Abstract
Background:
Surgical cytoreduction and endocrine blockade are important options for care for neuroendocrine liver metastases. We investigated the long-term survival of patients surgically treated for hepatic neuroendocrine metastases.
Methods:
Patients (n= 172) undergoing operations for neuroendocrine liver metastases from any primary were identified from a prospective liver database. Recorded data and medical record review were used to analyse the type of procedure, length of hospital stay, peri-operative morbidity, tumour recurrence, progression,and survival.
Results:
The median age was 56.8 years (range 11.5–80.7 years). 48.3% of patients were female. Median overall survival was 9.6 years (range 89 days to 22 years). On multivariate analysis, lung/thymic primaries were associated with worse survival [hazard ratio (HR): 15.6, confidence interval (CI): 4.3–56.8, P= 0.002]. Severe post-operative complications were also associated with worse long-term survival (P < 0.001). A positive resection margin status (R1) was not associated with a worse overall survival probability (P∼ 0.8).
Discussion:
Early and aggressive surgical management of hepatic metastases from neuroendocrine tumours is associated with significant long-term survival rates. Radiofrequency ablation is a reasonable option if a lesion is unresectable. R1 resections, unlike many other cancers, are not associated with a worse overall survival.
Keywords: neuroendocrine, carcinoid, liver metastases, liver resection, radiofrequency ablation, survival
Introduction
Neuroendocrine carcinoma is thought to have such a biologically indolent nature that initially the lesion of interest was referred to as ‘carcinoma-like’, not frank carcinoma.1 This distinction is not only of academic interest, but also extraordinarily important to the individual patient. The ‘risk–benefit ratio’ of surgery is predicated on the assumption that a patient's disease can be accurately (albeit, crudely) risk stratified. Clearly, there is a major difference between benign tumours and invasive cancer. Historically, patients with neuroendocrine lesions were felt to do relatively well, but once metastatic disease is present, systemic therapy is often the mainstay of treatment.2 Over 40 years ago, hepatic resection for metastatic carcinoid tumours was considered an option,3 yet the literature still suggests this is a controversial topic.4
There are multiple classifications for neuroendocrine tumours (NETs). Often, they are organized by foregut, midgut and hindgut NET. Likewise, NETs can be grouped by the prevalence of the primary location. These are gastrointestinal carcinoids (also called midgut neuroendocrine tumours), pancreatic islet cell tumours (also known as pancreatic neuroendocrine tumours, or PNETs) and neuroendocrine tumours arising in other sites (thoracic, thyroid, other ectodermic tissues). Most share the characteristic of longer survival than adenocarcinomas originating from the same organs. A recent retrospective analysis of patients with PNETs from the Surveillance, Epidemiology and End Results (SEER) database demonstrated a median survival of 9.5 years after primary pancreatic resection compared with 2.9 years when resection was declined by the patient.5 Likewise, another report demonstrated median disease-free survival ranges from 4.8 to 9.0 years after resection of primary gastrointestinal carcinoid tumours.6 Clearly, when possible, resection of NET tumours may prolong patient survival. Intriguingly, large database studies have suggested a survival benefit for resection of the primary neuroendocrine tumour even in the setting of stage IV disease.2,5,7
Often, metastases are the hallmark of aggressive disease. This is clearly the case in adenocarcinoma.8 Metastatic disease is advanced disease by definition,9 but we hypothesize that metastatic NETs isolated to the liver can be managed in a surgical fashion as ‘advanced regional disease.’
Methods
A consecutive series of 172 patients were identified from our prospective hepatobilliary database who underwent their first laparotomy for treatment of neuroendocrine or carcinoid liver metastases. All patients were diagnosed and underwent operation between 1978 and 2009 at The University of Texas M. D. Anderson Cancer Center in Houston, Texas. When patients underwent multiple resections, only data from the first procedure were utilized.
