Abstract
Vertebrobasilar ischaemia is a rare life-threatening complication in giant cell arteritis (GCA). We report three patients with bilateral vertebral artery occlusion. Neurovascular imaging, including CT-angiography, MR-angiography and colour-coded duplex sonography revealed flow reversal in the basilar artery as well as inflammation of the vertebral vessel wall. The first patient died from massive brainstem infarction, the other two patients survived the initial inflammatory phase of GCA. No stroke recurrence at 12 months’ follow-up on warfarin and steroid treatment was observed. Bilateral distal vertebral artery occlusion and retrograde basilar artery flow persisted.
Outcome in these patients is dependant on potent immunosuppression, concurrent atherosclerotic steno-occlusive disease and presence and/or rapid development of sufficient collateral pathways into the vertebrobasilar circulation. The identification of patients with high risk of ischaemia due to compromised vertebrobasilar flow may be important to select adjunct treatment to immunosuppression, such as anticoagulation in GCA.
BACKGROUND
Giant cell arteritis (GCA; temporal arteritis; Horton’s disease) is a systemic autoimmune granulomatous vasculitis of multifactorial origin. It is by far the most frequently diagnosed vasculitis of large and medium-sized arteries in the elderly favouring the female gender.1,2 Increasing evidence suggest a central role of T cell autoimmunity. Histopathology reveals predominantly CD4+ T cells and activated macrophages infiltrates with frequent formation of multinucleated giant cells.3 Disruption of the internal elastic lamina and hyperplasia of the intimal layer are additional characteristic features. Ischaemic optic neuropathy and ischaemic stroke or respective transient symptoms are dreaded manifestations usually occurring shortly or around the time of diagnosis. In particular, vertebral artery (VA) involvement with brainstem and/or cerebellar infarction is a rare but well-established and life-threatening type of GCA. In contrast to the anterior circulation in atherosclerotic disease evolving over months to years, collateralisation into the vertebrobasilar circulation is most often limited to the posterior communicating arteries and needs to be pre-existing. Ischaemic stroke has been repeatedly described to occur despite prompt initiation of corticosteroid treatment.4 Unfortunately, no prospective data or central registry exist.3,5 Standard treatment of GCA consists of immunosuppression with corticosteroids.6,7
Although inflammation of the arterial wall may result in significant lumen narrowing up to occlusion and also arterial thrombosis causing ischaemic injury to the retina or brain, few studies address the importance of adjunct antiplatelet or anticoagulation treatment in GCA despite evidence of a state of hypercoagulation; this maybe because ischaemic events could not be linked to elevated levels of pro-coagulatory Willebrand factor antigen (vWF:Ag), plasminogen activator inhibitor (PAI) and antiphospholipid antibody (aPL).8–11 Acetylic acid (ASA, aspirin) has experimentally been shown to synergise with corticosteroids in the suppression of the inflammation.12 In a retrospective study of GCA patients, its use as an antiplatelet agent was associated with fewer ischaemic events at the time of diagnosis and during follow-up;13 however, no comparison with anticoagulation in these special cases has been performed.
We report on three patients suffering from GCA with bilateral occlusion of the VAs with reversal of flow in the basilar artery (BA) demonstrated by multimodal neuroimaging (MR-angiography (MRA), CT-angiography (CTA), transcranial colour-coded sonography (TCCS)). All patients were admitted with symptoms attributable to transient or permanent brainstem or cerebellar stroke. Neuroimaging findings, treatment, short to mid-term outcome and pathophysiological consideration will be presented.
