Abstract
Objectives:
Cholangiocarcinoma (CCA) has a poor prognosis and its aetiology is inadequately understood. Magnetic resonance spectroscopy (MRS) of bile may provide insights into the pathogenesis of CCA and help identify novel diagnostic biomarkers. The aim of this study was to compare the chemical composition of bile from patients with CCA with that of bile from patients with benign biliary disease.
Methods:
Magnetic resonance spectra were acquired from the bile of five CCA patients and compared with MRS of control bile from patients with benign biliary disease (seven with gallstones, eight with sphincter of Oddi dysfunction [SOD], five with primary sclerosing cholangitis [PSC]). Metabolic profiles were compared using both univariate and multivariate pattern-recognition analysis.
Results:
Univariate analysis showed that levels of glycine-conjugated bile acids were significantly increased in patients with CCA, compared with the benign disease groups (P= 0.002). 7β primary bile acids were significantly increased (P= 0.030) and biliary phosphatidylcholine (PtC) levels were reduced (P= 0.010) in bile from patients with CCA compared with bile from gallstone patients. These compounds were also of primary importance in the multivariate analysis: the cohorts were differentiated by partial least squares discriminant analysis (PLS-DA).
Conclusions:
These preliminary data suggest that altered bile acid and PtC metabolism play an important role in CCA aetiopathogenesis and that specific metabolites may have potential as future biomarkers.
Keywords: bile acids, cholangiocarcinoma, metabonomic, magnetic resonance spectroscopy, phosphatidylcholine
Introduction
Cholangiocarcinoma (CCA) is the leading cause of death from a primary liver tumour in many developed countries.1,2 Although a relatively rare cancer, the incidence and mortality of CCA have been increasing on a global scale for reasons which remain to be determined.1–3 Cholangiocarcinoma has a high mortality and poor prognosis, with a 5-year survival of <5%, as a result of its late clinical presentation. Consequently, most tumours are at an advanced stage at the time of diagnosis.3
Confirmation of CCA diagnosis is often difficult and is currently based on a high index of clinical suspicion, depending on serum levels of the cancer antigen tumour marker, carbohydrate antigen (CA) 19-9, and hepatobiliary imaging, with additional histological or cytological verification if tumour tissue or bile can be reliably obtained. Although in widespread use, serum CA19-9 has a poor sensitivity (50–60%) for the diagnosis of CCA.4,5 Cytological examination of bile also has a poor detection rate for malignant biliary disease.6 Despite improvements in biliary cytological examination using digital image analysis (DIA) and fluorescent in situ hybridization (FISH), the ability to distinguish benign from malignant ductular epithelium remains sub-optimal.7 Tissue biopsies often yield negative findings as a result of marked fibrosis and because no pathognomonic immunohistochemistry exists.
In order to improve diagnosis and prognosis, there is a pressing need to identify biological markers for early disease detection, as well as to enhance the understanding of disease aetiopathogenesis. Sampling of bile for diagnostic purposes has become common clinical practice since the introduction of endoscopic retrograde cholangiopancreatography (ERCP). Exposure of the biliary epithelium to bile and its constituents make bile an ideal biofluid for analytical profiling studies in malignancy of the biliary tract. The analysis of the metabolic profile of bile may therefore provide insights into the pathogenesis of CCA, as well as identifying biochemical disease markers.
Magnetic resonance spectroscopy (MRS) is a non-invasive and sensitive analytical technique which can determine both chemical composition and molecular structural information from non-homogeneous biological samples without a priori knowledge. Specific metabolites of interest may be quantified and analysed using conventional univariate statistical methods; the data may also be analysed using a multivariate, pattern-recognition approach with techniques such as principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA). This has been termed a ‘metabonomic’ or ‘metabolomic’ approach.8In vitro MRS studies on bile have provided information on composition, structure and function, as well as on the metabolism and biliary excretion of xenobiotics.9–11 A major advantage of the technique is that the sample can be studied intact, which allows for subsequent study as required. Recently, MRS studies on bile in patients with pancreatic carcinoma observed an alteration in bile composition and also identified a potential cancer biomarker.12
However, bile collected at ERCP is frequently contaminated by the contrast agent used; two previously published studies investigating the metabolic composition of bile from CCA patients using MRS were flawed by such contamination, which resulted in dominating spectral resonances that may confound the measurement of metabolites.11,13 A secondary major drawback of these studies is that bile samples were analysed from patients with marked cholestasis, which could partially account for the spectral differences observed.
