INTRODUCTION
A prominent pathology textbook used in the United States includes an image illustrating the renal histopathology caused by malignant hypertension. The legend describes striking “onion skin” changes of a renal arteriole in the center of this figure. Curiously, a sea of mononuclear inflammatory cells surrounding this arteriole is overlooked both in the figure legend and in the related text. Moreover, nothing regarding inflammation or immune reactions is discussed. This lack of attention to inflammatory cells is, however, not surprising. While many experimental studies have implicated inflammation in hypertension, these have largely been performed in experimental animals and there is no proof that inflammation contributes to human hypertension. In fact, some anti-inflammatory or immune suppressing drugs (non-steroidal anti-inflammatory drugs and cyclosporine for example) paradoxically cause hypertension in humans, likely via off-target effects. Often the term “inflammation” is used in the context of cardiovascular disease as a catchall referring to non-specific phenomena such as elevation of C-reactive protein or the presence of macrophages in a tissue. Most clinicians and investigators find this vague and difficult to understand. Even more puzzling is that many studies now implicate the adaptive immune response, and in particular, lymphocytes in hypertension and vascular disease. Traditionally, bacterial, viral or tumor antigens activate this arm of immune defense. As such, it has been hard to imagine how adaptive immunity could be involved in a disease like hypertension. In this article, we will attempt to address some of these puzzling questions. We will briefly review components of the innate and adaptive immune response, discuss data from many groups, including our own, that suggest that common forms of hypertension are immune mediated, and provide a working hypothesis of how signals from the central nervous system trigger an immune response that causes hypertension.
General Concepts regarding inflammation and immunity
Innate immunity
The first line of defense against pathogens is the innate immune response. Important components of this system include epithelial cells, which prevent pathogen entry, professional phagocytes (neutrophils, macrophages), the complement system and pattern recognition receptors. Among the pattern recognition receptors are the Toll-like receptors (TLRs) that sense “danger signals” from various pathogens including double-stranded RNA, bacterial coat proteins, bacterial heat-shock proteins and other toxins. These are relevant to cardiovascular diseases because they cause dramatic changes in cell signaling that can alter cardiac and vascular function. As an example, oxidized lipoproteins, thought to be important in the genesis of atherosclerosis, share similarities to some bacterial coat proteins and can activate TLR4, which in turn signals a variety of inflammatory responses. Reactive oxygen and nitrogen species (ROS and RNS), which play critical signal roles in the cardiovascular system, are fundamental components of innate immunity.
Adaptive immunity
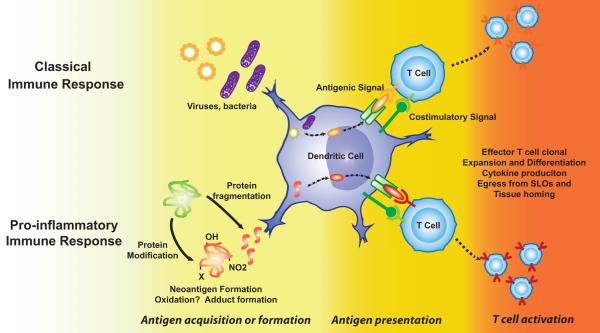
In contrast to the innate immune system, the adaptive immune system is highly specific. The traditional concept regarding adaptive immunity is that antigen-presenting cells in peripheral tissues take up foreign proteins, such as those of bacteria and viruses and process them into short peptides that are presented in the context of a major histocompatibility complex (MHC). Activation of CD4+ lymphocytes is predominantly mediated by dendritic cells, which process antigens in phagosomes and present the resultant antigenic peptides in within MHC II. Dendritic cells then migrate to secondary lymphoid organs, including the spleen and lymph nodes, where they seek a T cell that has a T cell receptor (TCR) that recognizes the antigenic peptide. The interaction of the MHC with the TCR occurs at a region termed the “immunological synapse”. Numerous other ligands and receptors interact at this site, and these promote a coordinated signal that affects both the APC and the T cell. An important additional interaction that occurs at this site, referred to as “co-stimulation”, involves B7 ligands on the APC (CD80 and CD86) with CD28 on the T cell. Other co-stimulatory molecules include members of the tumor necrosis factor (TNF) superfamily,1 and the inducible co-stimulator (ICOS), which helps to sustain T cell activation of B cells.2 In addition to dendritic cells, other cells that effectively present antigen include activated macrophages, B cells, and particularly relevant to cardiovascular biology, activated endothelial cells.3, 4 As a result of TCR ligation and co-stimulation, T cells proliferate, produce cytokines and alter expression of surface receptors that lead to their egress from the secondary lymphoid organs and homing to sites of peripheral inflammation. Helper T cells also bind to B cells and promote antibody formation. In contrast to CD4+ cells, CD8 cells are activated by peptides presented within type I major histocompatibility complexes, which are present not only on dendritic cells, but also on all nucleated cells. Activated CD8+ cells, referred to as cytotoxic T cells, produce killing molecules such as perforin and granzymes that cause death of adjacent cells. Like CD4+ cells, CD8+ cells can also produce cytokines that contribute to various pathophysiological processes. Aspects of this classical cellular adaptive immune response are summarized in Figure 1.
