Abstract
Background:
We have previously shown that galantide, a non-specific galanin receptor antagonist, ameliorates acute pancreatitis (AP) induced in mice. Octreotide, a somatostatin analogue, has been used in the treatment of AP with inconsistent outcomes. This study set out to compare the efficacy of a combined treatment of galantide and octreotide with the efficacy of each agent individually in experimental AP.
Methods:
Acute pancreatitis was induced in mice with 7-hourly caerulein injections. Galantide and/or octreotide were co-administered with each caerulein injection commencing with the first injection. Control animals received galantide, octreotide or saline alone. Pancreata were harvested for histological examination and estimation of myeloperoxidase (MPO) activity. Plasma amylase and lipase activities were measured.
Results:
Galantide significantly reduced AP-induced hyperenzymaemia by 39–45%. Octreotide alone, or in combination with galantide, did not significantly alter AP-induced hyperenzymaemia. Plasma enzyme activity in the control groups was comparable with pre-treatment activity. Galantide and octreotide administered individually reduced MPO activity by 79% and 50%, respectively; however their combination was without effect. Galantide, octreotide and their combination significantly reduced the percentage of abnormal acinar cells by 28–45%.
Conclusions:
Treatment with galantide alone ameliorated most of the indices of AP studied, whereas treatment with octreotide reduced pancreatic MPO activity and acinar cell damage. Combining the two peptides appears to negate their individual benefits, which suggests an interaction in their mechanism of action.
Keywords: peptide, somatostatin, inflammation
Introduction
Acute pancreatitis (AP) is a disease with high morbidity and mortality and a complex pathophysiology.1 Despite extensive investigation, to date there exists no specific medical treatment for AP.2 Given the numerous factors involved in the pathogenesis of AP, it appears that successful treatment of this disease may depend upon combining more than one therapeutic agent and on each agent targeting apparently different steps in AP. In this study, we investigated two possible therapeutic agents.
We have previously demonstrated that galanin is involved in the pathogenesis of caerulein-induced AP in mice and the Australian possum.3–7 Mice carrying a loss-of-function mutation in the galanin gene (galanin knockout, Gal KO) have less severe caerulein-induced AP.5 Galantide, a non-specific galanin receptor antagonist, is a peptide consisting of fragments of galanin and substance P (galanin-[1-12]-Pro-substance P-[5-11] amide).8 We and others have previously shown that galantide is able to antagonize the effects of galanin on pancreatic function6,8 and to ameliorate caerulein-induced AP in mice and the Australian possum.3–7
Octreotide, a somatostatin analogue, has been used clinically as a treatment for AP.9 However, its beneficial results in AP have been inconsistent: some studies have shown an improvement in all parameters studied (plasma enzyme reduction, inflammatory markers, histological benefit),9–12 whereas others suggest no benefit at all.13–15 These inconsistencies have been ascribed to the application of different animal models of AP, different doses of octreotide and different treatment protocols.
Peptide therapeutics is a growing field16 and the application of peptides in the treatment of AP has been very limited. Peptides offer several advantages as therapeutic agents, particularly the potential for minimal central side-effects.16 Galantide is one of several galanin receptor antagonists that are chimeric peptides.8 Similarly, octreotide is an octapeptide that mimics somatostatin pharmacologically.
In this study, we tested the hypothesis that a somatostatin analogue in combination with galantide, delivered at the induction of AP, would be a more effective treatment for AP than either agent alone. We aimed to investigate the effects of octreotide alone and in combination with galantide on the biochemical (plasma amylase and lipase and pancreatic myeloperoxidase [MPO]) and histological indices of AP induced by caerulein hyperstimulation in mice.
Materials and methods
All experiments were approved by the Animal Welfare Committee of Flinders University. For these studies we used the caerulein mouse model originally described by Niederau et al.17 Male Swiss mice aged 6–8 weeks and weighing 25–30 g were used. The mice were maintained in a facility with a controlled temperature (21–24 °C) and lighting (12 : 12 h light : dark cycle). Standard laboratory chow and drinking water were provided ad libitum. A minimum period of 2 days was allowed for animals to acclimatize before any experimental manipulations were undertaken. Mice were fasted for 12 h before the induction of AP and had free access to water.
The mice were allocated to seven groups (Table 1). On the day prior to AP induction, a blood sample was collected by retro-orbital sinus bleeding under anaesthesia and used to measure the basal plasma enzyme activity.3,5,18
Table 1.
