Abstract
Metastasis to the uterine corpus is uncommon and secondary colorectal tumours of the endometrium are rare. We describe a uterine tumour with components of both primary endometrial and metastatic colorectal carcinomata. In this case, a 72-year-old obese woman presented with a 2-week history of postmenopausal bleeding per vaginum and weight loss. She had an abdominoperineal resection 3 years previously for a Dukes stage B rectal carcinoma. A transvaginal ultrasonography showed a thickened endometrium. Histology immunophenotyping showed a CK7+, CK20+, CA125− and CEA+ colorectal metastasis (a profile consistent with her previous cancer) associated with a primary CK7+, CK20−, CA125+ and CEA− endometroid endometrial adenocarcinoma. We conclude this represents endometrial metastasis of colorectal carcinoma with coincident primary endometrial adenocarcinoma. We speculate as to whether the endometrial carcinoma arose de novo or was induced by the colorectal metastasis, or whether the primary endometrial tumour provided a fertile site for the colorectal metastasis.
Background
Although metastasis to the uterine corpus is uncommon, when it occurs, it is generally to the myometrium rather than the endometrium. Endometrial involvement by metastasis is almost always in conjunction with myometrial metastasis.1 Endometrial metastasis has been described in association with cancers of the breast, colon1 2 and stomach,3 4 as well as in association with malignant melanoma5; in most cases there is disseminated metastatic disease.6 We describe an unusual occurrence of isolated endometrial metastasis of a colorectal tumour, closely associated with a primary endometrial cancer.
Case presentation
A 72-year-old woman presented with a 2-week history of continuous fresh bleeding per vaginum and a 2-month history of a 3 kg weight loss. She denied urinary symptoms or change in bowel habit and there was no history of pain. Systemic review was unremarkable.
She was menopausal at age 58 and was gravida 4. There was no evidence of previous pelvic inflammatory disease and there was no history of hormonal therapy.
She had undergone an abdominoperineal resection of a colorectal carcinoma 3 years previously which proved to be a T3 N0 MX, well to moderately differentiated Duke's stage B adenocarcinoma with no other adverse prognostic features. Her postoperative recovery was long and complicated by a severe methicillin-resistant Staphylococcus aureus wound infection, wound dehiscence and poor diabetic control. Postoperative radiological examination showed no evidence of metastatic disease and the multidisciplinary team (MDT) decision was that no further treatment was necessary. Up-to-date follow-up colonoscopy showed no evidence of recurrence.
Her co-morbidities included poorly controlled type 2 diabetes mellitus, ischaemic heart disease, hypertension, a body mass index of 48 and non-alcoholic steatohepatitis. She was previously a heavy smoker, her diet was poor and she had poor exercise tolerance. Her regular medications were lisinopril 10 mg twice daily, metformin 500 mg twice daily and rosiglitazone 2 mg twice daily. She had no relevant family history.
On examination she was alert and well. Her abdomen was soft and non-tender and no masses or organomegaly were felt. Bimanual examination revealed a moderate cystocoele and the uterus could not be felt.
Investigations
Transvaginal ultrasonography showed a thickened endometrium at 39 mm, with a mixed echogenic pattern and there was a small collection in the pouch of Douglas, findings suggestive of endometrial carcinoma. Hysteroscopy showed a thickened, yellow endometrium with a partly necrotic and partly viable tumour. Colonoscopy was normal. CT of the thorax, abdomen and pelvis showed no evidence of lymphadenopathy or metastatic disease as demonstrated in figure 1. Skeletal surveys are not routinely performed in the initial work-up of suspected colorectal or endometrial cancer at our institution and therefore a bone scan was not carried out.
Figure 1.
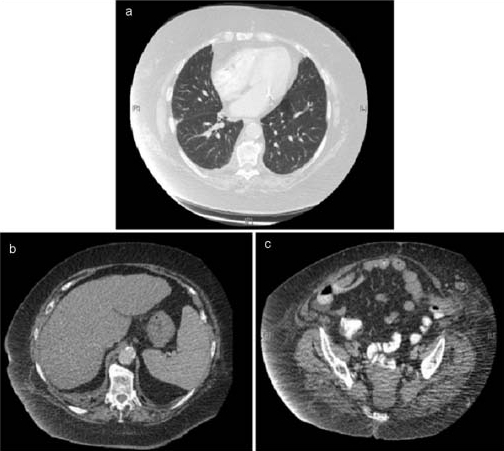
Computerised tomography images of (A) the thorax at the level of the pulmonary trunk (lung window), showing both lungs free of metastases, (B) the abdomen at approximately the level of T10 showing the liver also free of metastases (note the mildly cirrhotic appearance of the liver, most likely the sequelae of non-alcoholic steatohepatitis) and (C) the pelvis at approximately the level of the mid sacrum showing no apparent enlargement of the uterus or lymphadenopathy.