Figure 1.
T cell activation in response to foreign antigens and neoantigen. Classically, proteins of invading organisms, not recognized as self, are processed in antigen presenting cells, such as a dendritic cell and presented within a major histocompatibility complex. In conditions such as atherosclerosis and hypertension, it is hypothesized that endogenous proteins are modified, either by oxidation, fragmentation or other modifications, such that they are no longer recognized as self. These are processed in a manner similar to foreign proteins. T cells are activated and undergo clonal expansion and polarization. The activated T cells leave secondary lymphoid organs (SLO) and are targeted to sites of inflammation.
The interaction between innate and adaptive immunity
While it is convenient to think of innate and adaptive immunity as separate, there is actually enormous interplay between the two. The immunological synapse, described above, involves an interaction between a phagocytic cell of the innate immune system (the APC) and a highly specific T cell of the adaptive immune system. Nitric oxide and reactive oxygen species (ROS), which are components of innate immunity, can modulate T cell function and survival. Cytokines produced by macrophages, dendritic cells and other cells in the inflammatory milieu can influence T cell polarization and alter T cell function. Molecules such as nitric oxide, superoxide, cytokines and ligands for TLRs regulate expression of vascular adhesion molecules and chemokines that promote entry of T cells into target tissues. There is also interdependence between macrophages and T cells. As an example, it has recently been recognized that CD8 cell-derived IFN-γ promotes MMP12 expression and macrophage activation in emphysema induced by cigarette smoke.5 We have found that the TH17 cells modulate entry of other inflammatory cells into the vessel in the setting of hypertension (discussed below).6
The reader is referred to excellent textbooks edited by Abbas and Lichtman and Janeway’s Immunobiology for an in-depth review of the principles mentioned above.7, 8
Relationship between oxidation and inflammation
From the early 1990s to the present time, a great deal of research has been devoted to understanding how oxidative events contribute to hypertension. Many factors common to the hypertensive milieu, including angiotensin II, aldosterone, cytokines and altered mechanical forces like stretch and shear stress stimulate enzyme sources such as the NADPH oxidases, uncoupled nitric oxide synthase and the mitochondria to produce ROS which contribute to hypertension in many ways.9 In the central nervous system, ROS promote sympathetic outflow. In the vessel, ROS induce vasoconstriction whereas in the kidney, they cause sodium and volume retention. While these events alone could cause hypertension, they also enhance inflammatory responses, which as discussed below, further promote blood pressure elevation. ROS activate pro-inflammatory transcription factors such as Nrf2, NFκB, and AP110, 11 These in turn modulate expression of genes, including those encoding adhesion molecules and chemokines, that promote inflammation.12-15 Endothelial permeability is enhanced by oxidative injury, which increases entry of lipoproteins to the sub-endothelial space where they are oxidized and promote inflammation.16 Oxidized lipoproteins can interact with Toll-like receptors, in particular TLR4, to promote vascular disease.17 ROS can affect T cell polarization and cytokine secretion.18 Inflammatory cells such as macrophages and granulocytes can release ROS, further promoting an oxidative environment.