Experimental groups: protocols and animal numbers
| Group | Protocol | Mice per group |
|---|---|---|
| 1 | Control, no AP (saline) | 4 |
| 2 | Control, AP | 9 |
| 3 | GT alone | 6 |
| 4 | OT alone | 4 |
| 5 | AP + GT | 10 |
| 6 | AP + OT | 9 |
| 7 | AP + OT + GT | 12 |
AP, acute pancreatitis; GT, galantide (dose = 66 µg/kg/h); OT, octreotide (dose = 250 µg/kg/h)
Peptide stock solutions were prepared in 0.01% bovine serum albumin (BSA; Sigma-Aldrich Corp., St Louis, MO, USA) in saline. On the day of the experiment, AP was induced by seven intraperitoneal (i.p.) injections of caerulein (50 µg/kg) (American Peptide Co., Sunnyvale, CA, USA) administered in 150 µl of 0.9% NaCl at hourly intervals over 0–6 h. Each mouse was given a single subcutaneous injection of 100 µg/kg of buprenorphine-HCl (Temgesic Inject; Reckitt Benckiser Health Care UK, Hull, UK) at the induction of AP. This dose provided analgesia for the duration of the experimental period.18
Treatment protocols
Galantide (Bachem AG, Bubendorf, Switzerland) at a dose of 66 µg/kg was co-administered with each injection of caerulein commencing with the first caerulein injection (Fig. 1). Octreotide (American Peptide Co.) at a dose of 250 µg/kg was co-administered with caerulein at hourly intervals over 6 h (for a total of seven injections). The i.p. injection dose of octreotide (250 µg/kg) was previously used by Yuan et al. in a caerulein model of AP in mice.19 In the experiments in which the two peptides were combined, each peptide, including caerulein, was administered separately.
Figure 1.

Diagram illustrating the treatment protocol for administration of antagonists. The timeline shows doses of caerulein, galantide (GT) and octreotide (OT). GT and OT were administered at hourly intervals for 6 h starting from the first injection of caerulein. Mice were killed at 12 h
Mice were killed 6 h after the final caerulein injection under anaesthesia as previously described.3,5,18 Each pancreas was then harvested and divided into two pieces; one piece was placed in 10% buffered formalin solution for subsequent histological examination and the other piece was quickly blotted on gauze to remove excess blood and stored at −70 °C for subsequent MPO extraction and assay.18,20,21 Myeloperoxidase activity was expressed as IU per mg of protein. The plasma samples were assayed for amylase and lipase activity using an enzymatic colorimetric assay (Roche/Hitachi, Roche Diagnostics GmbH, Mannheim, Germany) using a Roche/Hitachi Modular Analyser (Hitachi High-Technologies Corp., Tokyo, Japan).
Histological analysis of pancreatic damage
Fixed pancreatic tissue was processed for standard haematoxylin and eosin histology. The histological analysis was performed using a point-counting morphometry method as previously described.3,18,22 The number of abnormal acinar cells was expressed as the percentage of total acinar cells counted. Interstitial space was expressed as a proportion of the number of points counted. Group data were expressed as mean ± standard error (SE) (n= 4–12 mice).
Statistical analysis
Statistical analysis was performed using spss Version 11.5 (SPSS, Inc., Chicago, IL, USA). The Mann–Whitney U-test was used and a P-value of <0.05 was regarded as significant.
Results
Plasma enzymes
The induction of AP produced an approximately six-fold increase in plasma amylase activity and an approximately nine-fold increase in plasma lipase activity (Fig. 2A, B). Treatment with galantide reduced the caerulein-induced elevation in plasma amylase and lipase activities by 39% and 45% (P < 0.007 and P < 0.009 compared with AP alone), respectively. Neither octreotide alone nor the combination of galantide and octreotide reduced the caerulein-induced hyperenzymaemia. Neither agent alone nor saline altered plasma enzyme activity compared with basal activity.
Figure 2.