In this study, uncontaminated bile was analysed from non-cholestatic patients. Our aims were to assess and quantify differences in the chemical composition of bile from patients with cancer of the biliary tree, compared with bile from patients with benign biliary disease, using in vitro proton (1H) MRS. The predominant lipid metabolites, bile acids and phosphatidylcholine (PtC), were selected for specific study, owing to their proposed role in cholangiocarcinogenesis.11,14,15 A further aim was to identify potential disease markers in bile in order to improve the diagnosis and prognosis of CCA.
Materials and methods
This study was approved by the Research Ethics Committee of Hammersmith Hospital, London (HHREC no. AM1073/0086). The study conformed to the ethical guidelines outlined in the 1975 Declaration of Helsinki. Written, informed consent was obtained from all patients. Four millilitres of contrast-free bile were obtained at ERCP, after an overnight fast, from 25 patients with malignant and benign conditions of the biliary tree.
MR sample preparation
Bile samples, stored at –80 °C and protected from light, were thawed to room temperature and pH was measured; 600 µl of bile were then transferred to a 5-mm glass nuclear magnetic resonance (NMR) tube. A sealed 4-mm stem coaxial NMR insert, containing 50 µl of an internal reference standard solution (35 µl of sodium trimethylsilyl-[2H4] propionate [TSP] 1 mg/ml dissolved in deuterium oxide), was placed inside the NMR tube.
MR data acquisition
In vitro1H MRS was performed using an ECP+ 500-Mhz NMR spectroscopy system (JEOL Instruments, Tokyo, Japan) and an 11.7-Tesla superconducting magnet. The 1H MR spectra were obtained using a pulse-collect sequence (90-degree pulse angle, acquisition duration 4.4 s, relaxation delay 20 s, 32 data collects), in combination with a water presaturation technique to reduce the dominant water signal. Magnetic resonance spectra were also acquired using the Hahn spin-echo (relaxation delay 2 s, time to echo 135 ms, 64 data collects) to aid metabolite peak assignments caused by the phase inversion of some of the coupled signals.
Spectral interpretation and analysis
The data were processed using the KnowItAll Informatics System Version 7.9 (Bio-Rad Laboratories, Philadelphia, PA, USA). Free induction decays were zero-filled, and multiplied by an exponential line-broadening function of 0.8 Hz and then subjected to Fourier transformation. The MR spectra were manually phased and a baseline correction was applied. Peaks were assigned on the basis of published literature relative to TSP (δ= 0.00 ppm).16 The cluster of peaks δ 0.70–1.10 ppm was assigned as one region with contributions from H-19 bile acid proton, H-21 bile acid proton and cholesterol. The peaks δ 3.09 ppm and δ 3.57 ppm were assigned to the taurine moiety in taurine-conjugated bile acids. The peak δ 3.71 ppm was assigned to the glycine moiety of glycine-conjugated bile acids. The peaks δ 3.24 ppm and δ 1.29 ppm were the most prominent resonances attributable to PtC, and assigned to the choline head group and (CH2)n chain, respectively.
For relative quantification, peak areas were manually integrated and expressed in arbitrary units (u), as a percentage ratio to the total spectral signal (range δ 10.00–0.20 ppm). The residual water signal (range δ 5.20–4.50 ppm) was excluded from the analysis.
Univariate statistical analysis was carried out using spss for Windows, Version 14.0 (SPSS, Inc., Chicago, IL, USA). The non-parametric Kruskal–Wallis test was used for comparisons of metabolite concentrations between the different disease groups. The Mann-Whitney U-test was used for comparisons between two independent disease groups.