CASE PRESENTATION
Case 1
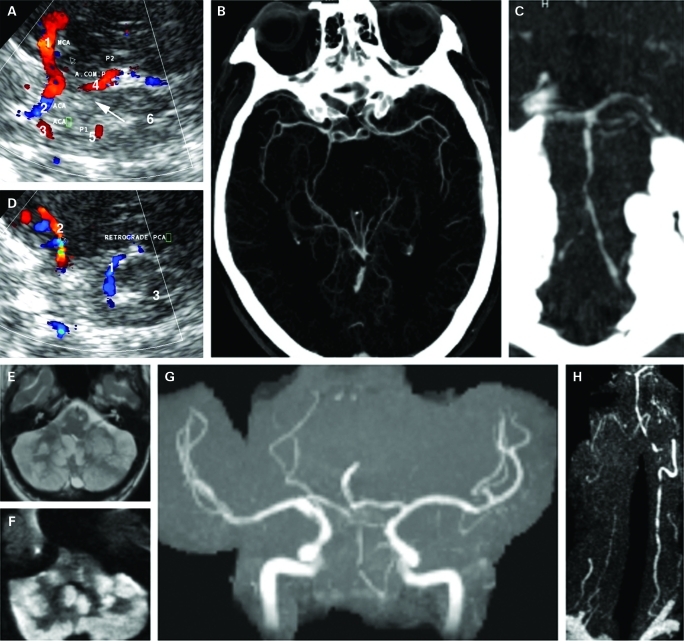
A 73-year-old patient was transferred to our neurology department with fluctuating dysarthria and paresis of the right arm. One month prior he was diagnosed with GCA with typical findings in a temporal artery biopsy after a 4-week history of pulsating headache, jaw claudication and general fatigue. Cerebral MRI was unremarkable at that time. He was treated with 100 mg prednisolone daily followed by successive tapering to 30 mg daily in addition to daily clopidogrel 75 mg for platelet inhibition. At presentation in our department, extracranial colour-coded sonography (ECCS) revealed a high resistance flow profile in the intervertebral segments of both VAs suggestive of distal occlusion next to generalised atherosclerotic disease due to several atherosclerotic risk factors. TCCS showed retrograde perfusion of the left posterior cerebral artery (PCA) in the pre-communicating and post-communicating segment (P1 and P2) in the absence of the left posterior communicating artery (PcomCA) indicative of reverse distal basilar artery flow. Therefore, primary collateralisation into the PCA was not via the PcomCA but most likely via atypical cortical or leptomeningeal anastomosis. Due to the compromised status of the patient, no digital subtraction angiography was performed. The right PCA originated directly from the PcomCA with absence of the P1 segment (fig 1). One day later, CT revealed bilateral patchy cerebellar infarcts and CTA showed significant basilar and vertebral artery lumen narrowing. Blood sedimentation rate was increased to 85 mm in the first hour and 155 mm in the second hour. Due to unusual location for atherosclerotic VA occlusion, inflammation of the VA was assumed and corticosteroid dose was increased to intravenous 500 mg daily prednisolone. However, the patient developed progressive ataxia, anisocoria and downbeat nystagmus and also severe pneumonia. At day 4, anticoagulation with intravenous heparin was started. Despite this, the patient’s status deteriorated and necessitated intubation. MRI at day 7 revealed space occupying cerebellar and pons infarction as well as “string-like” vertebrobasilar vessels. Escalation of immunosuppressive treatment with cyclophosphamide was initiated. The patient recovered slowly and could be extubated 3 days later. At day 12, however, the patient suffered from cardiorespiratory arrest and died 2 days later from massive brainstem and cerebellar infarction. Autopsy confirmed severe inflammation of the vertebral arteries extending even into the intradural segment but sparing the basilar artery. No thrombosis but significantly lumen narrowing of all basal cerebral vessels was observed.14
Figure 1.
A. On admission, transcranial colour-coded sonography, axial view of the mesencephalic image plane through the left temporal window: 1=retrograde filling of the left posterior cerebral artery (PCA) in blue away from the transducer (P1); 2=ipsilateral middle cerebral artery (MCA) with branches in blue. B. Transcranial colour-coded sonography, axial view of the mesencephalic image plane through the right temporal window: 1=right MCA (M1); 2=ipsilateral anterior cerebral artery (ACA) (A1); 3=contralateral ACA (A1); 4=filling of the right PCA (P2) via posterior communicating artery; 5=contralateral PCA (P1) now in red towards the transducer; 6=mesencephalic brainstem. Arrow marks missing right P1 segment due to either no flow, aplasia (fetal type) or hypoplasia. C. Maximum intensity projection of the circle of Willis from CT-angiography confirming fetal type origin of the right PCA and filling of the left PCA. D. Maximum intensity projection of the vertebrobasilar system showing multiple narrowing of the basilar and vertebral arteries. E. 7 day later, fluid attenuated inversion recovery (FLAIR) sequences showing massive ischaemia of the cerebellum. F. Diffusion-weighted MRI confirming acute cerebellar ischaemia. G. Time-of-flight MR-angiography (MRA) showing highly compromised flow in the vertebrobasilar system. H. Contrast-enhanced MRA showing multiple severe stenosis of the right vertebral artery, multiple distal high grade stenosis of the dominant left vertebral artery and basilar artery.