Effects of administration of galantide (GT) and octreotide (OT) on acute pancreatitis (AP)-induced (A) hyperamylasaemia and (B) hyperlipasaemia. A significant increase was noted in plasma amylase activity following the induction of AP. Treatment of AP with GT alone reduced plasma amylase and lipase activity. Treatment of AP with OT alone and GT + OT had no effect on AP-induced hyperenzymaemia. Treatment with either GT or OT alone had no effect on plasma enzyme activity. □ represents basal enzyme activity for each group;  represents post-treatment enzyme activity. Data are expressed as IU/l and are presented as mean + standard error (n= 4–12). *P < 0.05 compared with the saline group; †P < 0.05 compared with the AP-alone group
represents post-treatment enzyme activity. Data are expressed as IU/l and are presented as mean + standard error (n= 4–12). *P < 0.05 compared with the saline group; †P < 0.05 compared with the AP-alone group
Pancreatic MPO
The induction of AP resulted in a 28-fold increase in pancreatic MPO activity compared with saline alone (Fig. 3). Treatment with galantide or octreotide significantly reduced pancreatic MPO activity compared with AP alone by 79% and 50% (both P < 0.002), respectively. The reduction in MPO activity by galantide was significantly greater than that caused by octreotide (P < 0.011). Surprisingly, the combination of the two agents did not affect MPO activity compared with AP alone. Pancreatic MPO activity in the galantide-alone group was low but significantly greater (P < 0.011) than that in the saline group, whereas MPO activity in the octreotide-alone group did not significantly differ from that in the saline group (Fig. 3).
Figure 3.

Effects of administration of galantide (GT) and octreotide (OT) on acute pancreatitis (AP)-induced pancreatic myeloperoxidase (MPO) activity. Treatment of AP with either GT or OT alone reduced MPO activity compared with the AP-alone group. Treatment of AP with GT + OT did not reduce MPO activity compared with the AP-alone group. Treatment with GT alone did alter pancreatic MPO activity compared with the saline group, but treatment with OT alone did not. MPO activity is expressed as IU/mg protein. Data are presented as mean + standard error (n= 4–12). *P < 0.05 compared with the saline group; †P < 0.05 compared with the AP-alone group
Pancreatic damage
As expected, histological assessment of pancreatic tissue revealed abnormal acinar cells and interstitial oedema in the mice with AP (Figs 4A, B and 5). In the AP-alone group, 63% of the acinar cells were abnormal (Figs 4A, 5). Treatment with galantide significantly reduced the percentage of abnormal acinar cells by 34% (P < 0.001) compared with the AP-alone group. Similarly, octreotide treatment significantly reduced the percentage of abnormal acinar cells by 45% compared with the AP-alone group (P < 0.001). Treatment with the combination galantide + octreotide also significantly reduced the percentage of abnormal acinar cells by 28% (P < 0.001); this reduction was significantly less than that caused by AP treatment with octreotide alone (P < 0.023), but did not differ from that produced with galantide alone (P < 0.323). The galantide- or octreotide-alone control groups displayed a minor but statistically significant increase in the percentage of abnormal acinar cells compared with the saline group.
Figure 4.

Acute pancreatitis (AP)-induced pancreatic damage showing percentages of (A) abnormal pancreatic acinar cells and (B) pancreatic interstitial oedema in different experimental groups. Induction of AP resulted in a significant increase in the percentage of abnormal acinar cells. Treatment with galantide (GT) or octreotide (OT), individually and in combination, reduced the percentage of abnormal acinar cells compared with that in the AP-alone group. The OT- or GT-alone control groups showed a small but statistically significant increase in the percentage of abnormal pancreatic acinar cells compared with the saline group. The saline group displayed no abnormal pancreatic acinar cells. Only the treatment with OT significantly reduced pancreatic interstitial oedema compared with AP alone. Oedema in the OT- or GT-alone control groups did not significantly differ from that in the saline group. Data are presented as mean + standard error (n= 4–12). *P < 0.05 compared with the saline group; †P < 0.05 compared with the AP-alone group
Figure 5.
