Abstract
Background:
Only recently has a standard chemotherapy regimen, gemcitabine plus cisplatin, been established for advanced biliary tract cancers (BTCs) based on a phase III randomized study. The aim of this phase II single-institution trial was to assess the efficacy and safety of gemcitabine combined with carboplatin in the first-line treatment of patients with advanced BTCs.
Methods:
Patients with histologically proven BTCs, including cholangiocarcinoma or gallbladder and ampullary carcinomas, were treated with a maximum of nine cycles of intravenous (i.v.) gemcitabine at 1000 mg/m2 over 30 min on days 1 and 8 with i.v. carboplatin dosed at an area-under-the-curve (AUC) of 5 over 60 min on day 1 of a 21-day cycle.
Results:
A total of 48 patients with advanced BTCs (35 cholangiocarcinoma, 12 gallbladder and 1 ampullary cancer) were enrolled. A median of four cycles were administered (range: 1–9). The overall response rate for evaluable patients was 31.1%. Median progression-free survival, overall survival and 6-month survival rates are 7.8 months, 10.6 months and 85.4%, respectively. The most common grade 3–4 toxicities include neutropenia and thrombocytopenia. Grade 3 or 4 non-haematological toxicities were rare.
Conclusions:
Gemcitabine combined with carboplatin has activity against advanced BTCs. Our results are comparable to other gemcitabine-platinum or gemcitabine-fluoropyrimidine combinations in advanced BTCs.
Keywords: chemotherapy < cholangiocarcinoma, chemotherapy < gallbladder, cholangiocarcinoma < liver, gallbladder, biliary
Introduction
Cancers of the biliary tract (BTCs), including intra- and extra-hepatic cholangiocarcinomas and gallbladder cancers, are uncommon cancers accounting for approximately 4% of all gastrointestinal malignancies.1 An estimated 12 000 new cases of BTCs are diagnosed annually in the United States.2 Surgical resection remains the only curative treatment for BTCs, however, only a minority of patients diagnosed with these aggressive tumours present at an early, localized and surgically resectable stage. Unfortunately, disease recurrence rates are high despite curative-intent resection of BTCs. Adjuvant chemotherapy, with or without radiation, appears to improve outcomes after resection.3–5
As a result of the lack of early symptoms, most patients with BTCs present with locally advanced or metastatic disease. Prognosis is extremely poor for these patients with median survival times of less than 1 year.6–11 A small underpowered randomized study suggests that palliative chemotherapy may offer improvements in quality of life (QOL) and possibly prolong survival for patients with BTCs compared with best supportive care (BSC).12 Fluoropyrimydine monotherapy provided modest response rates of 0–21% in biliary tract tumours.13–16 Gemcitabine, as a single agent, and in combination with other antineoplastics, has been extensively studied in patients with BTCs. Single agent gemcitabine conferred response rates of 17% to 36% with median survivals of 6.5–11 months.17–26 Gemcitabine plus a fluoropyrimidine resulted in response rates of 9.5% to 29%.27–29 Multiple phase II studies combining gemcitabine with a platinum compound, either cisplatin or oxaliplatin in patients with advanced BTCs, resulted in response rate of 21% to 36% and median survivals of 8.4 months to 15 months.30–34 Moreover, a pooled analysis of 104 trials with 2810 patients demonstrated a superior response rate, tumour control rate and a trend towards improved time to progression for gemcitabine combined with a platinum compared with fluoropyrimydine-based regimens.35 Recently, the UK ABC-02 randomized Phase III trial demonstrated superior outcomes for patients treated with gemcitabine plus cisplatin compared with gemcitabine alone. The authors have recommended this regimen to be the new standard regimen for advanced biliary tract cancers.36
Compared with cisplatin or oxaliplatin, carboplatin has a better non-haematological toxicity profile and tolerability, less requirement for pre- and post- chemotherapy hydration and minimal risk for nephrotoxicity and cumulative peripheral neuropathy.37,38 Gemcitabine plus carboplatin has been evaluated in patients with other malignancies and found to be tolerable.38–40 We now report our phase II study combining gemcitabine with carboplatin for the first-line treatment of patients with advanced BTCs.