In keeping with this interaction between oxidative injury and inflammation, efforts to reduce ROS decrease inflammation. Antioxidants such as the superoxide dismutase-mimetic Tempol have anti-inflammatory effects in myriad models, including carrageenan-induced pleurisy,19 septic shock,20 peridontitis,21 colitis,22 and encephalitis.23 An example relevant to hypertension is the study of Liu et al, in which a peptide inhibitor of the NADPH oxidase was shown to lower blood pressure and prevent macrophage accumulation in rats during angiotensin II-infusion.24 Recently, Roson et al showed that the acute infusion of sodium caused an increase in renal levels of RANTES, NFκB, HIF1a and angiotensin II in the proximal tubules of rats and that Tempol markedly reduced these responses.25
There is substantial evidence to show that ROS modulate T cell function. Exogenously generated ROS cause apoptosis and suppress T cell proliferation and production of IL-2.26, 27 Of note, T cells also produce ROS endogenously via a Nox2 based NADPH oxidase,18 promoting a TH2 phenotype. Our group found that murine T cells produce angiotensin II endogenously, and that this stimulates the T cell NADPH oxidase that in turn drives production of TNFα. In this case, selective scavenging of superoxide, but not hydrogen peroxide, lowers TNFα production.28
Neoantigens and their potential role in cardiovascular diseases
The term “neoantigens” was first used in the cancer literature to refer to proteins detected by the presence of antibodies against tumor-associated epitopes.29 It is often used interchangeably with “autoantigen”, but connotes a special phenomenon in which an endogenous molecule is modified such that it is no longer recognized as self. This could occur in response to the release of a molecule that is generally intracellular, oxidative modification of an endogenous molecule, cleavage of a protein to expose intramolecular sites normally not available for immune attack, or by attachment of a xenobiotic to a molecule in a hapten-like fashion (figure 1). Such immunoreactive molecules were subsequently found in the serum of humans with cancer,30 and in inflammatory diseases such as halothane-induced hepatitis,31 some forms of glomerulonephritis,32 and osteoarthritis.33 Molecules suggested to elicit immune responses in atherosclerosis include oxidized LDL, heat shock proteins, platelet glycoproteins and others, although no specific antigen has been identified with certainty.34 A recent study has surprisingly shown that the unmodified protein ApoB100 of native LDL can promote an immune response in T cell hybridomas, while oxidation of ApoB 100 paradoxically decreases this response.35
Special mention should be made of the potential role of heat shock protein 70 (HSP70) in hypertension. This molecular chaperone has been intensely studied for more than 40 years,36 and has been implicated in the transport and delivery of antigenic peptides. Various epitopes of HSP70 are immunogenic and induce T cells with anti-inflammatory properties in a neoantigen-like fashion.37 More than 20 years ago, renal expression of HSP70 was found to be increased in hypertensive animals.38 HSP70 expression is increased by restraint stress in rats,39 and is elevated in lymphocytes of hypertensive humans.40 The precise role of heat shock proteins in hypertension remains to be defined but might involve antigen presentation and an ultimate immunologic response.
Early studies supporting a role of adaptive immunity in hypertension
Alterations of the immune response have been implicated in the genesis of hypertension for more than four decades. For the readers’ convenience, some of these are summarized in Table 1. In the 1960s, Grollman and co-workers showed that immunosuppression attenuates hypertension in rats with partial renal infarction.41 The investigators identified antibodies to renal tissue in these animals, and showed that transfer of lymph node cells from rats with renal infarction causes hypertension in normal recipient rats.42 Several early studies focused on immune perturbations in the spontaneously hypertensive rats (SHR) and suggested that T cell function is paradoxically depressed in this commonly studied model of genetic hypertension.43-45 Of note, Ba et al found that engraftment of normal thymus into SHR restored T cell function and lowered blood pressure.46 These investigators found that SHR harbored an antibody that was cytotoxic to thymocytes and proposed that this might produce immune suppression. These studies preceded the understanding that some T cells might be suppressive and did not examine T cell subtypes. It is therefore possible that the analyses of T cell function employed in these could have missed activation of certain T cell subtypes. Interestingly, the rate of nerve growth into the thymus in young SHR is enhanced compared to WKY rats,47 suggesting that neural activation of T cells is increased in hypertension. In keeping with a role of immunity in SHR, Bendich et al found that treatment with anti-thymocyte serum lowers blood pressure in these animals.48 The immunosuppressant cyclophosphamide also transiently lowers blood pressure in SHR.49
Table 1.