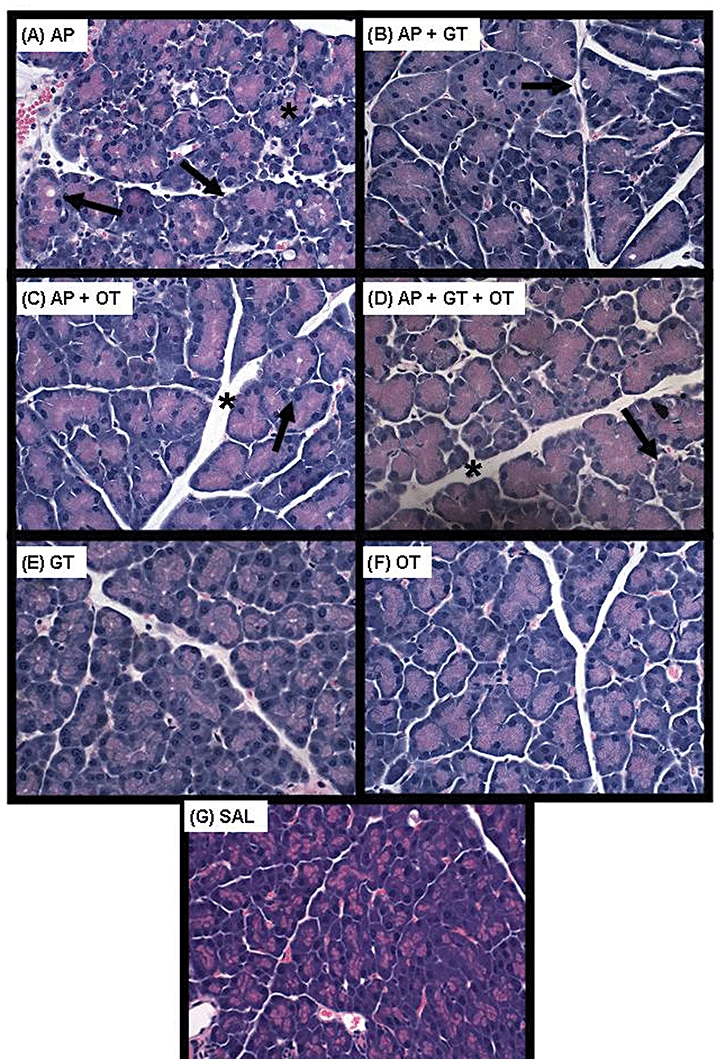
Representative haematoxylin and eosin stained photomicrographs of pancreatic sections for the various groups. (A) The induction of acute pancreatitis (AP) resulted in abnormal acinar cells (arrow) and interstitial oedema (*). Co-administration of (B) galantide (GT) (66 µg/kg), (C) octreotide (OT) (250 µg/kg) or (D) the combination of the two peptides with caerulein (AP + GT + OT) resulted in a significant reduction in abnormal acinar cells. Normal acinar cell architecture was seen following administration of (E) GT alone (66 µg/kg), (F) OT alone (250 µg/kg) and (G) saline (SAL). Original magnification ×40
Interstitial oedema was increased about three-fold in the AP-alone group compared with the saline control group (P < 0.005) (Figs 4B, 5). Treatment with octreotide alone significantly reduced the interstitial oedema compared with AP alone by 29% (P < 0.04), whereas treatment with galantide alone or the combination of the two peptides failed to alter the oedema compared with AP alone.
Discussion
This study demonstrated that treatment of AP with either galantide or octreotide, administered at the induction of AP, reduced most of the elevated markers of AP measured, including pancreatic damage. Interestingly, treatment with the combination of the two agents was much less effective.
To date there exists no specific treatment for AP. This may reflect the complexity of its pathophysiology, which involves multiple factors. Another important determinant of the course of the disease which may impact the success of any treatment is the time delay from the actual onset of the disease to the patient's presentation to the hospital. Although 50% of patients present in the first 12 h after the onset of disease, up to 30% of patients may present after 24 h of symptoms.23 Hence any strategy targeting a single agent or pathway may be insufficient to control the progress of the disease. An alternative strategy is to target multiple steps in the pathogenesis of the disease by combining more than one therapeutic agent. In this study, we attempted to explore for the first time the benefit of administering the two peptides galantide and octreotide (previously shown individually to ameliorate the indices of AP) at the induction of AP. The impact of our findings is two-fold: firstly, this research may provide us with a potential novel therapy in the prevention of endoscopic retrograde cholangiopancreatography (ERCP)-induced AP, and, secondly, it provides the impetus for further experiments exploring the role of the combination in a delayed setting in AP (a situation that closely mimics the actual clinical scenario in AP arising from most other causes).