Multivariate pattern-recognition analysis
Using an ‘intelligent bucketing’ algorithm in KnowitAll Version 7.9, each spectrum was divided into smaller regions (bins), of 0.04 ± 0.02 ppm, ensuring no overlap across binned regions. These regions were integrated and normalized to the total spectral signal and the data were mean-centred before multivariate analysis.
Principal component multivariate analysis
Principal component analysis is an ‘unsupervised’ technique in that it does not rely on a priori knowledge of the cohort to which samples belong. It facilitates the visualization of a multivariate dataset through data reduction, to identify and visualize inherent patterns of variance within the dataset. The first principal component is essentially a linear combination of the original variables, explaining the maximum amount of variance in the dataset; the second principal component describes the second greatest, and so on. Each sample is represented in the PCA scores plot generated; inspection of the corresponding loadings plot allows visualization of the metabolites responsible for the variation seen in the scores plot.
Partial least squares discriminant analysis
Data were then analysed by PLS-DA using Pirouette Version 4.0 (Infometrix, Inc., Woodinville, WA, USA). This linear regression technique relates the NMR spectroscopic variables, corresponding to metabolites, to the class membership of the sample, allowing the identification and visualization of metabolites responsible for differences between classes. It is thus ‘supervised’. The data filtering technique of orthogonal signal correction (OSC) was used to remove variation in the spectra not directly related to the physiological condition being studied, and to minimize the possible influence of inter-individual variation.17,18 The discriminatory power of each model was validated using a cross-validation technique whereby each sample in turn was excluded from the analysis, a model was created from the other samples and the class membership of the excluded sample was predicted (‘leave-one-out’ cross-validation).19,20
Results
Patient demographics
Five inoperable CCA patients (four male, one female; mean age 73 years, range 58–82 years) were recruited. Three had hilar bismuth grade 3 tumours and bile was collected downstream of the lesion. Two had distal tumours and bile was collected upstream. Of the five CCA patients, only two had an elevated CA19-9 (240 U/ml and 26 954 U/ml, respectively). Histological diagnosis was confirmed in two patients. Diagnosis in the other three patients was based on clinical presentation and imaging findings. Twenty patients with non-malignant biliary disease (seven with choledocholithiasis [gallstones], eight with sphincter of Oddi dysfunction [SOD] and five with primary sclerosing cholangitis [PSC] with biliary strictures) were included in the study. Importantly, bile samples were stratified according to ambient serum bilirubin levels. The mean serum bilirubin level for all the bile samples analysed was 16.2 mmol/l (standard deviation 13.2 mmol/l). The normal reference range was 3–17 mmol/l. The clinico-pathological details of patients are summarized in Table 1. The age and gender of the cohort were not normally distributed. The four groups were therefore analysed separately and in combination.
Table 1.
Clinico-pathological details of patients and bile samples (mean and range). Kruskal–Wallis tests showed significant differences between the four disease groups according to age (P= 0.011) and gender (P < 0.001)
| CCA | Gallstones | SOD | PSC | |
|---|---|---|---|---|
| Age, years | 73 (58–82) | 64 (45–79) | 47 (27–62) | 45 (28–66) |
| Sex, M : F | 4 : 1 | 0 : 7 | 0 : 8 | 5 : 0 |
| Bilirubin, µmol/l | 16 (8–31) | 22 (5–57) | 10 (2–33) | 19 (8–42) |
| ALP, IU/l | 637 (69–2423) | 222 (76–426) | 96 (50–130) | 242 (63–325) |
| pH of bile | 7.80 (7.26–8.40) | 7.80 (7.50–8.10) | 7.78 (6.8–8.45) | 8.02 (7.50–8.70) |
CCA, cholangiocarcinoma; SOD, sphincter of Oddi dysfunction; PSC, primary sclerosing cholangitis; M, male; F, female; ALP, alkaline phosphatase
Proton NMR spectroscopy of bile samples
The human bile 1H MR spectrum is dominated by broad resonances arising from bile acids, phospholipids and cholesterol. A representative 1H MR spectrum of bile from a patient with CCA is shown in Fig. 1. The region of interest δ 3.00–4.00 ppm has been expanded to illustrate the assignment to resonances arising from taurine- and glycine-conjugated bile acids (Fig. 2).