Early studies implicating T cells in hypertension.
| Model | Finding | Ref |
|---|---|---|
| Partial renal infarction (Rats) | Antibodies against renal tissue detected. Blood pressure reduced by 6-mercaptopurine or cortisone |
White and Grollman41 |
| Partial renal infarction (Rats) | Hypertension prevented by thymectomy or splenectomy. Induction of hypertension by transfer of lymph node cells from rat with renal infraction to normal rat. |
Okuda and Grollman42 |
| Partial renal infarction (Mice) | Hypertension not maintained in athymic nude mice. |
Svendsen90 |
| SHR | Reduced T cell rosette formation | Takeichi and Boone44 |
| SHR | Reduction of T cell rosette formation with age (opposite to that of WKY). Reduced delayed-type hypersensitivity. Reduced antibody formation. |
Takeichi et al91 |
| SHR | Impaired resistance to tumors induced by methylcholanthrene (MCA). Enhanced natural killer cell function. |
Takeichi et al92 |
| SHR | Antibody to thymocytes present in SHR. |
Takeichi et al93 |
| SHR | Reduction of blood pressure by thymus transplant from WKY. |
Ba et al46 |
| SHR | Chronic infections possibly suppress immunity in SHR. |
Takeichi et al43 |
| SHR | Reduction in blood pressure by anti- thymocyte serum. |
Bendich et al48 |
| SHR | Cyclophosphamide and implantation of WKY thymus grafts lower blood pressure. |
Dzielak49, 94 Norman and Dzielak94 |
| DOCA-salt (mice) | Hypertension not maintained in athymic nude mice. Thymus grafts from haired mice caused sustained hypertension. |
Svendsen50 |
| DOCA-salt (rats) | Transfer of spleen cells from hypertensive rats confers hypertension in normal rats. |
Olsen51 |
Several early studies suggested that T cells are also important in mineralocorticoid-induced hypertension. These showed that while the initial elevation in blood pressure in response to deoxycorticosterone acetate and salt administration is similar between athymic nude mice and normal mice, the athymic, immune deficient mice do not sustain hypertension.50 Subsequent experiments showed that transfer of splenocytes from rats with DOCA-salt hypertension raises blood pressure in recipient rats.51
It is of interest that despite these compelling early observations, there seemed to be lack of further advancement in understanding the role of immunity and inflammation in hypertension in the 1990s and early 2000s. This in part might have been due to the findings in SHR, where T cells seemed depressed. Additionally, until recent, few tools have been available to study the role of immunity in cardiovascular diseases. Several events have changed this research environment. The development of mouse models that allow study of specific aspects of immunity in vivo has been a major boon. Without these, further studies of immunity and hypertension would have been impossible. Technological innovations such as flow cytometry and commercially available kits to isolate cells, measure cytokines, stimulate cells and monitor immune responses are now commonly used. These powerful tools have allowed experiments that were previously not possible. Lastly, several leading investigators have defined a role of immune cells in atherosclerosis, thus providing somewhat of a template for parallel studies in hypertension.52-54
Recent observations regarding the adaptive immune response and hypertension
In the past few years, several studies have strengthened the concept that hypertension has an immunological basis. We initially studied RAG-1−/− mice, which lack both T and B cells.55 Surprisingly, the degree of hypertension caused by chronic angiotensin II infusion was markedly blunted in these animals. RAG-1−/− mice were also protected from the increase of vascular superoxide production and loss of endothelium-dependent vasodilatation that generally accompany angiotensin II infusion. Following adoptive transfer of T cells, but not B cells, the hypertension caused by angiotensin II was completely restored to levels observed in wild-type mice. Likewise, adoptive transfer of T cells led to impaired endothelium-dependent vasodilatation and increased vascular superoxide production when the mice were treated with angiotensin II.
In these studies, we found that chronic angiotensin II infusion increases the percent of cells CD69 and CCR5 positive and CD44high T cells in the circulation. These are markers of activated, effector T cells. Interestingly, angiotensin II also markedly increased vascular levels of the chemokine ligand 5 (also known as RANTES). Thus, like many inflammatory stimuli, hypertension has a dual effect. One is to promote T cell activation, and the second is to increase chemokine and adhesion molecule expression in target tissues to promote tissue entry of the activated inflammatory cells. In keeping with this, hypertension also causes a marked infiltration of CCR5+ cells into perivascular fat.55 In preliminary studies, we have also found that hypertension promotes RANTES expression in perivascular fat.
In addition to angiotensin II-induced hypertension, we have also found that T cells are essential for development of DOCA-salt and norepinephrine-induced hypertension.55, 56 These findings emphasize that many forms of hypertension, beyond that induced by angiotensin II, have an inflammatory component requiring T cells.