Methods
Eligibility
Patients 18 years or older with biopsy-proven advanced, adenocarcinoma of the biliary tract and measurable disease were eligible for this study. Other requirements included the following: Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, adequate haematological, renal and hepatic function [absolute neutrophil count (ANC) ≥1500/mm3, platelet count ≥100 000/mm3 and haemoglobin ≥9 g/dL, serum creatinine ≤2 mg/dL, serum bilirubin ≤3.0 mg/dL (biliary stents were allowed), serum transaminases ≤ fivefold the institutional upper limits]. Patients must not have received any prior chemotherapy for metastatic disease. Prior adjuvant radiation therapy and chemotherapy was allowed. At least 3 weeks needed to have elapsed since any surgery requiring general anaesthesia. Ineligibility criteria included co-existing severe medical illness and inability to sign consent. The study was approved by the institutional review board and informed consent was obtained from all participants prior to enrolment.
Treatment schedule
Gemcitabine at a dose of 1000 mg/m2 was administered as a 30-min intravenous (i.v.) infusion on day 1 and 8 of a 21-day cycle. Carboplatin, at an area-under-the-curve (AUC) of 5, was administered as a 1-h i.v. infusion on day 1 of a 21-day cycle. Gemcitabine was administered prior to carboplatin on day 1 of each cycle. Patients received a maximum of nine cycles of therapy. Treatments after the prescribed nine cycles were administered per treating physician discretion. Dose adjustments were made as per a study-defined dose modification table depending on the type and severity of toxicities associated with study treatment.
Assessment of efficacy and toxicity
At study entry, a full history and a physical examination were obtained, including vital signs, height and weight. Prior to enrolment, a complete blood count, comprehensive metabolic profile, β-human chorionic gonadotropin in females, lactic dehydrogenase as well as tumour markers including carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) were obtained. Within 28 days of enrolment, baseline tumour measurements were obtained. At the start of each cycle, a history and physical examination were once again performed, as well as a complete blood count, comprehensive metabolic profile and CA19-9 and CEA. Tumour measurements using spiral computed tomography (CT) scans for response were obtained every three cycles (every 9 weeks). Responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST).41 Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0.
For patients who developed treatment-related toxicities, the doses of gemcitabine and carboplatin were adjusted according to protocol-defined parameters. Treatments were delayed if the ANC was <1500 cells/µl and platelet counts were <100 000 cells/µl or for any Grade III non-haematological toxicities. Upon recovery, gemcitabine doses were reduced by 25% and carboplatin dose was reduced to the calculated AUC dose of four for all subsequent cycles. Two further dose modifications with another 25% reduction in the previous gemcitabine dose and reduction of the carboplatin calculated AUC dose to 3.5 and 3, were allowed. If any further toxicity requiring dose modification occurred after these dose reductions, the patient was removed from the study. The use of granulocyte-colony stimulating growth factors was not allowed, whereas erythropoietin used for anaemia was used at the discretion of the treating physician.
Statistical considerations
The primary objective of this phase II trial was to evaluate the response rate of gemcitabine plus carboplatin in patients with advanced BTCs. Secondary endpoints include tumour control rate, time to progression and overall survival. Descriptive statistical methods were used to summarize baseline patient characteristics and adverse event rates. Progression-free survival and overall survival rates were calculated using the Kaplan–Meier methodology.
The two-stage design proposed by Simon42 was used to calculate sample size to reject a response rate of <20% in favour of a response rate of 40%. A sample size of 43 patients was determined to be the number needed to validate the above hypothesis with an alpha error rate of 0.05 and a power of 0.8. Enrolment of a total of 48 patients was planned in the event of a 10% rate of inevaluable or ineligible patients.
Results
Patient characteristics
From March 2002 through to September 2006, 49 patients were enrolled: 35 patients (71%) had cholangiocarcinoma, 12 patients (25%) had gallbladder cancer, 1 patient (2%) had ampullary cancer and 1 patient (2%) was found ineligible after our institutional pathological review of outside slides revealed gallbladder melanoma. The distribution of various baseline patient characteristics is listed in Table 1. The median age at the time of study entry was 63 years (range, 31 to 85).
Table 1.