All patients had pathological confirmation of neuroendocrine disease. Pre-operative imaging included chest radiograph or chest computed tomography when indicated, as well as abdominopelvic imaging with conventional imaging (computed tomography or magnetic resonance imaging) in all patients. In addition, radio-nucleotide scintigraphy (i.e., octreotide scan) was performed as standard practice since 2004. Prior to that, many patients presented to our institution with scintigraphy from their primary oncologist. Patients selected for surgery had adequate functional reserve and lesion correlation on radio-nucleotide and conventional imaging. Patients with extra-hepatic lesions were excluded from surgery unless all disease could be simultaneously treated during surgery. Radiofrequency ablation (RFA) was utilized if a lesion could not be safely and completely resected. Likewise, small tumours were often treated with RFA if the patient was undergoing concomitant contralateral hepatectomy or trisectionectomy. Complete tumour resection or RFA with preservation of sufficient hepatic parenchyma was the key selection criterion for surgical treatment. Patients were considered for RFA even if they had a tumour abutting a major hepatic vein branch or the inferior vena cava. RFA was not performed for metastases adjacent to major biliary structures, particularly at the hepatic duct confluence.
All patients underwent resection and/or RFA during open laparotomy by a hepatobiliary surgeon. No patients were treated percutaneously, and intra-operative ultrasound was used in all patients. All patients who underwent RFA were treated using the RadioTherapeutics RF 2000 or RF 3000 generator system (Boston Scientific, Natick, MA, USA) using a standard algorithm.10,11 For tumours > 2.5 cm in diameter, the array was repositioned for multiple treatments to obtain complete destruction of the tumour and at least a 1-cm zone of surrounding liver parenchyma when possible.
The Brisbane 2000 terminology of liver anatomy and resections was used.12 Briefly, extended right hepatectomy (resection of Couinaud segments IV + V + VI + VII + VIII ± I), extended left hepatectomy (resection of Couinaud segments II + III + IV + V + VIII ± I), right hepatectomy (resection of Couinaud segments V + VI + VII + VIII ± I), left hepatectomy (resection of Couinaud segments II + III + IV ± I), bisegmentectomy/sectionectomy (i.e. partial resection of 2 or 3 adjacent Couinaud segments), or segmentectomy (resection of a single Couinaud segment) were performed. Resection was performed with the clamp-crush method or high-pressure water jet at the discretion of the attending surgeon. Vascular staplers are typically used for the hepatic veins whereas parenchymal staplers were only rarely used.
Statistical analysis
Patient demographics, primary tumour and liver tumour factors, operative factors, pathological findings, recurrence patterns and survival were analysed. The Cox proportional hazard model was used to analyse differences in risk factors for survival. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated as appropriate. Survival was estimated using Kaplan–Meier analysis; differences in survival were analysed using the Mantel–Cox log-rank test. Backward elimination was used to evaluate univariate variables in the multivariate model. Variables were kept in the model if P < 0.05. Differences in tumour recurrence rates between treatment groups were analysed using the Fisher's exact and two-sided chi-square tests. Aggregated data are presented as means; the uncertainties are standard deviations unless otherwise noted. Differences were considered to be statistically significant when the P-value was <0.05.
Results
Descriptive characteristics
The median patient age for the whole group was 56.8 years (range, 12 years to 81 years of age). 60.5% presented with synchronous primary and hepatic metastatic tumours (Table 1). 48.3% were female. 41.8% of patients received pre-operative chemotherapy with the majority receiving 5-florouracil and streptozocin, often prior to being evaluated at our institution. Median follow-up was 4.2 years (range 3 months to 22.9 years). All patients had pathologically proven primary and metastatic NET disease except for five patients who underwent RFA only without concurrent biopsy. These patients had pathologically proven primary disease and multiple types of abdominal imaging studies consistent with metastatic NET to the liver. Fifty-nine (34.3%) patients had only right-sided liver lesions, including isolated caudate lobe metastases. Twenty-seven (15.7%) patients had left-sided lesions and 84 (48.8%) had bilateral lesions. Finally, there were two patients (1.2%) who had NET metastases at the confluence/bifurcation with invasion into liver sections 4 and 5. Approximately half of the patients (n= 87, 50.6%) had intermediate grade (or higher) or moderately differentiated (or worse) metastatic NET whereas the other half had low grade or well-differentiated lesions (n= 85, 49.4%).
Table 1.