More recently, Crowley et al have examined the hypertensive response in mice that have severe combine immunodeficiency (SCID mice).57 These animals have a genetic abnormality leading to abnormal VJD recombination such that they do not develop T or B cells, in a manner very similar to RAG-1−/− mice. Crowley et al confirmed that T cells are essential for full development of angiotensin II-induced hypertension, and showed that these animals have reduced left ventricular hypertrophy, reduced cardiac fibrosis and reduced albuminuria following angiotensin II administration. Other histological parameters of renal injury are reduced or absent in SCID mice. Importantly, the investigators showed that the pressure diuresis and natriuresis caused by hypertension is greater in SCID mice than in wild-type mice. This is associated with a marked increase in expression of the endothelial isoform of nitric oxide synthase and NO production in kidneys of SCID mice.
Role of cytokines in hypertension
Based on our own studies and those such as Crowley’s, a working hypothesis has emerged in which hypertensive stimuli promote accumulation of activated T cells in the perivascular fat and in the kidney. In these sites, these cells release cytokines that affect adjacent vascular cells and tubular epithelium in the kidney. In keeping with this concept, several recent studies have supported the concept that cytokines produced by T cells and other inflammatory cells contribute to hypertension. The TNFα antagonist etanercept reduces the hypertension caused by fructose-feeding,58 prevents vascular dysfunction and blunts the hypertension caused by angiotensin II,55 and lowers blood pressure in an autoimmune model of chronic inflammation.59 In some cases, TNFα antagonism prevents end organ damage without lowering blood pressure. As examples, Etanercept prevent renal injury in salt-dependent hypertension without lowering blood pressure,60 and reduces albuminuria and renal inflammation in a transgenic hypertensive rats.61 Interleukin-6 has also been implicated in angiotensin II-induced, 62-64 but not salt-sensitive hypertension.65 More recently, we found that the novel, proinflammatory cytokine IL-17 contributes to hypertension. This cytokine is produced by TH17 cells, a subset of CD4+ cells, which are distinct from TH1 and TH2 cells. IL-17 has been implicated in a variety of diseases including rheumatoid arthritis, inflammatory bowel disease, psoriasis and airway inflammation.66 IL-17 is also made by CD8+ cells,67 neutrophils,68 and natural killer T cells.69 We found that the increase in blood pressure in mice lacking IL-17 (IL-17−/− mice) is similar to that observed in wild-type mice, but that IL-17−/− mice do not sustain hypertension. Moreover, the increase in superoxide production and reduction of endothelium-dependent vasodilatation observed in wild-type mice does not occur in IL-17−/− mice. IL-17 promotes chemotaxis of other inflammatory cells, in part by stimulating release of chemokines.70, 71 In keeping with this, we found that the vascular accumulation of leukocytes (including T cells) caused by angiotensin II is markedly reduced in IL-17−/− mice. Thus, IL-17 might contribute to the vascular pathophysiology of hypertension not only by direct effects, but also by recruiting other inflammatory cells to the perivascular tissue.
The role of T regulatory cells and IL10 in hypertension
In addition to TH17 cells, another subset of CD4+ cells that differ from the TH1 and TH2 subsets are T regulatory cells (Tregs). These cells, characterized by expression of the Forkhead Transcription Factor FOXP3 and surface expression of CD25, play a critical role in maintaining self-tolerance.72 Genetic deletion of these cells by ablation of FoxP3 leads to a severe, fatal lymphoproliferative disorder.73 Recent studies have suggested that Tregs have a protective effect in hypertension. Kvaken et al found that adoptive transfer of these cells did not affect the hypertensive response to angiotensin II, but had marked effects on the cardiac damage caused by angiotensin II. Treg adoptive transfer reduced the cardiac inflammation, hypertrophy and fibrosis caused by chronic angiotensin II-induced hypertension.74 The authors further showed that Treg adoptive transfer reduced the percent of circulating activated T cells and improved electrical stability during angiotensin II infusion.