Patient characteristics
| Characteristics | N (%) |
|---|---|
| No. of patients enrolled | 49 |
| Gender | |
| Male | 15 (31%) |
| Female | 34 (69%) |
| Performance status | |
| ECOG = 0 | 22 (45%) |
| ECOG = 1 or 2 | 27 (55%) |
| Age (years) | |
| median (range) | 63 (31 to 85) |
| Location of primary tumour (%): | |
| Cholangiocarcinoma | 35 (71%) |
| Gallbladder | 12 (25%) |
| Ampulla of Vater | 1 (2%) |
| Gallbladder melanoma | 1 (2%) |
| Sites of metastasis (%, n= 48) | |
| Locally advanced | 8 (17%) |
| Metastatic | 40 (83%) |
| Previous surgery | 19 (39%) |
| Curative intent | 9 (19%) |
| Cholecystectomy | 2 |
| Right hemihepatectomy | 3 |
| Right trisegementectomy | 2 |
| Whipple | 1 |
| Common bile duct resection | 1 |
| Palliative | 10 (20%) |
| Bypass surgery | 2 |
| Cholecystectomy | 6 |
| Whipple with liver resection | 1 |
| Omentectomy | 1 |
| Prior radiation | 6 (12%) |
| Prior adjuvant chemotherapy: | 5 (10%) |
| 5-FU | 3 (6%) |
| gemcitabine | 1 (2%) |
| gemcitabine-taxotere | 1 (2%) |
| Ca19-9 | |
| At baseline: median/range | 64 (2 to 600 435) |
Chemotherapeutic drug administration
A total of 227 cycles of chemotherapy were given among the 48 eligible patients. The median number of treatment cycles given to patients was 4 (range 1–9).
Sixteen patients (33%) completed the maximum of nine cycles. Other reasons for discontinuing study treatment include disease progression (29%), toxicity (33%) and consent withdrawal (4%).
During the course of therapy, 36 (75%) out of 48 patients required at least one dose modification secondary to haematological adverse events. Only one-level dose reduction to gemcitabine at 750 mg/m2 and carboplatin at AUC of 4 was required in 22 patients (46%), whereas 14 patients (29%) required 2 or more dose reductions.
Toxicity
All patients treated on study, including the ineligible patient with melanoma who received one cycle of therapy, were evaluable for toxicity. There was one possible treatment related death in an 80-year-old female patient with cholangiocarcinoma who completed three cycles of chemotherapy on protocol with evidence of stable disease. She subsequently died from non-neutropenic urosepsis and multi-system organ failure.
The grade and distribution of haematological and non-haematological toxicities are summarized in Table 2. The most common haematological toxicities were anaemia (91%), thrombocytopenia (67%) and neutropenia (59%). Grade 3 and grade 4 neutropenia and thrombocytopenia occurred in 37% and 20% of patients, respectively. Four patients developed febrile neutropenia.
Table 2.
Maximum severity, per patient, of grade 3 or 4 adverse events, n= 49
| Toxicity | Grade 3/Grade 4 | Total |
|---|---|---|
| Hematologic | ||
| Anaemia | 12% | 91% |
| Thrombocytopaenia | 20% | 67% |
| Neutropaenia | 37% | 59% |
| Lymphopaenia | 18% | 59% |
| Non-haematological | ||
| Fatigue | 6% | 61% |
| Transaminitis (AST/ALT) and increase in alkaline phosphatase | 4% | 41% |
| Nausea | 6% | 37% |
| Vomiting | 6% | 28% |
| Infection without neutropaenia | 4% | 13% |
| Hyperbilirubinaemia | 2% | 10% |
| Diarrhoea | 4% | 8% |
| Febrile neutropaenia with infection | 6% | 6% |
| Febrile neutropaenia without infection | 2% | 2% |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The most common non-haematological toxicities observed were fatigue (61%), transaminitis and elevated alkaline phosphatase (41%), nausea (37%), vomiting (28%) and infections without neutropenia (13%). However, grade 3–4 non-haematological toxicities were uncommon. (Table 2).
Eleven patients (22%) required hospitalization, most of which were because of disease progression or reasons unrelated to study therapy. The most common reasons for admission were cholangitis (3), febrile neutropenia (2), sepsis (2), biliary obstruction, suicidal ideation and depression, pain control, dehydration, nausea, ascites, obstructive jaundice, pancreatitis and deep vein thrombosis. Five patients required more than one hospital admission (10%).