Case 2
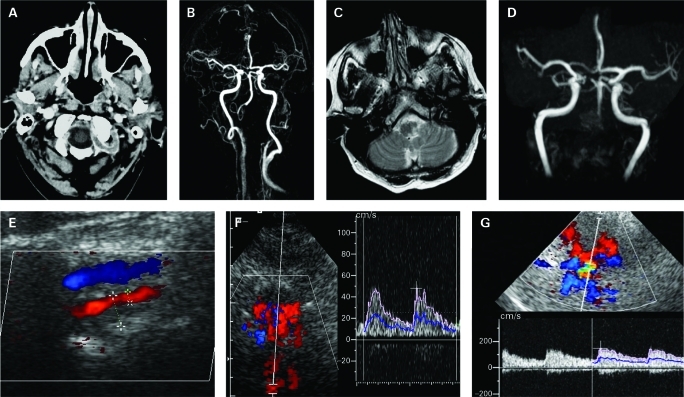
A 74-year-old patient was referred to our department with signs of brainstem ischaemia after she had been treated for GCA for 5 days at a general medicine intensive care unit. Intermittent headaches had started several weeks before with a single episode of partial loss of vision. Due to atrial fibrillation, she was already on oral anticoagulation. At the time of hospital admission she suffered from fluctuating symptoms of vertigo, sickness and cold sweating, with hypotensive episodes and reduced vigilance. She was given 50 mg prednisolone hemisuccinate for 5 days, at which time point further deterioration required an urgent referral to the neurological intensive care unit. She presented with a spontaneous nystagmus to the right, dysarthria, paresis of the right abducens nerve and left hemiataxia. An initial post-contrast CT scan revealed no signs of ischaemia but contrast-enhancement of both extradural distal VAs. A subsequent MRI 4 days later showed signs of posterior cerebral artery infarction and ischaemic lesions in both cerebellar hemispheres, the left cerebellar pedicle, the dorsolateral medulla oblongata as well as occlusion of both VAs in MRA. ECCS revealed hypoechoic lumen narrowing of the vertebral artery with minimal diastolic flow on the left and high-grade stenosis on the right side consistent with vessel wall inflammation (“halo”). There was only mild atherosclerosis predominantly in the common carotid arteries. Transnuchal insonation demonstrated retrograde flow of the basilar artery down to the vertebrobasilar junction. Contrast-enhanced TCCS showed retrograde flow in the left P1 segment of the PCA via PcomCA, which is indicative of a collateralisation into the BA (fig 2). Laboratory results showed an extremely high erythrocyte sedimentation rate (ESR), elevated c-reactive protein (26 mg/l) and interleukin-6 of 8.4 pg/ml plus negative results for other vasculitis parameter. Treatment of high-dose methylprednisolone (intravenously 1g daily) for 5 days was started. Fluoro-deoxyglucose positron emission tomography (FDG-PET) showed enhancement of the larger blood vessels caudal of the abdominal aorta, which is indicative for large vessel vasculitis. Along with improving clinical symptoms, steroids were slowly tapered to 12.5 mg/d within 6 months and anticoagulation continued. No superficial temporal artery biopsy was performed due to signs of vessel inflammation in post-contrast CT, FDG-PET, clinical and laboratory findings indicative for GCA, and because the patient was already on oral anticoagulation. Twelve months later both VAs were still occluded with persistent retrograde BA flow as shown by ultrasound examination. There was no residual focal neurological deficit apart from intermittent episodes of vertigo.
Figure 2.
A. Post-contrast cerebral computed tomography showing contrast-enhancement consistent with inflammation of both vertebral arteries. B. Contrast-enhanced MR-angiography (MRA) showing filling of the basilar artery (BA) down to the right posterior inferior cerebellar artery. C. Fluid attenuated inversion recovery (FLAIR) image showing left lateral medullar infarct. D. Time-of-flight MRA showing intracranial filling of the BA via left communicating artery. E. Extracranial colour-coded sonography showing hypoechoic lumen narrowing of the vertebral artery (red) above vertebral vein. F. Transnuchal contrast-enhanced duplex sonography: Doppler gate placed in the BA colour-coded in red running away from the transducer with Doppler spectrum showing retrograde filling of the BA with pulsatile slow flow. G. Contrast-enhanced transcranial duplex sonography through the left temporal bone window. The Doppler gate is placed in the proximal left posterior cerebral artery showing retrograde flow with flow values characteristic for the anterior circulation.