Galantide has been shown to ameliorate caerulein-induced severe AP in mice and in the Australian possum.3,5–7 Our studies to date indicate that galanin acts at several steps in the induction and progression of AP. The present study confirms our previous reports of the beneficial effects of galantide treatment in a mild model of AP. In the present study, galantide administered at AP induction ameliorated most of the indices of AP, except oedema. The effect of galantide on plasma amylase activity is consistent with an inhibition of the co-stimulatory/potentiating effect of galanin on caerulein-stimulated amylase secretion.24
Ischaemia involving the pancreatic microvasculature is a key trigger in the progression of pancreatic damage after the onset of AP.25 Galanin has been shown to induce pancreatic microvascular vasoconstriction6 and galantide has been shown to be vasodilatory, which may prevent the development of pancreatic necrosis. Moreover, galanin is known to inhibit the secretion of insulin.8 Therefore, it is also possible that galantide, by antagonizing galanin's inhibition of insulin release, may increase local concentrations of insulin. Insulin is important for the regeneration of acinar cells following AP26 and potentiates the expression of heat shock proteins including hsp 70.27,28 Insulin is also known to induce actin-cytoskeletal reorganization in order to mediate a wide variety of biological actions, including cell growth, migration and metabolism.29
A recent study by Schmidhuber et al.30 reported that adult mice carrying a loss-of-function mutation in the galanin gene (Gal KO) demonstrated an absence of the normal neurogenic inflammatory response upon treatment of the skin with either noxious heat or the vanilloid receptor 1 agonist capsaicin. These and other findings reported by the same authors suggest a role for galanin in neurogenic inflammation in murine skin.30 Neurogenic inflammation is a contributor to AP.31 Galantide may ameliorate AP by attenuating neurogenic inflammation by virtue of its antagonism of galanin's effects in the pancreas.3
Octreotide, a long-acting somatostatin analogue, has been extensively studied in relation to AP. The rationale behind the use of octreotide as a treatment for AP is based on several observations, including its inhibitory effects on pancreatic exocrine secretion32 and its ability to reduce the degree of leukocyte infiltration and focal areas of pancreatic tissue necrosis in experimentally induced AP.12 Its ability to increase the tone of the sphincter of Oddi has been used in the prevention and treatment of post-ERCP pancreatitis, in which it is believed to reduce the reflux of duodenal contents into the pancreatic duct.33
The effects of octreotide on pancreatic exocrine secretion have been studied in animals9–15 as well as humans34,35 in terms of its potential as a therapeutic agent for AP. The proposed mechanisms by which octreotide was shown to ameliorate AP in previous studies included the inhibition of basal and the stimulation of exocrine pancreatic secretion,36 by which it ‘puts the pancreas to rest’, reduces the degree of leukocyte infiltration and limits focal areas of pancreatic tissue necrosis in experimentally induced AP,12 the reduction of lipid peroxidation,37 and the production of histological changes consistent with organization and healing in established cases of AP.9 However, reports on the action of somatostatin and octreotide on pancreatic exocrine secretion are inconsistent and reflect the use of different species and concentrations and the complexity of the system under study.9,12,38,39
Our current findings of a lack of effect of octreotide on plasma enzyme levels in a caerulein model of AP are comparable with those of previous studies using a similar model.38,39 However, the mechanisms responsible are unclear. One possible mechanism could be that offered by Jenkins et al.40 These authors showed that following the initial inhibition of pancreatic enzyme and overall juice secretion by octreotide, continued administration of the drug resulted in a steep increase (‘escape phenomenon’) in the enzyme level despite a continued inhibition of overall juice secretion within 4–5 h. This ‘escape phenomenon’ may reflect the desensitization of the somatostatin receptors involved in the regulation of pancreatic amylase secretion. Using a mouse pancreatic lobule preparation under experimental conditions equivalent to those used in the present study, we recently showed that galanin potentiated amylase secretion induced by supramaximal concentrations of caerulein and that this response was partly mediated by endogenous somatostatin, indicating an interaction between galanin and somatostatin.24 Thus the caerulein-induced release of endogenous somatostatin may occupy the somatostatin receptors regulating acinar cell secretion, thereby preventing the binding of octreotide and consequently resulting in no change in AP-induced hyperenzymaemia with octreotide treatment. Another explanation may involve the inability of octreotide to overcome cholecystokinin (CCK)-stimulated amylase secretion, as reported by Garvin et al.41
In the present study we also noted an unexpected reduction in the efficacy of the agents when used in combination, suggesting interactions at one or more sites. As outlined above, interactions between galanin and somatostatin can occur in the presence of supramaximal concentrations of caerulein. Another explanation may relate to the reported potentiation of CCK-stimulated amylase secretion by somatostatin,42 which could mask or negate the effect of galantide and result in no significant change in hyperenzymaemia. Further studies are required to understand this unexpected result.
The lack of effect of the combination of these peptides on pancreatic MPO activity suggests opposing mechanisms of action, a proposal that is difficult to reconcile. These actions may occur at the neutrophil recruitment level and/or at the cellular/intracellular level to modulate the neutrophil activation state, and hence MPO activity, of the infiltrating neutrophils. As described above, Schmidhuber et al.,30 found that galanin is involved in neurogenic inflammation caused by substance P. Galantide contains moieties of galanin and substance P8 and could thus exert its effect of reducing MPO activity by a mechanism involving either galanin and/or substance P. Somatostatin has been shown to block substance P receptors43 and consequently may possibly prevent galantide from binding to these receptors and exerting its beneficial effects (via substance P antagonism). This may be an example of common receptor activity of these two peptides. Another reason for the decreased reduction in MPO activity seen with octreotide as well as with the combination of the two peptides may involve the contribution to MPO activity of activated monocytes and macrophages44 as octreotide has been shown to promote activation of the monocyte–macrophage system.45,46 Octreotide has also been shown to inhibit neutrophil chemotaxis induced by substance P.43
Our finding that the combination of the two peptides administered at the induction of AP has no benefit does not support further testing of the combination in a delayed setting (i.e. after the induction of AP).