Recently, Viel et al studied rats harboring the Dahl salt-sensitive (SS) genome except for chromosome 2 of the Brown Norway strain (SSBN2 rats).75 Chromosome 2 contains genes associated with both hypertension and inflammation and has quantitative trait loci for hypertension. The authors found that SSBN2 rats have reduced hypertension, fewer inflammatory cells in the aorta, and less vascular hypertrophy than Dahl SS rats. They further showed that the aorta of these animals has more aortic Treg cells as evidenced by an increase in mRNA for FoxP3b compared to Dahl SS animals. IL-10 represents an important anti-inflammatory cytokine that both induces and is produced by Treg cells. Tregs of SSBN2 rats were found to produce more IL-10 than Tregs from the Dahl SS rats. The authors concluded that Tregs play an important role in mitigating both blood pressure elevation and end organ damage in the SSBN2 animals. In keeping with an important protective role of IL-10, Didion and co-workers found that incubation with angiotensin II causes marked endothelial dysfunction of carotid arteries from IL-10−/− mice, while not altering endothelium-dependent vasodilatation of arteries from normal mice.76 These investigators further showed that angiotensin II increases vascular superoxide production in IL-10−/− mice, but not in wild-type animals.
Central Nervous System and Inflammation – concept of inflammatory “priming” in hypertension
Several studies have linked the central nervous system to inflammation. Lymph nodes and the spleen are richly innervated with sympathetic nerves that terminate in T cell rich areas.77, 78 The principal neurotransmitter released at the sympathetic nerve terminal is norepinephrine, which can both inhibit and stimulate T cell activation and proliferation.79 The pre-existing state of the T cell seems to determine the ultimate effect of β-adrenergic activation. Norepinephrine stimulates naïve CD4+ lymphocytes cultured under TH1-promoting conditions to produce 3 to 4-fold more IFNγ than non-stimulated cells.80 Importantly, Ganta et al. have shown that intracerebroventricular (ICV) administration of angiotensin II increases splenic sympathetic nerve activity, which in turn increases mRNA expression of IL-1, IL-2, IL-6, IL-16 and TGFβ-1 in splenocytes. Splenic sympathectomy abrogates these responses, clearly linking the central effects of angiotensin II to peripheral immune activation.81 Fannon and Phillips showed that prolonged infusions of either substance P or angiotensin II into the brains of Sprague-Dawley rats increased the percentage of circulating T cells, while decreasing circulating B cells.82
Recently, we performed additional studies to understand the link between central nervous system stimulation, inflammation and hypertension. In initial studies, we sought to enhance the central effects of angiotensin II by deleting the extracellular superoxide dismutase (ecSOD or SOD3) in the circumventricular organs (CVO). These regions surround the ventricular system and both send and receive input from cardiovascular control centers of the brainstem. Because the CVO lack a well-formed blood brain barrier, they are influenced by hormonal signals such as angiotensin II. The CVO, and in particular the subfornical organ (SFO) contain an NADPH oxidase which produce ROS that in turn promote sympathetic outflow. Administration of an adenovirus encoding superoxide dismutase abrogates both the acute and chronic effects of angiotensin II.83, 84 Likewise, administration of a dominant negative form of rac1, which prevents activation of the NADPH oxidase, prevents hypertension.85 We created mice with loxP sites flanking SOD3, which is highly expressed by cells of the SFO. By ICV injection of an adenovirus encoding Cre-recombinase, we were able to delete SOD3 specifically from the CVO in these mice.86 This increased sympathetic outflow, as estimated by analysis of heart rate and blood pressure variability, and caused a modest elevation of blood pressure at baseline and markedly enhanced the hypertensive response to a low dose of angiotensin II (140 ng/kg/min) that alone had minimal to no effect on blood pressure. More importantly, while this dose of angiotensin II did not affect activation of T cells or vascular inflammation alone, following deletion of SOD3 in the CVO, there was a marked increase in circulating T cells baring CD69 and CD44high and a striking increase in vascular infiltration of inflammatory cells. There was also a striking elevation of the percent of circulating double negative (CD3+, CD4−, CD8−) T cells. The precise role of these double negative T cells remains unclear, but in other settings they promote inflammation,87, 88 and we find that they represent up to one third of the vascular infiltrating T cells in hypertension. Thus, these experiments clearly show that central stimuli promote the systemic inflammatory response to angiotensin II.
