared. As a result of the blood work-up, the diagnosis of multiple myeloma was established; the macular lesions were thought to relate to the latter disease and represent subretinal deposition of immunoglobulin.
Background
This case is important as it illustrates a possible way in which patients with multiple myeloma may present to clinicians. Ophthalmic manifestations may be the first sign of multiple myeloma; thus, their recognition is important in order to establish an early diagnosis and treatment. This is the first case presented (there are no others in the literature) of macular lesions likely related to multiple myeloma with detailed evaluation, including fundus examination, fluorescein angiography, fundus autofluorescence and optical coherence tomography imaging, and long-term follow-up, and provides insight on the possible pathogenesis of the eye disease in this systemic condition.
Case presentation
A 73-year-old woman was referred to the Ophthalmology Department, Grampian University Hospitals-NHS Trust, by her general practitioner in February 2005 with slowly progressive bilateral visual loss. Her past ocular and family history was unremarkable. Her past medical history included high blood pressure. Her visual acuity was measured at 20/40, N6 right eye and 20/64, N6 left eye. Fundus examination disclosed bilateral oval-round, yellow-orange macular lesions with minimal subretinal fluid overlying them (figure 1A).
Figure 1.
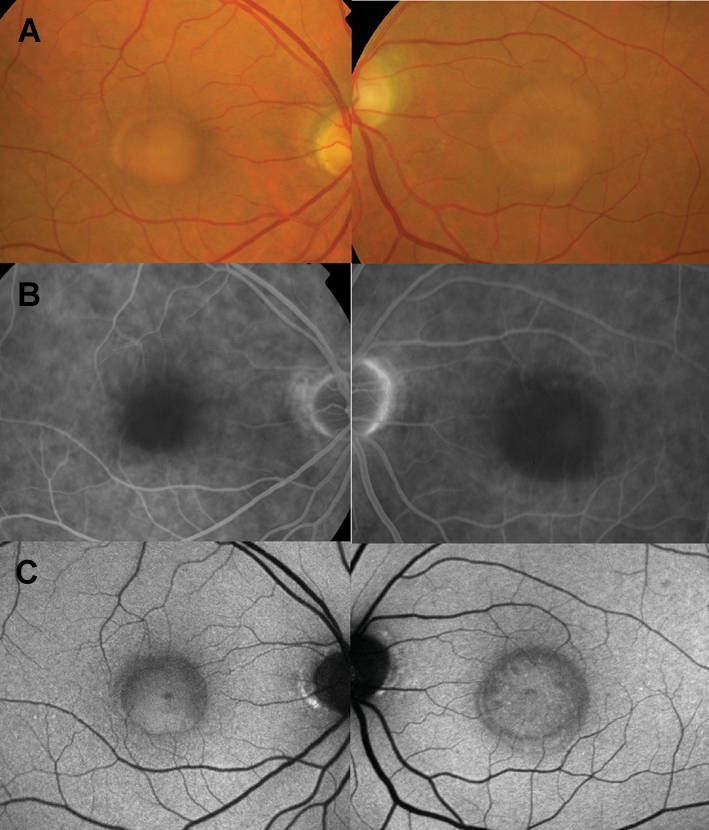
Findings at presentation (February 2005). (A) Colour fundus photographs showing bilateral yellow-orange lesions in the centre of the macula. (B) Fluorescein angiography showed blocked fluorescence at the site of the ‘pseudovitelliform’ macular lesions. (C) Fundus autofluorescence did not demonstrate increased autofluorescence signal.
Investigations
The macular lesions blocked fluorescence on fluorescein angiography (figure 1B) and did not demonstrate an increased autofluorescence signal on autofluorescence imaging (figure 1C). Electrophysiology, including pattern and full-field electroretinogram and electro-oculogram, was normal.
Differential diagnosis
Yellow-orange lesions at the macula similar to those described here can be observed in Best disease, adult-vitelliform foveo-macular dystrophy and basal laminar drusen. Unlike in the current case, in Best disease and adult vitelliform foveomacular dystrophy, fundus autofluorescence would disclose a marked increased autofluorescence signal when the yellow material is present; this increased autofluorescence signal progressively turns into reduced autofluorescence signal as the material disappears over time.1 2 Furthermore, the majority of patients with Best disease would have a reduced electro-oculogram. Patients with basal laminar drusen may develop ‘pseudovitelliform’ lesions similar to those described herein, which also show, when fully developed, no or mild increased autofluorescence signal. However, unlike the current case, fluorescein angiography would demonstrate in most cases a myriad of hyperfluorescent drusen as well as some staining (even if mild) in late stages of the angiogram of the subretinal material forming the pseudovitelliform lesion. Conditions that cause a neurosensory retinal detachment of the macula, including exudative age-related macular degeneration, central serous retinopathy, infectious, inflammatory or infiltrative choroidopathies, or malignant hypertension, could also simulate clinically the lesions presented here. In contrast to the current case, all previously mentioned diseases would be associated with angiographic leakage.
Treatment
No treatment was given initially until the diagnosis was established (see below).