Figure 1.
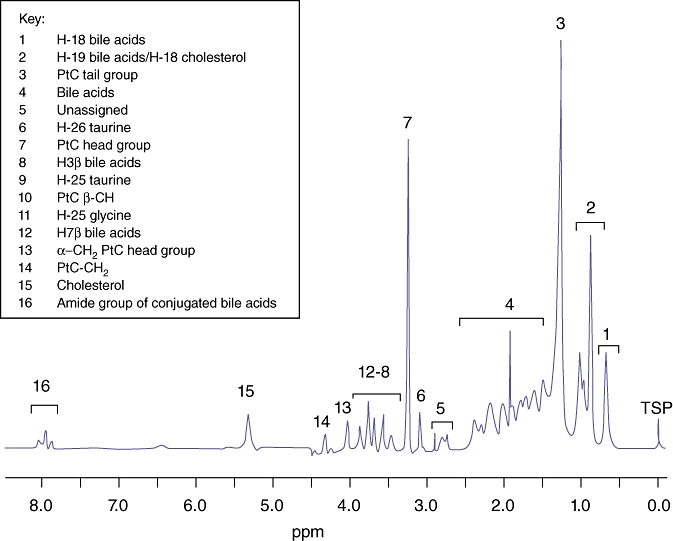
Typical 500 MHz proton MR spectrum of bile from a patient with cholangiocarcinoma illustrating the predominant metabolites
Figure 2.
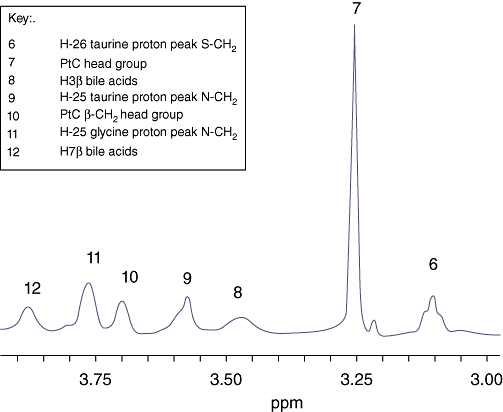
Relevant peak assignments in the proton MR spectrum of CCA bile in the expanded region 3.00–4.00 ppm
Univariate analysis
Biliary levels of glycine-conjugated bile acids (δ 3.71 ppm, relative to the total spectral integral) were significantly higher in patients with CCA than in the benign biliary disease groups (P= 0.002, Kruskal–Wallis test). The level of these glycine-conjugated bile acids was higher in the CCA group than in patients with SOD and PSC, at a median of 1.00 u (interquartile range [IQR] 0.78–1.59 u) vs. 0.70 u (IQR 0.47–0.85 u) (P= 0.030) and 0.56 u (IQR 0.44–0.74 u) (P= 0.056), respectively. By contrast, there was no difference in the levels of taurine-conjugated bile acids (δ 3.57 ppm) across the patient groups (P= 0.12, Kruskal–Wallis test). However, the relative ratio of glycine- to taurine-conjugated bile acids was greater in the CCA group, at 1.11, than in the other patient groups, in which it was reduced to 0.82 in the SOD group, 0.64 in the PSC group and 0.97 in the gallstones group. Median levels of the 7β primary bile acids (cholic acid and chenodeoxycholic acid, δ 3.45 ppm) were significantly elevated in the bile of patients with CCA vs. that of patients with gallstones: median 0.76 u (IQR 0.56–1.32 u) vs. 0.44 u (IQR 0.09–0.71 u) (P= 0.030). Levels of the peaks assigned to the choline moiety of the PtC head group δ 3.24 ppm and the (CH2)n tail group δ 1.29 ppm were significantly lower in bile from patients with CCA compared with bile from the gallstone control group, at a median of 4.03 u (IQR 3.94–4.66 u) vs. 4.61 u (IQR 4.32–6.09 u) (P= 0.018) and a median of 24.99 u (IQR 22.68–27.06 u) vs. 29.08 u (IQR 25.10–34.61 u) (P= 0.010), respectively.