Efficacy
Among the 48 eligible patients, 3 were inevaluable for response (discontinuation from study because of an adverse event prior to first radiological assessment). The overall response rate for the 45 eligible patients with BTCs was 31.1% [95% confidence interval (CI), 18.2%-46.7%]. Four patients (8.9%) achieved complete responses and 10 patients (22.2%) had partial responses. Twenty patients (44.4%) had stable disease. Thus, the overall tumour control rate (TCR = CR + PR + SD) was noted to be 75.6% (95% CI, 60.7–87.1%) (Table 3). Eleven patients (24.4%) met the criteria for progressive disease as their best response. A Waterfall plot of the best response detailing the percentage change from baseline tumour measurements for each individual patient is represented in Fig. 1.
Table 3.
Response rates, progression-free survival and overall survival for all patients, and by disease site
| Total patients | Cholangiocarcinoma | Gallbladder cancer | |
|---|---|---|---|
| Response | |||
| N | 45a | 33 | 11 |
| Response rate, % | 31.1% (95% CI, 18.2–46.7%) | 23.5% | 54.5% |
| Stable disease, % | 44.4% | 50% | 27.3% |
| Tumour-control rate, % | 75.6% (95% CI, 60.5–87.1%) | 73.5% | 81.8% |
| Survival (ITT) | |||
| n, eligible patients | 48b | 35 | 12 |
| Progression-free survival, months | 7.8 (95% CI, 5.8, 9.1) | 7.9 | 6.8 |
| Overall survival, months | 10.6 range = 1–45 (95% CI, 9–15) | 10.6 Range = 3–42 | 11.5 Range = 1–45 |
| 6-month survival, % | 85.4% (95% CI, 71.8–92.8%) | 83.3% | 91.7% |
| 12-month survival, % | 43.8% (95% CI, 29.6–57.1%) | 42.4% | 50.0% |
All evaluable patients including one patient with ampullary cancer.
All eligible patients, including one patient with ampullary cancer.
Figure 1.
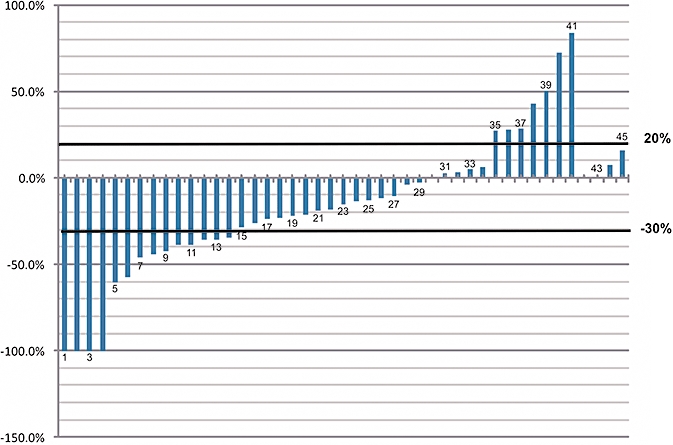
Waterfall Plot. Best objective tumour response for each patient: maximum change in the sum of the longest diameter of measurable disease from baseline. n= 45 complete/partial responders: >30% decrease; stable disease: <30% decrease to <20% increase; progressive disease: >20% increase or with new lesions
Among the 34 evaluable patients with cholangiocarcinoma, 8 patients (23.5%) had a radiological response (1 CR and 7 PR) and TCR of 73.5%. Six out of the 11 patients (54.5%) with gallbladder carcinoma responded to treatment (3 CR and 3 PR) while another 3 patients (27.3%) had SD (TCR = 81.8%).
Tumour marker responses, as defined by a ≥50% decrease of Ca 19-9 from an elevated abnormal baseline value, were observed in 14 out of 45 (31%) patients.
The median progression-free survival (PFS) for all 48 eligible patients was 7.8 months (95% CI, 5.8–9.1 months]. Among the 35 patients with cholangiocarcinoma, median PFS was 7.9 months (95% CI, 5.6–10.5 months] compared with 6.8 months for the 12 patients with gallbladder carcinoma (95% CI, 3.8–9.1 months).
Only one patient remains alive, now 42 months since study entry. Median overall survival for all 48 eligible patients was 10.6 months (95% CI, 8.8–14.2 months) with observed 6-month and 12-month survival rates of 85.4% (95% CI, 71.8–92.8%) and 43.8% (95% CI, 29.6–57.1%), respectively (Fig. 2). Table 3 summarizes the response to therapy and outcomes for all patients and among patients with cholangiocarcinoma and gallbladder cancer. Median survival for patients with gallbladder and cholangiocarcinoma are similar at approximately 11 months. Median overall survival for patients who achieved stable disease was 14.6 months (95% CI, 10.0–23.0 months], comparable to those who achieved a partial or complete response whose median survival was 12.1 months (95% CI, 9.0–19.0 months) (Fig. 3). Patients with progressive disease as their best response had median survival times of only 7.1 months (95% CI, 5.0–9.6 months).