Case 3
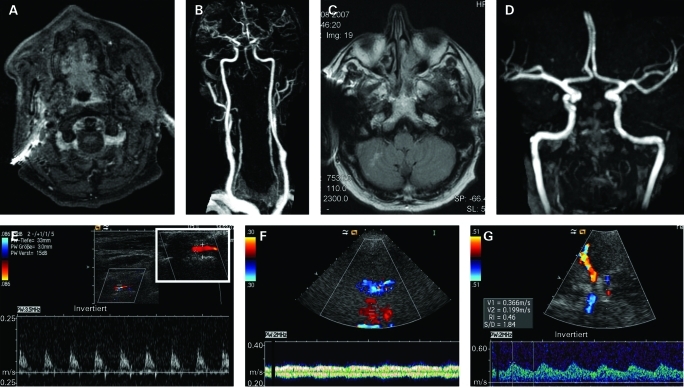
A 65-year-old patient presented with acutely worsening symptoms of vertigo and blurred vision. Medical history included hypertension, hypercholesterolemia and bilateral internal carotid endarterectomy for high-grade stenosis 3 and 5 years earlier. He had been diagnosed in a peripheral hospital with bilateral distal VA stenosis of atherosclerotic nature and treated with 100 mg ASA daily for suspected infarction in the vertebrobasilar circulation since week 1 and had been discharged 2 days prior admission. Due to a metal splinter no MRI had been performed. The patient had refused digital subtraction angiography (DSA) because of the associated peri-procedural risk. On examination, he only presented with blurred vision, an unsteady gait and right hemiataxia. The additional medical history revealed low dose corticosteroid treatment initiated for recurrent leg and shoulder pain and interpreted as polymyalgia rheumatica by a general physician 4 weeks ago. Serum levels showed a raised c-reactive protein of 22.7 mg/l and elevated ESRs above 100 mm in the first hour on various measurements. Two consecutive MRI scans with some metal artefacts revealed an old ischaemic infarction in the left middle cerebral artery territory and an old ischaemic lesion with minor haemorrhage in the right posterio inferior cerebellar artery (PICA) territory. Time-of-flight and contrast-enhanced MRA showed high-grade intracranial stenosis in both VAs directly proximal of the BA. ECCS showed high-resistance flow pattern in both VAs with atypical thickening of the vessel wall in the right V2 segment. TCCS demonstrated retrograde filling of the BA (fig 3). No biopsy was taken since the patient fulfilled all clinical criteria for GCA, including inflammation of the vertebral arteries as demonstrated by post-contrast MRI. It must be noted though, that the superficial temporal artery did not show signs of inflammation on ultrasound that may be attributed to skip lesions and the predominant involvement of the vertebrobasilar circulation. The patient was treated intravenously with 1 g methylprednisolone for 3 days followed by gradual tapering to 30 mg daily within 6 months. In addition, the patient was anticoagulated using intravenous heparin that was switched to warfarin treatment on discharge. Three months later, control MRI and neurosonography showed persistent distal occlusion of both VAs and retrograde perfusion of the BA. Twelve months later, flow in the right VA markedly increased while the left VA still revealed signs of distal occlusion and the BA was still retrograde on ultrasound. Anticoagulation and low-dose corticosteroid treatment was continued. The patient remained asymptomatic.
Figure 3.