Multivariate pattern-recognition analysis
Principal component analysis of all samples revealed no significant outliers. The analyses demonstrated the most notable clustering when CCA samples were compared with SOD samples (Fig. 3). Models were then constructed using OSC-PLS-DA to investigate the ability of this technique to distinguish between the cohorts studied. Using this method, with ‘leave-one-out’ cross-validation, the CCA samples were discriminated from all 20 non-malignant samples with a sensitivity of 80%, specificity of 95%, positive predictive value of 80% and negative predictive value of 95% (Table 2) (one CCA sample and one non-cancer patient were incorrectly predicted). The major metabolites contributing to this separation were PtC, H-18 bile acids (resonance δ 0.70 ppm) and taurine-conjugated bile acids.
Figure 3.
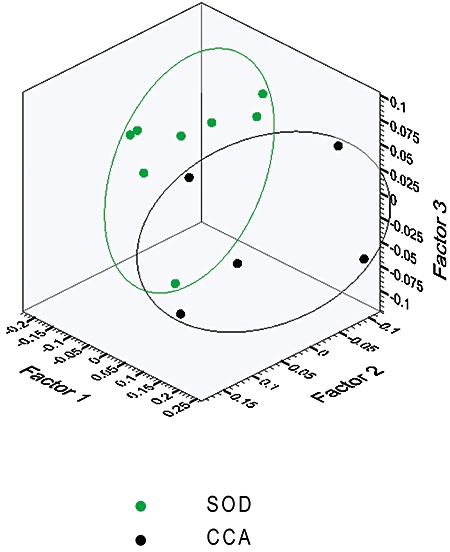
Principal component analysis demonstrated the most notable clustering when cholangiocarcinoma (CCA) samples were compared with sphincter of Oddi dysfunction (SOD) samples
Table 2.
Predictive abilities of the orthogonal signal correction partial least squares discriminant analysis (OSC-PLS-DA) models constructed
| CCA vs. all non-malignant | CCA vs. SOD | CCA vs. PSC | CCA vs. GS | |
|---|---|---|---|---|
| Sensitivity | 80% | 80% | 100% | 100% |
| Specificity | 95% | 100% | 100% | 86% |
| PPV | 80% | 100% | 100% | 83% |
| NPV | 95% | 89% | 100% | 100% |
CCA, cholangiocarcinoma; SOD, sphincter of Oddi dysfunction; PSC, primary sclerosing cholangitis; GS, gallstones; PPV, positive predictive value; NPV, negative predictive value
Other OSC-PLS-DA models were also constructed, comparing CCA with each of the control groups: the excellent discrimination achieved is demonstrated in Table 2, which shows the predictive abilities of the models constructed. The major discriminatory metabolites for these models were: glycine- and taurine-conjugated bile acids (CCA vs. PSC); PtC and taurine-conjugated bile acids (CCA vs. gallstones), and PtC (CCA vs. SOD).
Discussion
In this MR study, 25 contrast-free, non-cholestatic bile samples were analysed and compared using 1H MRS. Previous studies have been hampered by the contamination of samples with contrast agent, and by the marked cholestastic nature of the bile samples studied.11,13 Despite a small and heterogeneous sample population, our data demonstrated important differences in the biliary metabolic profile in comparisons between malignant and benign biliary disease. The patient study groups could be clearly distinguished using OSC-PLS-DA. The major findings of this study were significant differences in the levels of primary bile acids, their glycine conjugates and PtC in bile from patients with CCA compared with bile from non-malignant control groups. When analysed using univariate statistical methods, normalized signal levels of glycine-conjugated bile acids were significantly higher in CCA bile, compared with controls; these metabolic differences contributed strongly to the PLS-DA models and thus corroborated the findings. The greatest difference was seen when comparing CCA bile with SOD bile. It is also worth noting that the ratio of glycine- to taurine-conjugated bile acids was greater in the CCA bile compared with control bile. The ratio of conjugated to unconjugated bile acids, and the ratio of glycine to taurine bile acid conjugates, vary in specific hepatobiliary diseases and cholestasis,21,22 although until now there have been no published reports quantifying bile acid conjugates in bile from patients with CCA.