Conclusions
This study is the first to explore the efficacy of a combination of two peptides, galantide and octreotide, in the treatment of mild caerulein-induced AP in mice. From this study we conclude that treatment with galantide alone ameliorates mild caerulein-induced AP, whereas treatment with octreotide alone reduces pancreatic MPO activity and acinar cell damage. The combination of the two peptides appears to negate the individual benefits of the two agents, which may indicate complex interaction(s) in the mechanism of action of the two peptides at several sites.
Acknowledgments
The financial support of the Flinders Medical Centre Foundation is acknowledged.
Conflicts of interest
None declared.
References
- 1.Barreto SG, Rodrigues J. Acute pancreatitis in Goa – a hospital-based study. J Indian Med Assoc. 2008;106:575–576. discussion. [PubMed] [Google Scholar]
- 2.Braganza JM. Towards a novel treatment strategy for acute pancreatitis. 2. Principles and potential practice. Digestion. 2001;63:143–162. doi: 10.1159/000051884. [DOI] [PubMed] [Google Scholar]
- 3.Barreto SG, Carati CJ, Schloithe AC, Toouli J, Saccone GT. The combination of neurokinin-1 and galanin receptor antagonists ameliorates caerulein-induced acute pancreatitis in mice. Peptides. 2010;26:315–321. doi: 10.1016/j.peptides.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari M, Thomas A, Carati C, Kawamoto M, Toouli J, Saccone G. Galanin antagonism modifies hyperenzymaemia and pancreatic vascular perfusion (PVP) changes induced by acute pancreatitis (AP) in a possum model. Pancreas. 2006;33:447–448. [Google Scholar]
- 5.Bhandari M, Thomas AC, Hussey DJ, Li X, Jaya SP, Woods CM, et al. Galanin mediates the pathogenesis of caerulein-induced acute pancreatitis in the mouse. Pancreas. 2010;39:182–187. doi: 10.1097/MPA.0b013e3181bdc152. [DOI] [PubMed] [Google Scholar]
- 6.Brooke-Smith ME, Carati CJ, Bhandari M, Toouli J, Saccone GT. Galanin in the regulation of pancreatic vascular perfusion. Pancreas. 2008;36:267–273. doi: 10.1097/MPA.0b013e31815ac561. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto M, Bhandari M, Thomas A, Carati C, Toouli J, Saccone G. The galanin antagonists galantide and M35 but not M40 ameliorate caerulein-induced acute pancreatitis in mice. Pancreatology. 2007;7:231. doi: 10.1159/000314603. [DOI] [PubMed] [Google Scholar]
- 8.Lindskog S, Ahren B, Land T, Langel U, Bartfai T. The novel high-affinity antagonist, galantide, blocks the galanin-mediated inhibition of glucose-induced insulin secretion. Eur J Pharmacol. 1992;210:183–188. doi: 10.1016/0014-2999(92)90669-u. [DOI] [PubMed] [Google Scholar]
- 9.Baxter JN, Jenkins SA, Day DW, Roberts NB, Cowell DC, Mackie CR, et al. Effects of somatostatin and a long-acting somatostatin analogue on the prevention and treatment of experimentally induced acute pancreatitis in the rat. Br J Surg. 1985;72:382–385. doi: 10.1002/bjs.1800720516. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Wang SS, Tsay SH, Lee FY, Wu SL, Lu RH, et al. Effects of high dose octreotide on retrograde bile salt-induced pancreatitis in rats. Peptides. 1998;19:543–547. doi: 10.1016/s0196-9781(97)00453-1. [DOI] [PubMed] [Google Scholar]
- 11.Marton J, Szasz Z, Nagy Z, Jarmay K, Takacs T, Lonovics J, et al. Beneficial effect of octreotide treatment in acute pancreatitis in rats. Int J Pancreatol. 1998;24:203–210. doi: 10.1007/BF02788423. [DOI] [PubMed] [Google Scholar]
- 12.Zhu ZH, Holt S, el-Lbishi MS, Grady T, Taylor TV, Powers RE. A somatostatin analogue is protective against retrograde bile salt-induced pancreatitis in the rat. Pancreas. 1991;6:609–613. doi: 10.1097/00006676-199109000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Salem MZ, Cunha JE, Coelho AM, Sampietri SN, Machado MC, Penteado S, et al. Effects of octreotide pretreatment in experimental acute pancreatitis. Pancreatology. 2003;3:164–168. doi: 10.1159/000070086. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez J, Garcia JL, Gil JS, Oses AV, Coma del Corral MJ, Puerta CV, et al. Octreotide: effects in experimental severe acute pancreatitis. Analysis of survival, biochemical findings and histomorphometry. Rev Esp Enferm Dig. 1997;89:101–115. [PubMed] [Google Scholar]