Figure 2.
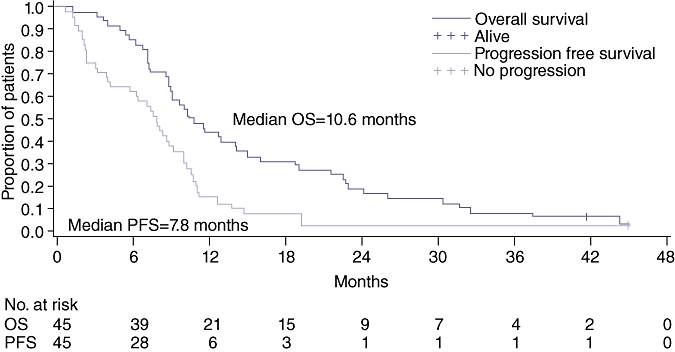
Overall and progression-free survival. n= 45
Figure 3.
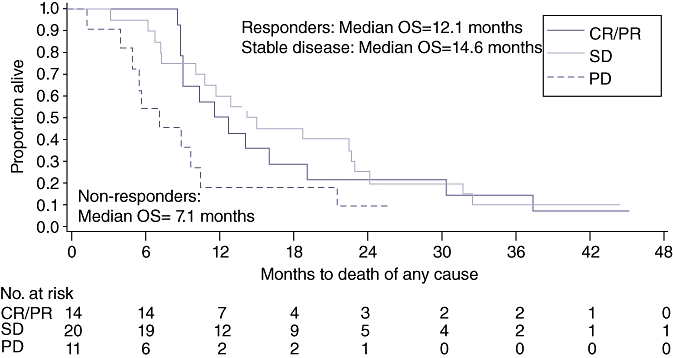
Overall survival based on response. n= 45
Among the 48 evaluable patients, 19 patients had prior surgery. Curative-intent oncological surgery was performed in nine patients who later experienced disease recurrence. Surgery for 10 patients was palliative in intent, as metastatic disease was noted during surgery. Median survival for the 19 patients with prior surgery, whether curative or palliative, was 10.1 months, slightly worse than those who had no prior surgery (12.1 months).
Discussion
Although biliary tract cancers are relatively uncommon, it is a devastating disease, usually presenting at advanced stages when surgery is not a treatment option. Few therapeutic advances have been made in the recent era with regard to new drug development for unresectable, locally advanced or metastatic biliary tract cancers. Multiple agents have been investigated as single or combination therapies in small uncontrolled studies. Historically, 5-fluorouracil (5-FU) has been the most studied agent and has the longest track record in treating patients with advanced biliary tract cancers. 5-FU, as single agent therapy, has a response rate of 0–21%.13–16 Other agents, such as mitomycin-C,43 methotrexate,44 etoposide,45 docetaxel,46 and doxorubicin,47 have been used with mixed results in biliary tract cancers. Only 10–20% of patients treated with the above chemotherapies showed a response.
Gemcitabine has been evaluated both as a single-agent and in combination with numerous agents. Most of these studies were limited as a result of small sample sizes (Table 4). A recent pooled analysis of 104 clinical trials evaluating gemcitabine plus a platinum regimen in biliary tract cancer35 reported that the pooled RR and TCR was 22.6% and 57.3%, respectively. Significant correlations of RR and TCR with survival times were found.35
Table 4.