In vitro studies have shown that bile acids play a role in CCA aetiopathogenesis and, furthermore, bile acid abnormalities and toxicity have been implicated in other biliary diseases, such as primary biliary cirrhosis and PSC, in addition to colorectal carcinoma.23–25 The in vitro MR data presented in this study suggest that primary bile acids and their glycine conjugates may be elevated in CCA bile in the absence of clinical cholestasis and therefore these agents are potential disease biomarkers, requiring further evaluation in larger-scale studies.
Multivariate analysis highlighted PtC as the major metabolite in bile distinguishing the CCA group from the disease control groups. Levels of PtC were significantly lower in CCA bile compared with bile from patients with gallstones. We have previously shown differences in phospholipid metabolites that may help to distinguish between malignant and non-malignant causes of pancreaticobiliary obstruction; a reduced PtC resonance was seen in bile from the majority of patients with hepatobiliary cancer compared with bile from patients with non-malignant indications for ERCP.11 This finding has also more recently been confirmed by Albiin and co-workers in a comparison of bile from patients with CCA with bile from patients with PSC.13 An in vivo NMR study of bile after biliary decompression suggested that the altered metabolism of phosphorus-containing metabolites may be a useful indicator of malignancy in jaundiced patients with hepatobiliary disease.26 Phosphatidylcholine, a cytoprotective and dominant biliary phospholipid, is synthesized in the hepatocyte and transported into the biliary canaliculus by the flippase multidrug resistant protein 3 (MDR3).27 Animal studies have shown that MDR2 knockout mice (mdr 2−/−) develop CCA after prolonged bile acid exposure.28 In humans, genetic mutations in the ABCB4 gene (which encodes the protein MDR3) lead to reduced or absent phospholipid export into the bile, thus exposing the biliary epithelium to ‘toxic’ bile, and it has been postulated that these individuals may be predisposed to developing CCA.23,29
In conclusion, these preliminary data have highlighted the potential importance of bile acid and PtC metabolism in patients with CCA. These findings suggest important alterations in biomolecular mechanistic pathways in CCA and require further evaluation. The putative role of bile acids as potential disease biomarkers is novel and requires further validation in larger studies in order to improve current diagnostic techniques aimed at distinguishing malignant and benign biliary pathology. This remains a major challenge for the clinician.
Acknowledgments
The authors are grateful to the National Institute for Health Research (NIHR) Biomedical Facility at Imperial College London for infrastructure support and to the staff of Hammersmith Hospital Endoscopy Department for the collection of bile samples. The study was supported by generous grants from the Alan Morement Memorial Fund (http://www.ammf.org.uk), Imperial College London Healthcare Trustees (London, UK) and the Broad Medical Research Program (Los Angeles, CA, USA). AWS's salary was supported by the Arthur and Violet Payne Memorial Fund of the Hammersmith Hospital League of Friends, London, UK and by a charitable donation from the Gastroenterology Research Trust established by DSB and AVT. We are also grateful for a charitable donation from Mr and Mrs Barry Winter towards the running costs of this study.
Conflicts of interest
None declared.