Outcome and follow-up
In July 2006 it was noted that the yellow-orange material started to break up in both eyes and then, progressively, disappeared; in October 2006 it was nearly undetectable (figure 2A). At this stage, visual acuity was 20/126, N10 right eye (OD) and 20/126, N12 left eye (OS). Concurrently, as the material disappeared clinically, multiple small foci of very highly increased autofluorescence appeared (figure 2B).
Figure 2.
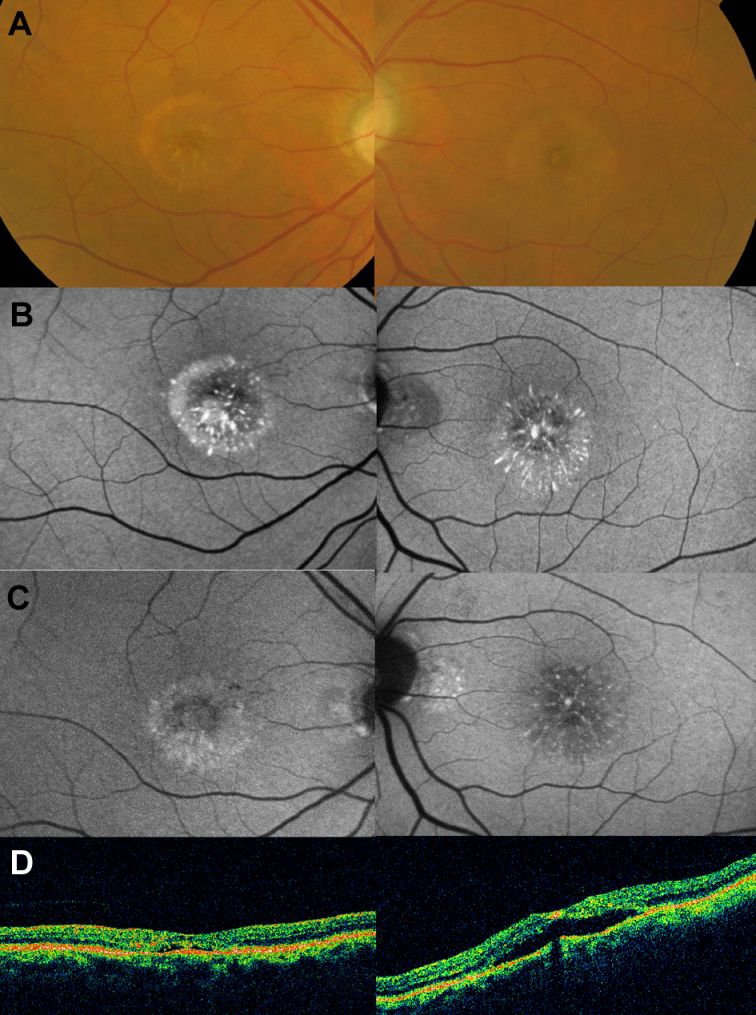
Fundus appearance and autofluorescence findings 18 months following the initial visit (October 2006, see text). (A) Colour fundus photographs show disappearance of most of the subretinal material. (B) Autofluorescence imaging demonstrates small foci of very highly increased signal at the site of the macular lesions. In April 2008, a mildly increased autofluorescence signal was evident in the right eye at the macula surrounding a central area of mildly reduced atrial fibrillation; optical coherence tomography demonstrated a lack of foveal depression and a minimal amount of subretinal fluid (C). In the left eye, areas of reduced autofluorescence signal and few remaining foci of increased autofluorescence were seen; optical coherence tomography demonstrated a neurosensory retinal detachment (NSRD; D).
In April 2007, only mild retinal pigment epithelial (RPE) changes were seen clinically at the macula but few retinal haemorrhages were present and, thus, blood work-up was arranged. On serum protein electrophoresis, a paraprotein band, typed as immunoglobulin G (IgG) and measuring 12 g/l, was detected. Serum IgG level was 21 g/l (normal range 6–16). Bone marrow biopsy disclosed 25% plasma cells with aggregates of small atypical plasma cells. At this stage, the diagnosis of stage II (South West Oncology Group staging) multiple myeloma was made. Treatment with melphalan and thalidomide was given. This treatment did not appear to modify the further progression of the macular lesions.
In April 2008, fundus examination remained unchanged. A mildly increased autofluorescence signal was evident in the right eye (figure 2C) and optical coherence tomography demonstrated a lack of foveal depression and minimal amount of subretinal fluid (figure 2D). In the left eye, areas of reduced autofluorescence signal and few remaining foci of increased AF were seen; optical coherence tomography demonstrated a neurosensory retinal detachment at the site previously occupied by the pseudovitelliform lesions (figure 2C,D).