Curettings were taken and microscopical examination of these showed a polyp associated with two separate tumour fragments of distinct histological and immunohistochemical phenotypes, as illustrated in figures 2 and 3. The first fragment had a mixed glandular and pseudopapillary pattern with extracellular mucin production, positive for CK7, CK20 and CEA but negative for CA125, ER and vimentin, suggesting a colorectal aetiology. The second fragment showed some solid areas with a distinct micropapillary pattern, positive for CK7, CA125, vimentin and ER positive but CK20 and CEA negative, consistent with endometrial cancer.
Figure 2.
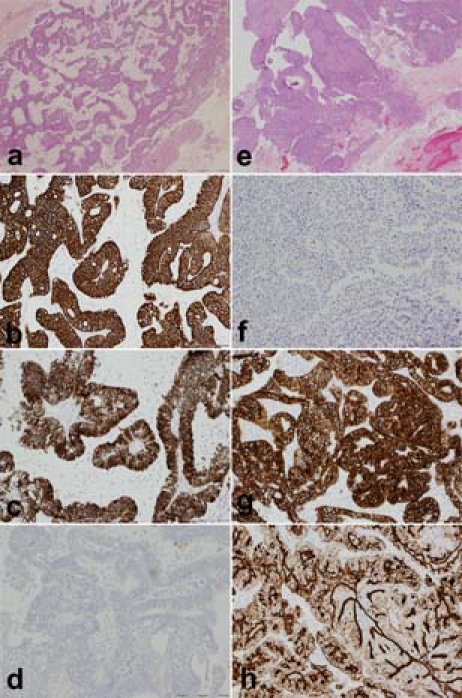
Low power view of the metastatic colorectal carcinoma which has a loose glandular pattern with extracellular mucin production (A; ×2, scale bar 1 mm, H&E) in contrast to the more solid and partly papillary high grade serous endometrial carcinoma (E; ×2, scale bar 1 mm, H&E). Immunohistochemistry shows that the colorectal metastasis is CK20+ (B; ×10, scale bar 200 µm), CK7+ (C; ×10, scale bar 200 µm) and CA125− (D; ×10, scale bar 200 µm). The endometrial carcinoma by contrast is CK20− (F; ×10, scale bar 200 µm), CK7+ (G; ×10, scale bar 200 µm) and CA125+ (H; ×4, scale bar 500 µm).
Figure 3.
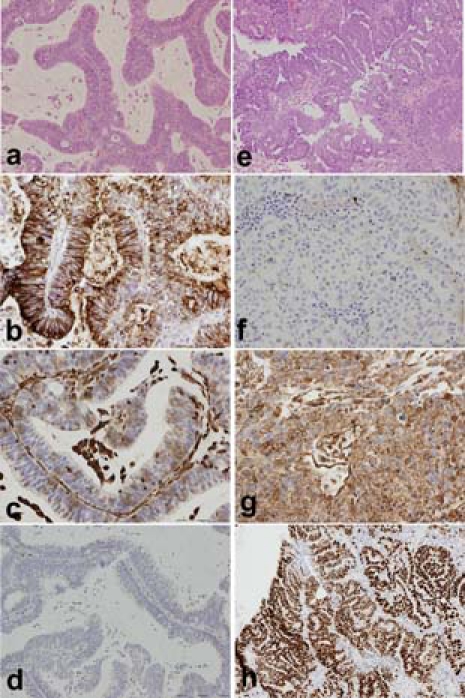
Higher power views of the colorectal metastasis (A; ×10, scale bar 200 µm, H&E) and the endometrial carcinoma (E; ×10, scale bar 200 µm, H&E) with features as described in figure 1. The colorectal metastasis is CEA+ (B; ×20, scale bar 100 µm) but vimentin negative (C; ×20, scale bar 100 µm) and ER− (D; ×10, scale bar 200 µm). The endometrial carcinoma however is CEA− (F; ×20, scale bar 100 µm) and vimentin positive (G; ×20, scale bar 100 µm) and ER+ (H; ×10, scale bar 200 µm).
Immunohistochemical profiling of her previous adenocarcinoma was then carried out as illustrated in figure 4. This proved her previous tumour to be positive for CK7, CK20 and CEA, but negative for CA 125, ER and vimentin, consistent with the metastatic (but not endometrial) phenotype.
Figure 4.