References
- 1.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsson E, Kilander A, Olsson R. CA19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999;19:501–508. doi: 10.1111/j.1478-3231.1999.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 5.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 6.Harewood GC, Baron TH, Stadheim LM, Kipp BR, Sebo TJ, Salomao DR. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004;99:1464–1469. doi: 10.1111/j.1572-0241.2004.30845.x. [DOI] [PubMed] [Google Scholar]
- 7.Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287–1296. doi: 10.1111/j.1365-2036.2006.02900.x. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 9.Cox IJ. Nuclear magnetic resonance spectroscopy. Br J Hosp Med. 1988;40:165. [PubMed] [Google Scholar]
- 10.Cox IJ, Sharif A, Cobbold JF, Thomas HC, Taylor-Robinson SD. Current and future applications of in vitro magnetic resonance spectroscopy in hepatobiliary disease. World J Gastroenterol. 2006;12:4773–4783. doi: 10.3748/wjg.v12.i30.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan SA, Cox IJ, Thillainayagam AV, Bansi DS, Thomas HC, Taylor-Robinson SD. Proton and phosphorus-31 nuclear magnetic resonance spectroscopy of human bile in hepatopancreaticobiliary cancer. Eur J Gastroenterol Hepatol. 2005;17:733–738. doi: 10.1097/00042737-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bezabeh T, Ijare OB, Albiin N, Arnelo U, Lindberg B, Smith IC. Detection and quantification of D:-glucuronic acid in human bile using (1)H NMR spectroscopy: relevance to the diagnosis of pancreatic cancer. MAGMA. 2009;22:267–275. doi: 10.1007/s10334-009-0171-5. [DOI] [PubMed] [Google Scholar]
- 13.Albiin N, Smith IC, Arnelo U, Lindberg B, Bergquist A, Dolenko B, et al. Detection of cholangiocarcinoma with magnetic resonance spectroscopy of bile in patients with and without primary sclerosing cholangitis. Acta Radiol. 2008;49:855–862. doi: 10.1080/02841850802220092. [DOI] [PubMed] [Google Scholar]
- 14.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 16.Gowda GA, Ijare OB, Somashekar BS, Sharma A, Kapoor VK, Khetrapal CL. Single-step analysis of individual conjugated bile acids in human bile using 1H NMR spectroscopy. Lipids. 2006;41:591–603. doi: 10.1007/s11745-006-5008-7. [DOI] [PubMed] [Google Scholar]
- 17.Westerhuis JA, de Jong S, Smilde AK. Direct orthogonal signal correction. Chemometr Intell Lab Syst. 2001;56:13–25. [Google Scholar]
- 18.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, et al. Rapid and non-invasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 19.Mahadevan S, Shah SL, Marrie TJ, Slupsky CM. Analysis of metabolomic data using support vector machines. Anal Chem. 2008;80:7562–7570. doi: 10.1021/ac800954c. [DOI] [PubMed] [Google Scholar]
- 20.Williams HR, Cox IJ, Walker DG, North BV, Patel VM, Marshall SE, et al. Characterization of inflammatory bowel disease with urinary metabolic profiling. Am J Gastroenterol. 2009;104:1435–1444. doi: 10.1038/ajg.2009.175. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa H, Nakashima T, Inaba K, Mitsuyoshi H, Nakajima Y, Sakamoto Y, et al. Proton magnetic resonance assay of total and taurine-conjugated bile acids in bile. J Lipid Res. 1999;40:1920–1924. [PubMed] [Google Scholar]
- 22.Ijare OB, Bezabeh T, Albiin N, Arnelo U, Bergquist A, Lindberg B, et al. Absence of glycochenodeoxycholic acid (GCDCA) in human bile is an indication of cholestasis: a 1H MRS study. NMR Biomed. 2009;22:471–479. doi: 10.1002/nbm.1355. [DOI] [PubMed] [Google Scholar]
- 23.Komichi D, Tazuma S, Nishioka T, Hyogo H, Chayama K. Glycochenodeoxycholate plays a carcinogenic role in immortalized mouse cholangiocytes via oxidative DNA damage. Free Radic Biol Med. 2005;39:1418–1427. doi: 10.1016/j.freeradbiomed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329–3340. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon RM. NMR studies of phospholipid metabolism in hepatic lymphoma. NMR Biomed. 1998;11:370–379. doi: 10.1002/(sici)1099-1492(1998110)11:7<370::aid-nbm514>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 28.Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of non-suppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 29.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]