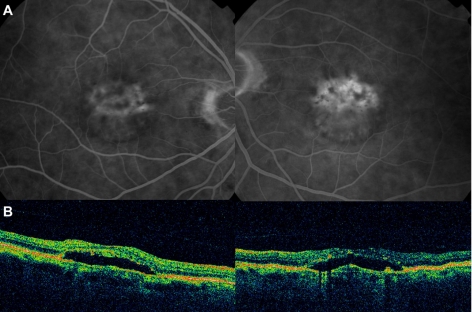
Little change was noted in the fundus appearance until May 2009 when bilateral neurosensory retinal detachments were noted on fundus examination and optical coherence tomography (figure 3A,B). At this stage, hyperfluorescence was observed on fluorescein angiography bilaterally (figure 3A). Treatment with oral acetazolamide (250 mg twice a day) was given in an attempt to reduce/resolve the subretinal fluid; however, no response at this stage of the disease was observed. The systemic status of the patient had remained unchanged with no progression of the multiple myeloma detected.
Figure 3.
Fluorescein angiography and optical coherence tomography findings on May 2009. (A) Leakage was observed at the macula on fluorescein angiography and (B) optical coherence tomography disclosed a neurosensory retinal detachment in both eyes.
Discussion
Although rare, the occurrence of what has been described as bilateral macular detachments in patients with benign monoclonal or polyclonal gammopathy, Waldenstrom macroglobulinemia and multiple myeloma has been reported. In two previously published cases, macular detachments were present before the diagnosis of multiple myeloma was established;3 4 in all other cases, they were found in patients known to have this malignancy.5 6 Characteristically, no hyperfluorescence was observed on fluorescein angiography.
Herein, yellow-orange vitelliform-like bilateral macular lesions, which blocked fluorescence on fluorescein angiography and did not demonstrate an increased autofluorescence signal, were observed at presentation in a patient who was later diagnosed of having multiple myeloma. Most likely, the macular lesions occurred as a result of the systemic disorder; however, the possibility for them being just a coincidental finding cannot be ruled out. Unlike the case reported by Ho et al,6 the patient presented here had only mildly increased IgG levels.
The lack of increased autofluorescence signal at the macular lesions at presentation indicates the absence of lipofuscin or other autofluorescent fluorophores on them. As deposition of Ig in the subretinal space has been demonstrated in a case of Waldenstrom's macroglobulinemia,7 it could be then hypothesised that the macular lesions observed in our patient represented also accumulation of Ig subretinally (submacularly). As the lesions disappeared clinically, small foci of increased AF were seen in a pattern resembling that recently described in an experimental animal model and shown to derive from lipofuscin-loaded subretinal microglia.8 It could be hypothesised that microglial cells could migrate subretinally to help RPE cells to clear Ig and damaged/descamated photoreceptors and outer segments deposited in the subretinal space. Following microglial ingestion, these materials could have given rise to lipofuscin or other fluorophores. As the disease progressed, damage to RPE cells may have ensued with a subsequent alteration in their function maintaining the outer retinal barrier and with the subsequent accumulation of fluid subretinally manifested as the neurosensory retinal detachments observed in late stages of the disease.
Learning points
-
▶
Eye symptoms and signs may be the earliest indication of multiple myeloma.
-
▶
Patients with multiple myeloma may present to clinicians with retinal changes in the form of ‘pseudovitelliform’ lesions as the initial manifestation of the disease and prior to systemic involvement being recognised.
-
▶
Multiple myeloma should be included in the differential diagnosis of pseudovitelliform lesions.
-
▶
If systemic treatment to the multiple myeloma is not initiated early (such as it occurred in the case presented here), progression of the retinal disease occurs and damage to pigment epithelium and likely photoreceptor cells occurs with subsequent visual loss.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Preising M. Fundus autofluorescence in best disease. In: Lois N, Forrester JV, eds. Fundus Autofluorescence. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:197–205 [Google Scholar]
- 2.Downes SM. Fundus autofluorescence in pattern dystrophy. In: Lois N, Forrester JV, eds. Fundus Autofluorescence. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:184–96 [Google Scholar]
- 3.Brody JM, Butrus SI, Ashraf MF, Rabinowitz AI, Whitmore PV. Multiple myeloma presenting with bilateral exudative macular detachments. Acta Ophthalmol Scand. 1995;73:81–2 [DOI] [PubMed] [Google Scholar]
- 4.Leys A, Vandenberghe P. Serous macular detachments in a patient with IgM paraproteinemia: an optical coherence tomography study. Arch Ophthalmol. 2001;119:911–13 [PubMed] [Google Scholar]
- 5.Knapp AJ, Gartner S, Henkind P. Multiple myeloma and its ocular manifestations. Surv Ophthalmol 1987;31:343–51 [DOI] [PubMed] [Google Scholar]
- 6.Ho AC, Benson WE, Wong J. Unusual immunogammopathy maculopathy. Ophthalmology. 2000;107:1099–103 [DOI] [PubMed] [Google Scholar]
- 7.Berta A, Beck P, Mikita J. IgM paraprotein in the subretinal fluid of a patient with recurrent retinal detachment and Waldenstrom's macroglobulinaemia. Acta Med Hung 1985;42:179–86 [PubMed] [Google Scholar]
- 8.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin and subretinal microglia in experimental mice. Aging Cell 2008;7:58–68 [DOI] [PubMed] [Google Scholar]