Demographic characteristics of all patients in the study
| Clinical characteristics of study population (n= 172) | ||
|---|---|---|
| Characteristic | Median | Range (Min–Max) |
| Age at surgery (years) | 56.8 | 11.5–80.7 |
| Length of stay (days) | 7 | 1–30 |
| Characteristic | Number (n) | % Patients |
| Gender | ||
| Female | 83 | 48.3% |
| Male | 83 | 48.3% |
| Ethnicity | ||
| Asian | 2 | 1.1% |
| Black | 8 | 4.7% |
| Hispanic | 15 | 8.7% |
| Other | 1 | 0.6% |
| Caucasian | 146 | 84.9% |
| Synchronous liver metastases at diagnosis | ||
| No | 68 | 39.5% |
| Yes | 104 | 60.5% |
| Synchronous non-liver resection | ||
| No | 88 | 51.2% |
| Yes | 84 | 48.8% |
‘Synchronous non-liver resection’ refers to procedures where the metastatic and primary disease (or regional lymphadenectomy) were simultaneously resected except for a single revision pancreaticojejunostomy.
Operative characteristics
One-hundred and forty patients (81.4%) underwent a hepatic resection of some type (Table 2). Thirteen additional patients (7.6%) were found to have diffuse metastases not amenable to surgical treatment at the time of operation while 18 patients (10.5%) received RFA alone without hepatic resection (where RFA was used as definitive therapy with curative intent). The most common primary sites of NETs (Table 3) were the small bowel (n= 65, 37.8%) and the pancreas (n= 55, 32.0%). The eight patients diagnosed with metastatic hepatic NETs without known primary sites were diagnosed based on a metastatic pattern of liver disease with biopsy proven neuroendocrine characteristics of those lesions.
Table 2.
Major and secondary surgical treatments were performed simultaneously for metastatic neuroendocrine tumours to the liver (n= 172)
| Major operative interventions | n | % |
|---|---|---|
| Trisectionectomy (extended hepatectomy) | 22 | 12.8% |
| Hepatectomy | 49 | 28.5% |
| Bisegmentectomy/sectionectomy (partial hepatectomy) | 34 | 19.8% |
| Segmentectomy | 21 | 12.2% |
| Wedge resection | 14 | 8.1% |
| RFA alone | 18 | 10.5% |
| Cholecystectomy without resection | 1 | 0.6% |
| No hepatic intervention | 13 | 7.5% |
| Secondary interventions | ||
| Wedge resection | 17 | 9.9% |
| RFA | 23 | 13.4% |
| Segmentectomy | 17 | 9.9% |
Secondary procedures typically managed contralateral lesions not encompassed in the simultaneous major resection because of location or expected final liver volume. An additional 34 patients (19.8%) also underwent a prophylactic cholecystectomy to prevent chemical cholecystitis from future treatments, such as transarterial embolization.
Table 3.
The majority of neuroendocrine tumours metastatic to the liver are from gastrointestinal primaries
| Primary location | n | % |
|---|---|---|
| Small bowel | 65 | 37.8% |
| Pancreas | 55 | 32.0% |
| Appendix/cecum | 8 | 4.7% |
| Colon | 13 | 7.6% |
| Liver | 6 | 3.5% |
| Lung/thymus | 6 | 3.5% |
| Rectum | 2 | 1.2% |
| Thyroid | 4 | 2.3% |
| Ovary/mesentery | 5 | 2.9% |
| Unknown | 8 | 4.7% |
All were biopsy-proven primaries with the exception of unknown primaries where hepatic lesions were biopsied prior to treatment and the pattern was consistent with neuroendcrine metastatic disease. Hepatic NET lesions found subsequent to primary hepatic NET resection were classified as metastatic hepatic NET. Pancreatic primaries include functional, non-functional, islet cell and non-islet cell NET.
There were no staged hepatectomies. Four patients in this series had a diagnosis of cirrhosis. Furthermore, no patient in this series underwent pre-operative portal vein embolization. One hundred and seventeen patients (84.2%) had negative margins (R0) whereas 22 patients (15.8%) had positive margins (R1 or R2).
Of the 41 patients who underwent RFA, 18 patients (43.9%) received no other major procedure (Table 2). The median number of tumours treated with RFA per patient was two (range 1 to 12). Nineteen patients had three or more tumours ablated while eight patients had five or more tumours ablated. Tumours were ablated in all eight Couinaud segments.
There were four intra-operative surgical complications (2.3%) while the postoperative surgical complication rate was 22.1% (Table 4). The majority of complications were Grade I or II according to the Dindo–Clavien classification (Table 4).13 There were no peri-operative deaths. Eighteen patients received adjuvant chemotherapy after liver resection.