A. Post-contrast MRI showing contrast-enhancement consistent with inflammation of both vertebral arteries (VAs). B. Contrast-enhanced MR-angiography showing filling of the basilar artery (BA) down to both distal VAs. C. Fluid attenuated inversion recovery (FLAIR) image showing small right cerebellar infarct. D. Time-of-flight MRA suggesting absence of flow in the vertebrobasilar system. E. Extracranial colour-coded sonography high-resistance flow pattern in the left VA. Inlay demonstrating “halo” sign, hypoechoic vessel wall inflammation leading to significant lumen reduction. F. Transnuchal contrast-enhanced duplex sonography: Doppler gate placed in the BA colour-coded in red running away from the transducer with Doppler spectrum showing retrograde filling of the BA with slow flow with venous-like pulsatility. Note the distal VAs with orthograde flow coloured in blue. G. Contrast-enhanced transcranial duplex sonography through the left temporal bone window. Large red vessel: ipsilateral middle cerebral artery (MCA) with normal flow towards the transducer. Small red vessel: ipsilateral posterior cerebral artery (PCA) with retrograde flow away from the transducer into the BA. Large blue vessel: contralateral MCA with normal flow away from the transducer. Small blue vessel: contralateral PCA with retrograde flow away from the transducer into the BA.
DISCUSSION
Bilateral VA occlusion is a life-threatening complication in patients suffering from GCA. In addition to effective immunosuppression, short-term outcome and long-term prognosis in these patients appears to be dependant on a) the control of the underlying vessel wall inflammation and b) the presence and/or development of sufficient collateral pathways into the vertebrobasilar circulation. In the surviving two patients, no recanalisation of the occluded distal VA was observed and BA flow remained retrograde at 12 months’ follow-up, while both patients recovered well and had no new symptoms on tapered corticosteroid and oral anticoagulation treatment. The rare finding of flow reversal in the basilar artery, a rare finding per se, often implies slow “pseudo-venous” flow velocities at least in the early and pro-coagulatory phase, and, since high-dose corticosteroid treatment further increases the risk of thrombosis, we suggest that additional anticoagulation may be considered in these cases. This is in contrast with recommendations in intracranial atherosclerotic artery disease with platelet activation by shear stress in high-flow tight stenosis, among other factors, in which anticoagulation is not superior to antiplatelet treatment.15
Although the incidence involvement of brain supplying arteries is estimated up to 15%, stroke syndromes caused by GCA are rarely reported or misdiagnosed given the high degree of overlap with atherosclerotic cerebrovascular disease in this age group.16,17 Bilateral VA occlusion is a rare and serious manifestation of GCA.18 In contrast to GCA and atherosclerosis in the carotid artery territory, atherosclerotic vertebrobasilar disease is often considered relatively benign in contrast to atherosclerotic carotid artery disease. This is most often due to the existence of dominant posterior communicating arteries, the re-activation of cervical embryonic collaterals over time and, in rare cases, persistent carotido-basilar anastomosis such as the persistent trigeminal artery.19–21 In a large cerebrovascular registry, only 8 out of 430 patients (1.8%) had bilateral intracranial VA occlusion and none of these experienced stroke progression or death.22 Bogousslavsky et al studied a series of 17 patients with angiographically proven VA occlusion.23 In contrast, patients suffering from GCA with bilateral VA occlusions appear to have higher mortality. Including our patients to others in the literature, 10 out of 19 patients died from brainstem and/or cerebellar infarction.24–27 The main differences of atherosclerotic VA disease to VA involvement in GCA are, first, VA occlusion in GCA often occurs in the distal VA segments where the only intracranial collaterals are the PcomCAs and, second, GCA is a rapid process over days to weeks allowing little time for the development of collateral pathways.