Selected published studies of various regimens for biliary tract cancer
| Author, year [reference] | Regimen | Dose and Schedule | N | Response Rate | TCR | Median PFS, months | Median OS, months |
|---|---|---|---|---|---|---|---|
| Choi et al., 200016 | Leucovorin 5-FU | 25 mg/m2/day 1−5, 375 mg/m2/day 1−5, q28d | 28 | 32.1% | – | – | 6 |
| Arroyo et al., 200125 | Gemcitabine | 1000 mg/m2 on D1, 8, 15 q28 days | 42 | 36% | 64% | 3.9 | 6.5 |
| Alberts et al., 200527 | Gemcitabine 5-FU Leucovorin | 1000 mg/m2 on D1, 8,15 600 mg/m2 IVP on D1 25 mg/m2 on D1, q28d | 42 | 9.5% | 23% | 4.6 | 9.7 |
| Riechelmann et al., 200728 | Gemcitabine Capecitabine | 1000 mg/m2 on D1, 8 650 mg/m2 PO BID x14d, q21d | 75 | 29% | 78% | 6.2 | 12.7 |
| Koeberle et al. 200829 | Gemcitabine Capecitabine | 1000 mg/m2 on D1,8 650 mg/m2 PO BID, D1-14 | 44 | 25% | 80% | 7.2 | 13.2 |
| Kim et al., 200632 | Gemcitabine Cisplatin | 12 500 mg/m2 on D1, 8 60 mg/m2 on D1, q21d | 29 | 34.5% | 48.3% | 3.0 | 11 |
| Harder et al., 200633 | Gemcitabine Oxaliplatin | 1000 mg/m2 on D1, 8,15 100 mg/m2 on D1, 8 q28d | 31 | 26% | 71% | 6.5 | 11 |
| Kim et al., 200926 | Gemcitabine Oxaliplatin | 1000 mg/m2 on D1, 15 85 mg/m2 on D2, 16 q28d | 40 | 15% | 52.5% | 4.2 | 8.5 |
| Eckel et al., 200734 | Gemcitabine platinum | Various | 2810 | 22.6% | 57.3% | 4.1 | 8.2 |
| Valle et al., 200935 | Gemcitabine Gemcitabine Cisplatin | 1000 mg/m2 on D1,8,15 q 28d 1000 mg/m2 on D1,8 25 mg/m2 on D1,8 q21d | 206 204 | 16% 25.7% | 71.2% 79.1% | 6.5 8.4 | 8.3 11.7 |
| Williams-Current study | Gemcitabine Carboplatin | 1000 mg/m2 D1,8 AUC 5 D1, q 21d | 48 | 31.1% | 75.6% | 7.8 | 10.6 |
5-FU, 5-fluoropyrimidine; PFS, progression-free survival; N, number of patients; OS, overall survival; TCR, tumour control rate.
Recently, gemcitabine in combination with cisplatin has been evaluated in 410 patients with advanced or metastatic cholangiocarcinoma, gallbladder cancer and ampullary cancers in a large, randomized phase III clinical trial.36 Gemcitabine plus cisplatin conferred a significantly superior median overall survival (OS) compared with those treated with single-agent gemcitabine [11.7 months vs. 8.2 months, P= 0.002, hazard ratio (HR) = 0.68, 95% CI 0.53–0.86]. The median PFS was also significantly greater with gemcitabine/cisplatin than gemcitabine alone (8.5 vs. 6.5 months, P= 0.003, HR = 0.70, 95% CI, 0.56–0.88). Although the response rate for the combination arm was higher (25.7% vs. 16%), this was not statistically significant compared with gemcitabine alone. Toxicities were similar in both arms, however, neutropenia was found to be more common in the combination arm. This large phase III study established a new standard first-line regimen using gemcitabine with cisplatin for advanced biliary cancer.
In this single institution phase II clinical trial we demonstrate that gemcitabine with carboplatin has activity in biliary tract cancer with an overall response rate of 31.1%, tumour control rate of 75.5%, median time to progression of 7.8 months and median overall survival of 10.6 months. The 6-month and 1-year survival rates were 85.4% and 43.8%, respectively. These results are comparable to those reported by Valle et al. in their randomized study using gemcitabine and cisplatin in a similar population of patients with biliary tract tumours.36 These results are also consistent with other phase II studies using gemcitabine-based combinations and the pooled analysis data published by Eckel35 (Table 4).
As there appears to be differences in the clinicopathological behaviour of the different tumour types comprising these rare biliary tract malignancies, a subset analysis of outcomes for patients with cholangiocarcinomas and gallbladder cancers was done. Although the clinical response rate appears to be higher among patients with gallbladder cancer, compared with patients with cholangiocarcinoma, OS for both groups are identical at approximately 11 months. Patients with stable disease as their best response still achieved comparable survival times similar to those who achieved partial or complete responses. Similar to the clinical experience in pancreatic cancer, significant benefits in outcome can be observed despite low response rates to chemotherapy. Unfortunately, most patients progress and die of their malignancy, including the four patients who achieved a complete radiological response to treatment. Patients with prior surgery, whether curative or palliative, appear to have a shorter median survival time compared with those who never had any prior surgery. However, this observation needs to be validated in larger studies.