Table 4.
Peri-operative surgical complications for the 90-day period after surgical therapy occurred in a minority of patients
| Peri-operative surgical complications | n | % | Grade(s) |
|---|---|---|---|
| None | 134 | 77.9% | – |
| Cardiac | 4 | 2.3% | I–II |
| Non-infectious intra-abdominal fluid collection | 4 | 2.3% | I |
| Partial portal venous thrombosis or non-fatal hepatic insufficiency | 4 | 2.3% | II–IIIa |
| Gastrointestinal | 4 | 2.3% | I |
| Pulmonary | 10 | 5.8% | I–II |
| Infectious | 5 | 2.9% | I–II |
| Others | 7 | 4.1% | I–IVa |
| Death | 0 | 0.0% | – |
They are listed here by organ system. Cardiac complications were typically transient atrial fibrillation. Intra-abdominal fluid collections with positive bacterial cultures were categorized as ‘Infectious’ with urinary tract infections, abscess, and wound infections. Gastrointestinal complications were primarily conservatively managed ileus and dysphagia at discharge. Pulmonary complications included effusions (all conservatively managed) and pneumonia. ‘Others’ included, bladder/urinary dysfunction, transient renal insufficiency and transient thrombocytopenia. Classification is based on the Dindo–Clavien classification.13
Recurrence of hepatic metastases after surgical treatment
Eighty patients (46.5%) had recurrence of their liver metastases after surgical therapy and their tumours were re-resected or ablated. With a median follow-up of 5.2 years (range 0.7 years to 22.5 years), 62.5% (50/80) were still alive. 25% (20/80) of these recurrent patients underwent a third resection/ablation for metastatic hepatic disease. 60% (12/20) of these patients remained alive with a median follow-up 7.5 years (range 2.7 years to 22.5 years). Eight patients recurred after their third treatment and underwent a fourth resection. Of this very small population, 50% (4/8) are alive with a median follow-up of 7.6 years (range 4.9 years to 22.5 years).
Survival analysis
The median overall survival was 9.64 years (range 89 days to 22 years). Five-year overall survival was 77.4% whereas 10-year overall survival was 50.4%. On univariate analysis (Table 5), increasing the metastatic interval (hazard per year), increased length of stay (hazard per day), thoracic or rectal primary site and liver-related post-operative complications were all significantly associated with a decrease in overall survival probability. Less aggressive procedures were associated with a trend towards improved overall survival (Table 5). Despite this, the intra-hepatic location did not yield a statistically significant association with survival on univariate or multivariate analysis.
Table 5.
These are all of the variables from Tables 1 and 2 where the univariate P-value was significant at P≤ 0.10
| Overall survival | HR (95% CI) | P value |
|---|---|---|
| Univariate analysis | ||
| Increasing interval (per year) from primary resection to hepatic metastases | 1.11 (1.04–1.18) | 0.003 |
| Synchronous disease at diagnosis | 0.55 (0.30–1.01) | 0.05 |
| Increasing length of stay (per day) | 1.06 (1.00–1.12) | 0.04 |
| Primary site | ||
| Pancreas | 0.53 (0.24–1.13) | 0.10 |
| Lung/thymus | 5.69 (1.86–17.43) | 0.002 |
| Rectum | 4.76 (1.07–21.24) | 0.04 |
| Primary procedure | ||
| Partial hepatectomy | 0.11 (0.01–0.91) | 0.04 |
| Segmentectomy | 0.37 (0.12–1.15) | 0.09 |
| RFA | 0.35 (0.11–1.09) | 0.07 |
| Post-operative complications | ||
| Fluid collection | 3.83 (0.89–16.48) | 0.07 |
| Hepatic | 9.88 (2.13–45.88) | 0.004 |
| Multivariate analysis | ||
| Increasing interval from primary resection to hepatic metastases | 1.11 (1.02–1.20) | 0.01 |
| Primary site | ||
| Lung/thymus | 15.63 (4.30–56.8) | 0.002 |
| Post operative complications | ||
| Fluid collection | 15.89 (3.10–81.4) | 0.0009 |
| Hepatic | 22.94 (4.39–120.0) | 0.0002 |
These univariate variables were entered into a multivariate, saturated model utilizing backward elimination to remove the insignificant variables (P > 0.05) in a multiple regression analysis. Interestingly, margin status (R0 vs. R1 vs. R2) and primary procedure did not meet the criteria to be included in the multivariate model (i.e. P > 0.05).