In light of the frequency and severity of ischaemic complications in GCA, the selection of antiplatelet or anticoagulation medication in addition to standard corticosteroid treatment is a major unresolved issue. The mechanism of ischaemia in GCA is considered to be primarily due to vessel occlusion or high-grade stenosis. Inflammation, however, is a pro-coagulatory state per se and raised levels of vWF:Ag, a carrier protein for coagulation factor VIII and promoter for platelet adhesion, have been described in the acute phase of GCA in 63 patients compared with controls.9 In the study vWF:Ag did not correlate with ischaemic events nor did the levels of PAI, which differed considerably among the study population. However, in Ruegg et al, autopsy was performed in two patients revealing thrombosis of the VAs (patients 1 and 2) indicative of pro-coagulatory state.24 Kumar and Costa described a patient with patchy thrombotic occlusion of both VAs and speculate that the thrombotic material developed on the background of the vasculitic process.27 Finally, Salvarani et al describe a patient with intracranial involvement of GCA with thrombosis of the internal carotid artery.28 A retrospective trial investigated the incidence of cerebrovascular accidents in GCA adding either adding low-dose ASA or anticoagulation with warfarin to standard corticosteroid treatment.29 Altogether, 86 out of 143 patients (60.1%) were treated with either ASA, clopidogrel or warfarin but, among these, 18 patients did not start treatment until after an ischaemic event. Bleeding complications were low and logistic regression analysis revealed a statistically significant effect for prevention of ischaemic events for either ASA or warfarin when adjusted for age, sex and the presence of cerebrovascular risk factors. The study is in line with an earlier retrospective study showing fewer ischaemic complication at the time of diagnosis when the patients were already on ASA or anticoagulation treatment by the time of developing GCA.13 Experimentally, Weyand et al have shown that ASA synergises with corticosteroids by suppressing the interferon-gamma driven cellular inflammation of GCA, which is largely corticosteroid resistant.12 This may be relevant both for the frequency of positive biopsy findings in corticosteroid-treated patients and the occurrence of central nervous system or eye ischaemia in spite of seemingly prompt clinical “responses” upon treatment initiation (case 1). ASA might, therefore, be preferable to other platelet inhibition agents such as clopidogrel in patients with GCA. Ruegg et al described three GCA patients with bilateral VA occlusion but unfortunately lacking any haemodynamic assessment of the vertebrobasilar system.24 In our patients, compromised and even retrograde BA flow was demonstrated by TCCS adding important information for the individual management. Patients with a combination of systemic or vessel wall inflammation with low, pseudo-venous, blood flow velocities, as in cases of retrograde BA flow, may have a high risk of developing thrombosis.
Vertebrobasilar perfusion may improve due to increasing collateral pathway sufficiency over time—especially the posterior communicating arteries. To date, little is known about the long-term outcome in patients with bilateral VA occlusion due to GCA. In patients 2 and 3, VA occlusion persisted at 12 months. We currently follow two cases: one with an asymptomatic vertebral artery and one with a common carotid artery inflammation in biopsy proven GCA. Both showed no signs of cerebral ischaemia despite >50% stenosis and were treated with ASA in addition to corticosteroids. After 9 months of treatment, we could observe significant reduction of the halo sign on ultrasound. Hence, vasculitic VA occlusions may lead to fibrosis with persistent occlusions, which is indicative for life-long anti-platelet or anticoagulation treatment.
Non-invasive imaging techniques, especially MRI and positron emission tomography, are increasingly used to identify the vessel wall inflammation in GCA.30,31 For routine examination, ultrasound techniques offer an inexpensive and widely available alternative. However, they have only been prospectively evaluated to detect the characteristic dark halo around the lumen of the temporal superficial artery.32,33 Yet, only a skilled sonographer, given the high operator dependence, should perform the ultrasound examination. Visualisation of vessel wall inflammation of the vertebral, carotid, subclavian, axillary and brachial arteries by means of ECCS have also been described and allow for accurate follow-up during immunosuppressive treatment.17,34 Of note, the halo sign cannot be seen in the distal vertebral arteries since low frequency (2 MHz) phased array transducers are routinely used and B-mode information is negligible. The strength of TCCS and ECCS lies in the detection of abnormal flow patterns as described in our patients. No studies exist on the use of post-contrast CT to detect arterial wall inflammation as demonstrated in patients 2 and 3. We feel that this technique should be employed after CTA or post-contrast MRI has detected a distal VA occlusion in patients with other characteristics of GCA.35
In contrast to GCA, cerebrovascular events occur often in Takayasu arteritis (TAK): a large vessel vasculitis of the young predominantly affecting the aorta and its main branches.36 Non-invasive imaging, including colour Doppler, ultrasound has gained significance in screening as well as long-term follow-up of these patients.37 Ultrasound may offer visualisation of vessel inflammation with high resolution as well as assessment of collateral pathways intracranial and extracranial brain supplying vessels.38 Similar to the patients presented here, Grosset et al described a case with reversal of flow in the basilar artery in TAK detected by multimodal neuroimaging including transcranial Doppler.39 Yoneda et al described five cases of TAK with subclavian steal syndrome yet without vertebrobasilar ischaemia. Both articles demonstrate the importance of functional end organ perfusion assessment as opposed to structural angiographic changes.39,40
In conclusion, vertebrobasilar ischaemia represents a life-threatening complication of GCA. Neurovascular examinations yield detailed information on vessel morphology, vessel wall inflammation (post-contrast MRI, MRA, post-contrast CT, CTA, positron emission tomography, duplex sonography) as well as the flow and perfusion abnormalities (duplex sonography, TOF-MRA, pwi-MRI, perfusion CT). Given the lack of relevant randomised controlled treatment trials in GCA, individual management decisions in complicated situations such as bilateral VA involvement should account for data on anatomical and functional neurovascular imaging. While low dose aspirin should generally be considered as an adjunct to immunosuppressive treatment, at least in cases with prior ischaemic events, anticoagulation might be preferable in low-flow states such as retrograde BA flow. Eventual restoration of flow in occluded VA is rare and did not occur in our two surviving patients within 12 months of follow-up stressing the need for long-term or life-long oral anticoagulation.