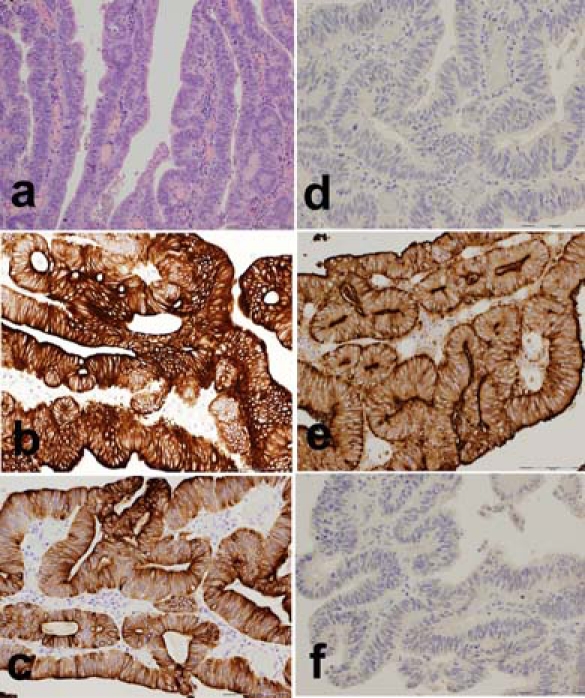
Histological (A; ×10, scale bar 200 µm, H&E) and immunohistochemical profile of the previous colorectal carcinoma demonstrating the similarities to that of figure 3. This tumour is CK20+ (B; ×20, scale bar 100 µm), CK7+ (C; ×20, scale bar 100 µm), CA125− (D; ×20, scale bar 100 µm), CEA+ (E; ×20, scale bar 100 µm) and ER− (F; ×20, scale bar 100 µm).
Differential diagnosis
From the histology and immunohistochemical profiling we concluded that this patient had two distinct tumours, the first being a high grade serous endometrial carcinoma and the second, based on comparative histology with her previous adenocarcinoma and their identical immunohistochemical profiles, a coincident colorectal carcinoma metastasis without radiological evidence of local, or general, tumour dissemination.
Treatment
Her histological diagnosis was discussed with the gynaecological MDT and subsequently with the colorectal MDT and the joint consensus was that metastasis from the previous primary gave her a poor prognosis from a colorectal cancer point of view, although a potentially curable stage I endometrial cancer. It was also apparent, however, that since her initial surgery her diabetic control had worsened, her weight had increased and her exercise tolerance had deteriorated and thus these factors combined with a likely surgically difficult, fibrotic abdomen made resection a very unfavourable option; chemotherapy or radiotherapy remained possible options but with limited potential for remission.
Due to her previously difficult postoperative recovery, our patient did not want to undergo another difficult operation and was also reluctant to undergo the ordeal of primary chemotherapy, leaving us with radiotherapy as her remaining option. Further investigation at this point therefore would not alter her management and thus no further imaging or biopsies were sought.
In the end our patient opted for a palliative course of external beam radiotherapy which was delivered in 10 daily sessions, each consisting of 10 meV fractions at anterior, left lateral, posterior and right lateral positions to a combined total of 30 Gy.
Outcome and follow-up
After this course of radiotherapy our patient remained stable for some 6 months, however she then rapidly declined and did not want any further intervention. Her final CT imaging showed para-aortic nodal disease, but again no dissemination and further investigation at this point would have been futile. After she died the coroner felt that a postmortem examination was not indicated and the family did not want a hospital autopsy.
Discussion
Postmenopausal per vaginum bleeding must be considered cancer until proven otherwise and therefore must be investigated urgently. There are around 4500 new cases of primary endometrial cancer each year in the UK, but per vaginum bleeding secondary to a metastatic cause is rare.6 Other than in disseminated disease or familial colorectal cancer syndromes, breast cancers are the only tumours known to commonly metastasise to the endometrium.7 In our patient there was good immunohistochemical evidence that this was a rare example of an isolated endometrial metastasis of colorectal origin.8 This may represent concurrent metastasis of a colorectal carcinoma to a de novo endometrial tumour or it is possible that the endometrial primary had provided a fertile site for metastasis. This idea has been suggested before in sites of cancer,9 10 chronic inflammation, high cell turnover11 or local immunosupression by tumours,10 12 although there are no reports of this in the female genital tract, making this case unusual. It is also possible that the colorectal metastasis may have induced an endometrial carcinoma.
Immunohistochemical panels, where available, are now standard practice worldwide in the diagnosis of endometrial cancer, thus we have accordingly used the recognised markers in this case.8 13 However, there is growing evidence as regards multiclonal or heterogenous tumours, whereby antigen expression is mixed. It should be emphasised that there are emerging data which suggest there may be distinct antigenic subtypes of endometrial carcinoma14 15; at this time, however, the evidence is limited and its clinical relevance is unclear. We feel therefore, given this and the distinct (exclusive) immunohistochemical profiles demonstrated, that multiclonality is not likely to be a significant factor in this case.