Although severe non-hematological toxicities were uncommon, haematological toxicities associated with gemcitabine and carboplatin, as administered in this protocol, appear higher compared with the toxicities reported using weekly cisplatin and gemcitabine in the ABC-02 study (Grade 3–4 anemia 12% vs. 6.3%; grade 3–4 thrombocytopenia 20% vs. 8.2% and grade 3–4 neutropenia 37% vs. 22.6%).36 Haematological toxicities associated with this regimen were also higher than other combinations such as gemcitabine with capecitabine.29
Gemcitabine in combination with carboplatin has activity against advanced biliary tract cancers comparable to other gemcitabine-platinum and gemcitabine fluoropyrimidine combinations. However, haematological toxicities associated with this combination are significant. This combination should be evaluated among patients who may be intolerant or not suitable for cisplatin. Further large multicentre randomized trials, preferably with pharmacogenomic and tissue correlative studies, are necessary to establish more efficacious and tolerable regimens to treat patients with these rare malignancies.
Acknowledgments
Steve Nicol for reviewing the manuscript. ClinicalTrials.gov identifier NCT00660140.
Conflicts of interest
Supported by Eli Lilly & Company.
References
- 1.Ahlgren J. Neoplasms of the Hepatobiliary System. New York: McGraw-Hill; 1993. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Picus J, Myerson R, Drebin J, Strasberg S. A Phase II trial of continuous infusion (CIVI) 5-FU with 3-D conformal radiation in the adjuvant treatment of pancreatic, ampullary and biliary cancers. Proc Am Soc Clin Oncol. 1998;17:266a. [Google Scholar]
- 4.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 5.Czito BG, Hurwitz HI, Clough RW, Tyler DS, Morse MA, Clary BM, et al. Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 2005;62:1030–1034. doi: 10.1016/j.ijrobp.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Shani M, Hart J, Modan B. Cancer of the biliary system: a study of 445 cases. Br J Surg. 1974;61:98–100. doi: 10.1002/bjs.1800610205. [DOI] [PubMed] [Google Scholar]
- 7.Hamrick RE, Jr, Liner FJ, Hastings PR, Cohn I., Jr Primary carcinoma of the gallbladder. Ann Surg. 1982;195:270–273. doi: 10.1097/00000658-198203000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo CJ, Pitt HA, Cameron JL. Cholangiocarcinoma. Surg Clin North Am. 1990;70:1429–1447. doi: 10.1016/s0039-6109(16)45293-x. [DOI] [PubMed] [Google Scholar]
- 9.Bengmark S, Ekberg H, Evander A, Klofver-Stahl B, Tranberg KG. Major liver resection for hilar cholangiocarcinoma. Ann Surg. 1988;207:120–125. doi: 10.1097/00000658-198802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871–877. doi: 10.1001/archsurg.1993.01420200045008. discussion 77–9. [DOI] [PubMed] [Google Scholar]
- 11.Kraybill WG, Lee H, Picus J, Ramachandran G, Lopez MJ, Kucik N, et al. Multidisciplinary treatment of biliary tract cancers. J Surg Oncol. 1994;55:239–245. doi: 10.1002/jso.2930550408. [DOI] [PubMed] [Google Scholar]
- 12.Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 13.Davis HL, Jr, Ramirez G, Ansfield FJ. Adenocarcinomas of stomach, pancreas, liver, and biliary tracts. Survival of 328 patients treated with fluoropyrimidine therapy. Cancer. 1974;33:193–197. doi: 10.1002/1097-0142(197401)33:1<193::aid-cncr2820330128>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769–1774. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965–969. doi: 10.1002/1097-0142(19840915)54:6<965::aid-cncr2820540603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Choi CW, Choi IK, Seo JH, Kim BS, Kim JS, Kim CD, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000;23:425–428. doi: 10.1097/00000421-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Gebbia V, Giuliani F, Maiello E, Colucci G, Verderame F, Borsellino N, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol. 2001;19:4089–4091. doi: 10.1200/JCO.2001.19.20.4089. [DOI] [PubMed] [Google Scholar]
- 18.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 19.Raderer M, Hejna MH, Valencak JB, Kornek GV, Weinlander GS, Bareck E, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology. 1999;56:177–180. doi: 10.1159/000011961. [DOI] [PubMed] [Google Scholar]