On multivariate analysis, most of these factors were found to be insignificant (Table 5). Two notable exceptions include primary lung/thymus site and increasing disease-free interval (per year) after primary resection; both were statistically associated with increasing hazard ratios for death (Table 5). In addition, late occurrences of hepatic metastases were associated with an increased hazard for death (HR: 1.11, 95th percentile CI: 1.04–1.18, P= 0.003). Finally, primary lesions from the colon were associated with an increased recurrence-free survival after hepatic metastatic resection (HR: 0.25, 95th percentile CI: 0.07–0.80, P= 0.02).
On multivariate analysis, recurrence-free survival after hepatic resection of metastases was decreased for Asian patients (HR: 29.6, 95th percentile CI: 2.45–358,
P= 0.008) and those with post-operative intra-abdominal fluid collection (HR: 6.45, 95th percentile CI: 1.42–29.3, P= 0.02), but improved for patients with colonic primaries (HR: 0.22, 95th percentile CI: 0.06–0.77, P= 0.02).
Positive margins (R1 or R2) were not associated with a significantly worse overall (P∼ 0.40) or recurrence-free survival (P∼ 0.80) probability on univariate or multivariate analysis. In addition, no statistically significant differences based on histology or site of origin of the primary NET were identified regarding survival. However, having an unknown primary site led to an improved overall survival (P < 0.001, Fig. 1) while a thoracic primary was associated with a significantly worse survival. Although there was decreased overall survival 8 years after a peri-operative complication (Fig. 2), this did not reach statistical significance (P∼ 0.10).
Figure 1.
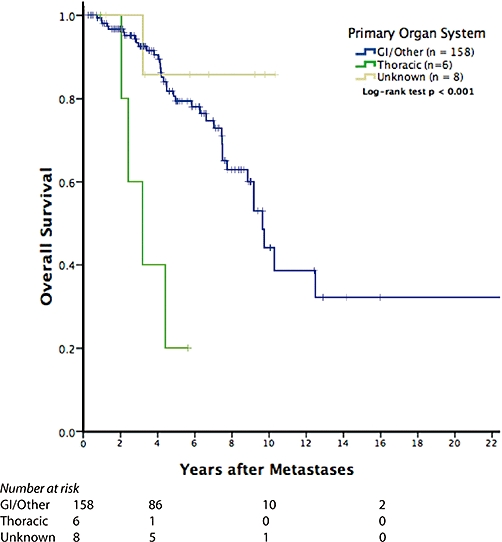
Patients with surgically treated isolated hepatic metastatic neuroendocrine tumours (NET) have very good long-term overall survival. In addition, the few patients with unknown primaries did extremely well after surgery. However, primary lesions in the thorax (lung and thymus) did strikingly poorly after resection. These small numbers do not permit accurate generalizations as to the cause of this phenomenon. As deaths are from any cause in the overall survival analysis, it is possible that the primary resection (i.e. lung resection) contributed to long-term morbidity and mortality
Figure 2.
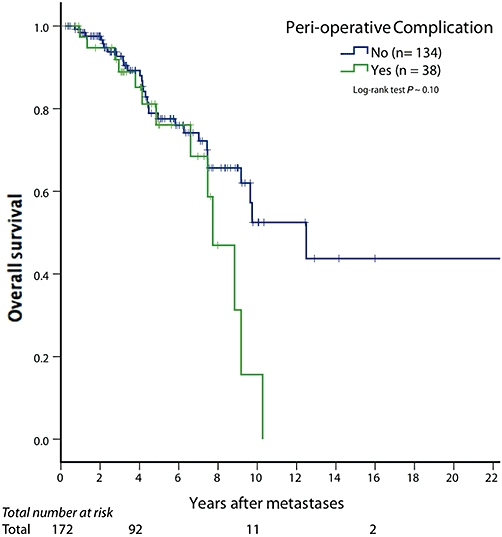
Although peri-operative complications seem to be associated with decreased long-term overall survival, this did not reach statistical significance. Furthermore, there is no discernable difference for the first 8 years after surgical treatment
Of the 18 patients who received chemotherapy (non-somatostatin analogue) after surgical treatment of hepatic metastases, 61.1% (11/18) remained alive with a median follow-up of 4.6 years (range 1.0–9.2 years).