Alternative conclusions should also be further considered. As mentioned, uterine metastases are most commonly found in disseminated metastatic disease, but no clinical or radiological evidence of this was ever demonstrated. The possibility of sample contamination was considered, however auditing showed no other colorectal pathology was processed that day in the dissection room or laboratory. The blocks and slides were also checked to exclude clerical error.
Finally, there was no evidence of intercurrent breast cancer and, although endometrial metastases are seen in familial colorectal cancer syndromes, a lack of family history made this very unlikely. It should be noted that the nosology of this group of diseases is evolving and Lynch syndrome is becoming recognised as a distinct entity where the occurrence of synchronous hereditary non-polyposis colorectal cancer-type tumours is recognised by diagnostic criteria alone16; this may therefore raise the need for genetic screening of this woman's family.
In conclusion, we describe an unusual case of metastasis of a primary colorectal adenocarcinoma to the endometrium with simultaneous primary serous endometrial adenocarcinoma. Such a case poses significant implications for surgical and oncological management, which should be determined on an individual basis.
Learning points.
-
▶
Endometrial metastatic disease is a rare aetiology of postmenopausal per vaginum bleeding.
-
▶
The environment of a cancer may alter local molecular and cellular biology and have the potential to induce abnormal growth.
-
▶
Equally that environment may provide fertile ground for metastatis.
-
▶
Multiple pathology, while rare, will pose unique challenges which need to be managed by a multidisciplinary team on a case-by-case basis.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Kumar NB, Hart WR. Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer 1982;50:2163–9 [DOI] [PubMed] [Google Scholar]
- 2.Zannoni GF, Vellone VG, Fadda G, et al. Colonic Carcinoma Metastatic to the Endometrium: The Importance of Clinical History in Averting Misdiagnosis as a Primary Endometrial Carcinoma. Int J Surg Pathol 2009 [DOI] [PubMed] [Google Scholar]
- 3.Pasini A, Mandelli P, Belloni C. Endometrial metastases from gastric adenocarcinoma: a case report. Tumori 1995;81:383–6 [DOI] [PubMed] [Google Scholar]
- 4.Neumayr G, Gänzer H, Duregger M, et al. Endometrial curettage with metastasis of a clinically inapparent stomach cancer–a case report. Pathologe 1993;14:58–60 [PubMed] [Google Scholar]
- 5.Berker B, Sertcelik A, Kaygusuz G, et al. Abnormal uterine bleeding as a presenting sign of metastasis to the endometrium in a patient with a history of cutaneous malignant melanoma. Gynecol Oncol 2004;93:252–6 [DOI] [PubMed] [Google Scholar]
- 6.Barakat RR, Park RC, Grigsby PW, et al. Corpus: epithelial tumours. In: Hoskins WJ, Perez CA, Young RC, eds. Principles and Practice of Gynecologic Oncology. Philadelphia, PA: Lippincott-Raven; 1997:859–97 [Google Scholar]
- 7.Manipadam MT, Walter NM, Selvamani B. Lobular carcinoma metastasis to endometrial polyp unrelated to tamoxifen. Report of a case and review of the literature. APMIS 2008;116:538–40 [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG. Immunohistochemical and functional biomarkers of value in female genital tract lesions. Int J Gynecol Pathol 2006;25:101–20 [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Matsumori M, Nakamura K, et al. Metastasis of cancer to cancer: report of a case of esophageal carcinoma metastasizing to early gastric cancer. Jpn J Surg 1991;21:352–6 [DOI] [PubMed] [Google Scholar]
- 10.Saito K, Kato H, Fukai Y, et al. Esophageal granular cell tumor covered by intramucosal squamous cell carcinoma: report of a case. Surg Today 2008;38:651–5 [DOI] [PubMed] [Google Scholar]
- 11.Altorjay A, Gonda G, Ereifej S, et al. Metastasis of an esophageal carcinoma to a giant gastric ulcer. Hepatogastroenterology 1999;46:981–2 [PubMed] [Google Scholar]
- 12.Larkin JO, Collins CG, Martin ST, et al. Paraesophageal lymph node metastasis from prostatic adenocarcinoma in a patient with esophageal squamous carcinoma. Dis Esophagus 2005;18:124–6 [DOI] [PubMed] [Google Scholar]
- 13.McCluggage WG, Sumathi VP, McBride HA, et al. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol 2002;21:11–5 [DOI] [PubMed] [Google Scholar]
- 14.Kounelis S, Kapranos N, Kouri E, et al. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol 2000;13:379–88 [DOI] [PubMed] [Google Scholar]
- 15.Halperin R, Zehavi S, Habler L, et al. Comparative immunohistochemical study of endometrioid and serous papillary carcinoma of endometrium. Eur J Gynaecol Oncol 2001;22:122–6 [PubMed] [Google Scholar]
- 16.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