- 20.Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12:183–186. doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- 21.Tsavaris N, Kosmas C, Gouveris P, Gennatas K, Polyzos A, Mouratidou D, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193–198. doi: 10.1023/B:DRUG.0000011797.09549.53. [DOI] [PubMed] [Google Scholar]
- 22.von Delius S, Lersch C, Schulte-Frohlinde E, Mayr M, Schmid RM, Eckel F. Phase II trial of weekly 24-hour infusion of gemcitabine in patients with advanced gallbladder and biliary tract carcinoma. BMC Cancer. 2005;5:61. doi: 10.1186/1471-2407-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JS, Oh SY, Kim SH, Kwon HC, Kim JS, Jin-Kim H, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005;35:68–73. doi: 10.1093/jjco/hyi021. [DOI] [PubMed] [Google Scholar]
- 24.Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57:647–653. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 25.Gerardo Arroyo JG, Rubio B, Orlandi L, Yañez M, Gamargo C, Ahumada M, et al. Sociedad de Oncologia del NOA; GETICS, Salta, Argentina; Oncologia Clinica Alemana, Santiago, Chile; Universidad de Chile, Santiago, Chile; Sociedad de Oncologia del NOA; GETICS, Tucuman, Argentina; Sociedad de Oncologia del NOA, Jujuy, Argentina; GETICS, Viedma, Argentina. Gemcitabine in advanced biliary tract cancer. Experience from Chile and Argentina in Phase II trials. Proc Am Soc Clin Oncol. 2001;20:157a. [Google Scholar]
- 26.Kim HJ, Lee NS, Lee SC, Bae SB, Kim CK, Cheon YG, et al. A phase II study of gemcitabine in combination with oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. Cancer Chemother Pharmacol. 2009;64:371–377. doi: 10.1007/s00280-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 27.Alberts SR, Al-Khatib H, Mahoney MR, Burgart L, Cera PJ, Flynn PJ, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer. 2005;103:111–118. doi: 10.1002/cncr.20753. [DOI] [PubMed] [Google Scholar]
- 28.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–1312. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 29.Koeberle D, Saletti P, Borner M, Gerber D, Dietrich D, Caspar CB, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2008;26:3702–3708. doi: 10.1200/JCO.2008.16.5704. [DOI] [PubMed] [Google Scholar]
- 30.Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16(Suppl 2):ii93–ii96. doi: 10.1093/annonc/mdi712. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53:564–570. doi: 10.1007/s10620-007-9885-2. [DOI] [PubMed] [Google Scholar]
- 32.Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 33.Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–1346. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 34.Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95:848–852. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 37.Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 38.Dogliotti L, Carteni G, Siena S, Bertetto O, Martoni A, Bono A, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 40.Yardley DA, Burris HA, 3rd, Simons L, Spigel DR, Greco FA, Barton JH, et al. A phase II trial of gemcitabine/carboplatin with or without trastuzumab in the first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2008;8:425–431. doi: 10.3816/CBC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Simon R. Optimal two-stage designs for Phase II trials. Controll Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 43.Taal BG, Audisio RA, Bleiberg H, Blijham GH, Neijt JP, Veenhof CH, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol. 1993;4:607–609. doi: 10.1093/oxfordjournals.annonc.a058597. [DOI] [PubMed] [Google Scholar]
- 44.Kajanti M, Pyrhonen S. Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol. 1994;17:223–226. doi: 10.1097/00000421-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Ekstrom K, Hoffman K, Linne T, Eriksson B, Glimelius B. Single-dose etoposide in advanced pancreatic and biliary cancer, a phase II study. Oncol Rep. 1998;5:931–934. doi: 10.3892/or.5.4.931. [DOI] [PubMed] [Google Scholar]
- 46.Papakostas P, Kouroussis C, Androulakis N, Samelis G, Aravantinos G, Kalbakis K, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract. A multicentre phase II study. Eur J Cancer. 2001;37:1833–1838. doi: 10.1016/s0959-8049(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 47.Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol. 1984;2:1245–1248. doi: 10.1200/JCO.1984.2.11.1245. [DOI] [PubMed] [Google Scholar]