Discussion
We report on the long-term survival rate of a large consecutive series of patients with isolated neuroendocrine hepatic metastases managed with surgery. Most importantly, there is significant long-term survival after resection of these patients categorized with Stage IV disease. It is the unique patient who has this ‘limited metastatic disease,’ but these patients clearly have the potential to benefit from an aggressive surgical approach. This patient population represents approximately 24.5% of all stage IV NET seen at our institution during this time frame. Furthermore, even in the presence of a peri-operative complication (Table 4), there is no difference in survival until over 8 years after treatment (Fig. 2), and even then, it remains statistically insignificant (P= 0.10). As microscopic margin status was not associated with survival, patients benefit from an approach to remove as much NET as possible, even if it is not an R0 resection.
Interestingly, on univariate analysis (Table 5), partial hepatectomy, segmentectomy and RFA alone were associated with a trend towards decreased hazards (i.e. increased overall survival probability). Although this was not significant on multivariate analysis, these patients have good long-term survival rates even after multiple resections or surgical RFA (clearly, a highly selected population). In addition, negative margins (R0) did not yield a statistically significant improvement in survival probability. This suggests that better survival is not associated with more aggressive resection, and NET liver metastatic patients do well with anatomic or non-anatomic resection that address their particular metastatic disease. Specifically, as is seen in liver surgery in general, patients with higher volumes of healthier liver parenchyma have improved outcomes. This is consistent with the relatively indolent nature of NET. They are, as has been described, carcinoma like, but clearly not of the same aggressive nature. A larger patient population and longer follow-up may eventually yield an association with degree of resection (or location of metastases, number of segments involved, etc.), but herein, none of this was significant in the multivariate analysis. The only two post-operative complications found to be associated with a decreased overall survival rate were intra-abdominal fluid collection and liver-related complications (insufficiency or portal venous thrombosis). It is important to not only classify the amount of future liver remnant, but also evaluate the effect of any previous treatments (i.e. systemic chemotherapy, chemoembolization, etc.) on the health of the liver and evaluate any other liver disease, particularly cirrhosis. As there is no evidence that chemotherapy is of major benefit, we do not recommend its use until surgical management is excluded for fear of long-term hepatotoxicity associated with surgical treatment after neoadjuvant chemotherapy.14 However, even after resection, there is no major evidence that systemic chemotherapy is beneficial suggesting a very real higher risk than potential benefit ratio. Patients should receive systemic chemotherapy only as part of a clinical trial.
We believe the use of a core liver biopsy before proceeding with liver resection is reasonable in the uncommon situation where the health of the future liver remnant is questioned. This may even be performed as a frozen-section at the beginning of a procedure if there is clinical evidence of unexpected liver dysfunction before proceeding to resection.
Non-infectious post-operative intra-abdominal fluid collections were found to carry a high hazard ratio (15.9, 95% confidence interval: 3.1–81.4) for death. This was surprising, and may relate to overall liver health in the post-operative period. We hypothesize that a globally injured liver may have increased sinusoidal pressure (and hence increased intra-abdominal fluid) that portends long-term liver dysfunction that either directly or indirectly leads to a premature death. Patients with hepatic NET metastases are not the same as patients with classic colorectal hepatic metastases, so it is difficult to make direct comparisons or assertions.
Late recurrences are also associated with an increased hazard for death. Even though this disease is indolent in nature, having late hepatic metastases is more hazardous than an early or synchronous occurrence. Long-term surveillance for recurrence is warranted. Although the most effective modality is unknown, magnetic resonance imaging is attractive as the radiation is non-ionizing. Until this is proven, however, local customs should dictate the follow-up imaging modality of choice. Our practice is to continue surveillance with CT or MR imaging on a yearly basis for the life of the patient.