- 15.Tulassay Z, Kisfalvi K, Papp M. The effect of the long-acting somatostatin analogue octreotide on caerulein-induced pancreatic injuries in rats. Z Gastroenterol. 1995;33:99–102. [PubMed] [Google Scholar]
- 16.McGregor DP. Discovering and improving novel peptide therapeutics. Curr Opin Pharmacol. 2008;8:616–619. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88(5 Pt 1):1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- 18.Rifai Y, Elder AS, Carati CJ, Hussey DJ, Li X, Woods CM, et al. The tripeptide analogue feG ameliorates severity of acute pancreatitis in a caerulein mouse model. Am J Physiol Gastrointest Liver Physiol. 2008;294:1094–1099. doi: 10.1152/ajpgi.00534.2007. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Gong Z, Lou K, Tu S, Di Z, Xu J. Effects and mechanisms of somatostatin analogues on apoptosis of pancreatic acinar cells in acute pancreatitis in mice. J Gastroenterol Hepatol. 2001;16:683–688. doi: 10.1046/j.1440-1746.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, et al. Treatment with neutralizing antibody against cytokine-induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–844. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore-Olufemi SD, Xue H, Attuwaybi BO, Fischer U, Harari Y, Oliver DH, et al. Resuscitation-induced gut oedema and intestinal dysfunction. J Trauma. 2005;58:264–270. doi: 10.1097/01.ta.0000133571.64393.d2. [DOI] [PubMed] [Google Scholar]
- 22.Howard C, Reed M. Unbiased Stereology, Three-Dimensional Measurement in Microscopy. Oxford: Bio Scientific; 2000. pp. 20–65. [Google Scholar]
- 23.Toh SK, Phillips S, Johnson CD. A prospective audit against national standards of the presentation and management of acute pancreatitis in the south of England. Gut. 2000;46:239–243. doi: 10.1136/gut.46.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreto SG, Carati CJ, Schloithe AC, Toouli J, Saccone GT. Galanin potentiates supramaximal caerulein-stimulated pancreatic amylase secretion via its action on somatostatin secretion. Am J Physiol Gastrointest Liver Physiol. 2009;297:1268–1273. doi: 10.1152/ajpgi.00342.2009. [DOI] [PubMed] [Google Scholar]
- 25.Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518–530. doi: 10.1002/bjs.5316. [DOI] [PubMed] [Google Scholar]
- 26.Hegyi P, Rakonczay Z, Jr, Sari R, Gog C, Lonovics J, Takacs T, et al. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004;10:2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Currie RW, Ali IS. Insulin potentiates expression of myocardial heat shock protein 70. Eur J Cardiothorac Surg. 2004;26:281–288. doi: 10.1016/j.ejcts.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Ting LP, Tu CL, Chou CK. Insulin-induced expression of human heat-shock protein gene hsp70. J Biol Chem. 1989;264:3404–3408. [PubMed] [Google Scholar]
- 29.Tobe K, Asai S, Matuoka K, Yamamoto T, Chida K, Kaburagi Y, et al. Cytoskeletal reorganization induced by insulin: involvement of Grb2/Ash, Ras and phosphatidylinositol 3-kinase signalling. Genes Cells. 2003;8:29–40. doi: 10.1046/j.1365-2443.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmidhuber SM, Starr A, Wynick D, Kofler B, Brain SD. Targeted disruption of the galanin gene attenuates inflammatory responses in murine skin. J Mol Neurosci. 2008;34:149–155. doi: 10.1007/s12031-007-9015-9. [DOI] [PubMed] [Google Scholar]
- 31.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. discussion 559–560. [DOI] [PubMed] [Google Scholar]