LEARNING POINTS
Vertebrobasilar ischaemia is a serious complication of giant cell arteritis.
Retrograde basilar artery flow has not been described given the lack of ultrasound investigations but solely morphological neuroimaging.
Haemodynamic assessment is mandatory to identify patients with high risk of vertebrobasilar stroke.
Colour duplex ultrasound is an important adjunct to morphological studies using CT-angiography or MR-angiography.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Gonzalez-Gay MA, Garcia-Porrua C. Epidemiology of the vasculitides. Rheum Dis Clin North Am 2001; 27: 729–49 [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003; 139: 505–15 [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Cantini F, Boiardi L, et al. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002; 347: 261–71 [DOI] [PubMed] [Google Scholar]
- 4.Staunton H, Stafford F, Leader M, et al. Deterioration of giant cell arteritis with corticosteroid therapy. Arch Neurol 2000; 57: 581–4 [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Rodriguez J, Font C, Garcia-Martinez A, et al. Development of ischemic complications in patients with giant cell arteritis presenting with apparently isolated polymyalgia rheumatica: study of a series of 100 patients. Medicine (Baltimore) 2007; 86: 233–41 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Gay MA, Garcia-Porrua C, Miranda-Filloy JA. Giant cell arteritis: diagnosis and therapeutic management. Curr Rheumatol Rep 2006; 8: 299–302 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman GS, Cid MC, Rendt-Zagar KE, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146: 621–30 [DOI] [PubMed] [Google Scholar]
- 8.Vrij AA, Rijken J, van Wersch JW, et al. Coagulation and fibrinolysis in inflammatory bowel disease and in giant cell arteritis. Pathophysiol Haemost Thromb 2003; 33: 75–83 [DOI] [PubMed] [Google Scholar]
- 9.Nordborg E, Andersson R, Tengborn L, et al. von Willebrand factor antigen and plasminogen activator inhibitor in giant cell arteritis. Ann Rheum Dis 1991; 50: 316–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa G, Tassies D, Font J, et al. Antiphospholipid antibodies and thrombophilic factors in giant cell arteritis. Semin Arthritis Rheum 2001; 31: 12–20 [DOI] [PubMed] [Google Scholar]
- 11.Spiera RF, Spiera H. Therapy for giant cell arteritis: can we do better? Arthritis Rheum 2006; 54: 3071–4 [DOI] [PubMed] [Google Scholar]
- 12.Weyand CM, Kaiser M, Yang H, et al. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 2002; 46: 457–66 [DOI] [PubMed] [Google Scholar]
- 13.Nesher G, Berkun Y, Mates M, et al. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004; 50: 1332–7 [DOI] [PubMed] [Google Scholar]
- 14.Haas S, Jurgens T, Vatankhah B, et al. [Multiple ischemic vertebrobasilar lesions in temporal arteritis]. Nervenarzt 2005; 76: 1527, 1529–31 [DOI] [PubMed] [Google Scholar]
- 15.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–63 [DOI] [PubMed] [Google Scholar]
- 16.Wiszniewska M, Devuyst G, Bogousslavsky J. Giant cell arteritis as a cause of first-ever stroke. Cerebrovasc Dis 2007; 24: 226–30 [DOI] [PubMed] [Google Scholar]
- 17.Pfadenhauer K, Esser M, Berger K. Vertebrobasilar ischemia and structural abnormalities of the vertebral arteries in active temporal arteritis and polymyalgia rheumatica—an ultrasonographic case-control study. J Rheumatol 2005; 32: 2356–60 [PubMed] [Google Scholar]
- 18.Caselli RJ, Hunder GG, Whisnant JP. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology 1988; 38: 352–9 [DOI] [PubMed] [Google Scholar]
- 19.Fisher CM. Occlusion of the vertebral arteries causing transient basilar symptoms. Arch Neurol 1970; 22: 13–19 [DOI] [PubMed] [Google Scholar]