We have not described interventional vascular therapies for liver lesions, such as hepatic artery embolization; however, others have reported moderate success with this treatment, including symptom palliation.2,15,16 When compared to formal resection, embolized patients do not survive as long, suggesting that this is an inferior procedure.2 However, patient selection is probably related to this finding; it is difficult to declare unequivocally that embolization therapy is inferior. Clearly from our data, we recommend surgical therapy for properly selected, medically fit patients. Others have suggested that embolization in addition to resection is beneficial, but this has not been conclusively demonstrated.4 Finally, there does not seem to be a clear benefit of chemoembolization over bland hepatic artery embolization.16 Although we did not explicitly investigate the benefit of multidisciplinary care teams, we are in agreement with previous studies that have demonstrated the benefit from their use.17
Nearly all of our patients received a somatostatin analogue at some point in their therapy, so it is difficult to extrapolate the benefit of this therapy in this patient population. However, as part of multimodality management of unresectable and widely metastatic NETs, it is extremely valuable and is associated with improved survival.2 We are not opposed to its use provided resection is not delayed. The PROMID study group demonstrated a near doubling in time to progression with octreotide compared with placebo in patients with unresectable NET.18 Although unproven, it may be possible to utilize octreotide to ‘control’ the tumour and reduce symptoms from excess hormone release while formal workup for other metastatic disease is undertaken. Therefore, we recommend surgical management as soon as extra-hepatic metastatic disease is excluded in an otherwise healthy patient.
This study demonstrates the appropriateness of early, aggressive therapy to remove or destroy focal hepatic metastases in otherwise reasonably healthy NET patients without evidence of extrahepatic disease. In this series, there were no peri-operative deaths after surgery for hepatic metastases. Patients who are at a slightly higher risk for complications may benefit from the surgery despite that higher risk. In a large volume centre, primary tumour and metastatic resections can be safely performed at the same time. RFA is a viable option for partially resectable multicentric disease where the number or location of metastases prevents complete resection. The presence of pre-existing conditions may also prevent a safe resection, but permit an ablation of lesions. Finally, the use of adjuvant chemotherapy is unproven, but it is even more unclear as R1 margin positive patients do relatively well after resection.
Acknowledgments
The authors would like to extend appreciation to Kristine Ash, Yolanda Brittain and Vickie G. Ellis for administrative support on this project.
Conception and design: Glazer, Tseng, Curley.
Data acquisition: Tseng, Al-Refaie, Solorzano, Abdalla, Vauthey.
Analysis and interpretation of data: Glazer, Tseng, Al-Refaie, Liu, Willborn, Curley.
Drafting of manuscript: Glazer, Tseng, Curley, Al-Refaie.
Revision of manuscript: Glazer, Willborn, Abdalla, Vauthey, Curley.
Statistical expertise: Glazer, Tseng, Liu.
Supervision: Vauthey, Curley.
Partially funded by NIH T32 training grant #T32 CA09599 (ESG).
Conflicts of interest
None declared.
References
- 1.Modlin IM, Sandor A, Tang LH, Kidd M, Zelterman D. A 40-year analysis of 265 gastric carcinoids. Am J Gastroenterol. 1997;92:633–638. [PubMed] [Google Scholar]
- 2.Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 3.Zeegen R, Rothwell-Jackson R, Sandler M. Massive hepatic resection for the carcinoid syndrome. Gut. 1969;10:617–622. doi: 10.1136/gut.10.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landry CS, Scoggins CR, McMasters KM, Martin RC., 2nd Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol. 2008;97:253–258. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 5.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741–751. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 6.Van Gompel JJ, Sippel RS, Warner TF, Chen H. Gastrointestinal carcinoid tumors: factors that predict outcome. World J Surg. 2004;28:387–392. doi: 10.1007/s00268-003-7019-3. [DOI] [PubMed] [Google Scholar]
- 7.Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–42. doi: 10.1016/s0002-9610(99)80107-x. discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 8.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. [Google Scholar]
- 10.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–347. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belgihiti J, Clavien PA, Gadzijev E, Garden JO, Lau W, Makuuchi M, et al. The brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 2000;2:333–339. [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 15.Ahlman H, Westberg G, Wangberg B, Nilsson O, Tylen U, Schersten T, et al. Treatment of liver metastases of carcinoid tumors. World J Surg. 1996;20:196–202. doi: 10.1007/s002689900030. [DOI] [PubMed] [Google Scholar]
- 16.Pitt SC, Knuth J, Keily JM, McDermott JC, Weber SM, Chen H, et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg. 2008;12:1951–1960. doi: 10.1007/s11605-008-0640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 18.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]