- 32.Uhl W, Anghelacopoulos SE, Friess H, Buchler MW. The role of octreotide and somatostatin in acute and chronic pancreatitis. Digestion. 1999;60(Suppl. 2):23–31. doi: 10.1159/000051477. [DOI] [PubMed] [Google Scholar]
- 33.Di Francesco V, Angelini G, Bovo P, Casarini MB, Filippini M, Vaona B, et al. Effect of octreotide on sphincter of Oddi motility in patients with acute recurrent pancreatitis: a manometric study. Dig Dis Sci. 1996;41:2392–2396. doi: 10.1007/BF02100133. [DOI] [PubMed] [Google Scholar]
- 34.Andriulli A, Leandro G, Clemente R, Festa V, Caruso N, Annese V, et al. Meta-analysis of somatostatin, octreotide and gabexate mesilate in the therapy of acute pancreatitis. Aliment Pharmacol Ther. 1998;12:237–245. doi: 10.1046/j.1365-2036.1998.00295.x. [DOI] [PubMed] [Google Scholar]
- 35.Andriulli A, Leandro G, Niro G, Mangia A, Festa V, Gambassi G, et al. Pharmacologic treatment can prevent pancreatic injury after ERCP: a meta-analysis. Gastrointest Endosc. 2000;51:1–7. doi: 10.1016/s0016-5107(00)70377-4. [DOI] [PubMed] [Google Scholar]
- 36.Guan D, Maouyo D, Sarfati P, Morisset J. Effects of SMS 201-995 on basal and stimulated pancreatic secretion in rats. Endocrinology. 1990;127:298–304. doi: 10.1210/endo-127-1-298. [DOI] [PubMed] [Google Scholar]
- 37.Wenger FA, Kilian M, Heukamp I, Foitzik T, Jacobi CA, Guski H, et al. Effects of octreotide in acute haemorrhagic necrotizing pancreatitis in rats. J Gastroenterol Hepatol. 2007;22:1872–1876. doi: 10.1111/j.1440-1746.2006.04627.x. [DOI] [PubMed] [Google Scholar]
- 38.Cindoruk M, Kayhan B, Gorgul A, Kandilci U. Comparison of the efficacy of the somatostatin analogue SMS 201-995 and electromagnetic fields in caerulein-induced pancreatitis in rats. Curr Ther Res. 2001;62:254–260. [Google Scholar]
- 39.Paran H, Klausner J, Siegal A, Graff E, Freund U, Kaplan O. Effect of the somatostatin analogue octreotide on experimental pancreatitis in rats. J Surg Res. 1996;62:201–206. doi: 10.1006/jsre.1996.0196. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins SA, Nott DM, Baxter JN. Fluctuations in the secretion of pancreatic enzymes between consecutive doses of octreotide: implications for the management of fistulae. Eur J Gastroenterol Hepatol. 1995;7:255–258. [PubMed] [Google Scholar]
- 41.Garvin PJ, Burton FR, Reese JC, Dysarz FA, III, Lingle D, Niehoff ML, et al. Effect of somatostatin and octreotide acetate on OP-CCK-stimulated exocrine secretion in the denervated canine pancreas. Pancreas. 1996;13:304–310. doi: 10.1097/00006676-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Singh J, Adeghate E, Salido GM, Pariente JA, Yago MD, Juma LO. Interaction of islet hormones with cholecystokinin octapeptide-evoked secretory responses in the isolated pancreas of normal and diabetic rats. Exp Physiol. 1999;84:299–318. doi: 10.1111/j.1469-445x.1999.01733.x. [DOI] [PubMed] [Google Scholar]
- 43.Kolasinski SL, Haines KA, Siegel EL, Cronstein BN, Abramson SB. Neuropeptides and inflammation. A somatostatin analogue as a selective antagonist of neutrophil activation by substance P. Arthritis Rheum. 1992;35:369–375. doi: 10.1002/art.1780350402. [DOI] [PubMed] [Google Scholar]
- 44.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Chao TC, Cheng HP, Walter RJ. Somatostatin and macrophage function: modulation of hydrogen peroxide, nitric oxide and tumour necrosis factor release. Regul Pept. 1995;58:1–10. doi: 10.1016/0167-0115(95)00051-c. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins SA, Baxter JN, Day DW, Al-Sumidaie AM, Leinster SJ, Shields R. The effects of somatostatin and SMS 201-995 on experimentally induced pancreatitis and endotoxaemia in rats and on monocyte activity in patients with cirrhosis and portal hypertension. Klin Wochenschr. 1986;64(Suppl. 7):100–106. [PubMed] [Google Scholar]