- 20.Larsen WJ. Human embryology. Churchill Livingstone Inc: Oxford, 1997 [Google Scholar]
- 21.Burger IM, Siclari F, Gregg L, et al. Bilateral segmental agenesis of the vertebrobasilar junction: developmental and angiographic anatomy. AJNR Am J Neuroradiol 2007; 28: 2017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin HK, Yoo KM, Chang HM, et al. Bilateral intracranial vertebral artery disease in the New England Medical Center, Posterior Circulation Registry. Arch Neurol 1999; 56: 1353–8 [DOI] [PubMed] [Google Scholar]
- 23.Bogousslavsky J, Gates PC, Fox AJ, et al. Bilateral occlusion of vertebral artery: clinical patterns and long-term prognosis. Neurology 1986; 36: 1309–15 [DOI] [PubMed] [Google Scholar]
- 24.Ruegg S, Engelter S, Jeanneret C, et al. Bilateral vertebral artery occlusion resulting from giant cell arteritis: report of 3 cases and review of the literature. Medicine (Baltimore) 2003; 82: 1–12 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Porrua C, Pego-Reigosa R, Martinez-Vazquez F, et al. Bilateral vertebral artery occlusion in giant cell arteritis. Clin Exp Rheumatol 2006; 24: S101. [PubMed] [Google Scholar]
- 26.Pego-Reigosa R, Garcia-Porrua C, Pineiro A, et al. Predictors of cerebrovascular accidents in giant cell arteritis in a defined population. Clin Exp Rheumatol 2004; 22: S13–7 [PubMed] [Google Scholar]
- 27.Kumar A, Costa DD. Insidious posterior circulation stroke with rapid deterioration due to vertebral giant cell arteritis. Age Ageing 2007; 36: 695–7 [DOI] [PubMed] [Google Scholar]
- 28.Salvarani C, Giannini C, Miller DV, et al. Giant cell arteritis: involvement of intracranial arteries. Arthritis Rheum 2006; 55: 985–9 [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Smith SD, Galor A, et al. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum 2006; 54: 3306–9 [DOI] [PubMed] [Google Scholar]
- 30.Dasgupta B, Hassan N. Giant cell arteritis: recent advances and guidelines for management. Clin Exp Rheumatol 2007; 25: S62–5 [PubMed] [Google Scholar]
- 31.Both M, Ahmadi-Simab K, Reuter M, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis 2008Has this now been published. Please give volume and page numbers. [DOI] [PubMed] [Google Scholar]
- 32.Karahaliou M, Vaiopoulos G, Papaspyrou S, et al. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther 2006; 8: R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt WA, Kraft HE, Vorpahl K, et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997; 337: 1336–42 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt WA, Seifert A, Gromnica-Ihle E, et al. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology 2008; 47: 96–101 [DOI] [PubMed] [Google Scholar]
- 35.Kuker W. Cerebral vasculitis: imaging signs revisited. Neuroradiology 2007; 49: 471–9 [DOI] [PubMed] [Google Scholar]
- 36.Maffei S, Di Renzo M, Bova G, et al. Takayasu’s arteritis: a review of the literature. Intern Emerg Med 2006; 1: 105–12 [DOI] [PubMed] [Google Scholar]
- 37.Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol 2004; 16: 31–7 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt WA. Takayasu and temporal arteritis. Front Neurol Neurosci 2006; 21: 96–104 [DOI] [PubMed] [Google Scholar]
- 39.Grosset DG, Patterson J, Bone I. Intracranial haemodynamics in Takayasu’s arteritis. Acta Neurochir (Wien) 1992; 119: 161–5 [DOI] [PubMed] [Google Scholar]
- 40.Yoneda S, Nukada T, Tada K, et al. Subclavian steal in Takayasu’s arteritis. A hemodynamic study by means of ultrasonic Doppler flowmetry. Stroke 1977; 8: 264–8 [DOI] [PubMed] [Google Scholar]