To further study the role of the CNS in peripheral vascular inflammation, we created lesions of the anteroventral 3rd ventricular (AV3V) region in mice.56 Lesions in this region prevent almost all forms of experimental hypertension,89 and we found that they markedly blunted the hypertensive response to high-dose angiotensin II (490 ng/kg/min). AV3V lesions also prevented activation of circulating T cells and the infiltration of leukocytes caused by angiotensin II. This finding was quite revealing, because it showed that the direct actions of angiotensin II on T cells and peripheral tissues are not responsible for the inflammation caused by this octapeptide, but that its central actions are required. In contrast to angiotensin II, AV3V lesions did not prevent the hypertension, circulating T cell activation or the leukocytic vascular infiltration caused by chronic norepinephrine infusion. These findings could have been explained in two ways. First, it is possible that the systemic inflammation caused by angiotensin II is due to increased sympathetic outflow, or perhaps other central signals, which were blocked by the AV3V ablation. In this case, norepinephrine administration “bypassed” the effect of the central lesion by direction acting on peripheral adrenergic receptors in a fashion suggested by Ganta et al.81 Another possibility is that angiotensin II-induced T cell activation and vascular inflammation is a direct effect of blood pressure elevation, which was prevented by AV3V-lesioning. To differentiate between these two, we administered hydralazine to prevent the hypertensive response to either angiotensin II or norepinephrine. In both cases, hydralazine completely prevented T cell activation and vascular accumulation of inflammatory cells. This was not due to a direct effect of hydralazine on T cell activation, as hydralazine did not alter the immunological response in another model of ovalbumin immunization.
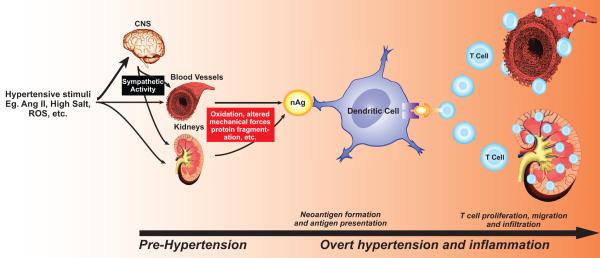
Based on these studies in which we both increased and decreased the central effects of angiotensin II, we have proposed a new paradigm to explain how hypertensive stimuli promote inflammation and elevations in blood pressure in a two-step, feed forward fashion. This working hypothesis is summarized briefly in Figure 2. We suggest that stimuli such as angiotensin II, sodium and others cause a modest elevation in blood pressure to values of about 135 to 140 mmHg. These initial elevations in pressure are largely due to central actions, but also require direct effects of angiotensin II on peripheral sites. This first phase of modest pressure elevation, often referred to as pre-hypertension, brings about an inflammatory response, likely by generating neoantigens that activate T cells. This inflammatory response leads to entry of effector-like T cells into the perivascular fat and the kidney. Macrophage infiltration is also promoted, in part due to signals from T cells. Cytokines and other inflammatory mediators released by these cells work in concert with the direct effects of angiotensin II, catecholamines and salt to cause vascular and renal dysfunction, promote vasoconstriction, vascular remodeling, a shift in the pressure-natriuresis curve and sodium retention, promoting a second phase of severe, sustained hypertension. The inflammatory response in hypertension is very dependent on oxidative events, and is modulated by up and down regulation of critical ROS-generating enzymes such as the NADPH oxidase and by administration of antioxidants such as Tempol, superoxide dismutase or Ebselen. One possibility is that oxidative modification of proteins, lipids or DNA causes neoantigen formation, which initiates the second wave of hypertension illustrated in Figure 2.
Figure 2.
Proposed role of T cells and inflammation in hypertension. Hypertensive stimuli such as angiotensin II and salt cause a modest elevation in pressure (pre-hypertension), in large part because of central stimuli and via direct effects on the kidney and vasculature. We hypothesize that this leads to neoantigen formation, promoting T cell activation as shown in figure 1. Activated T cells enter the kidney and vasculature. T cell derived signals such as IL-17 promote entry of other inflammatory cells, such as macrophages. These inflammatory cells release cytokines that cause vasoconstriction and promote sodium and water absorption, ultimately causing severe hypertension.
Conclusions
This review summarizes a growing body of research supporting a role of inflammation and immunity in hypertension and cardiovascular disease. As reflected in figure 2, we propose that inflammation and immune activation represent responses to modest elevations of blood pressure that are generally considered benign. We emphasize that the paradigm shown in figure 2 represents working hypothesis, and is likely simplistic. Our data and those of others however currently support this proposal and support the importance of the clinical condition commonly referred to as “pre-hypertension”, which while controversial, likely represents a condition in which inflammation initiates a more severe hypertensive state. This emphasizes the benefit of lowering blood pressure by virtually any therapeutic approach and by preventing even the most modest elevations in resting blood pressure. More importantly, it is conceivable that immunotherapy might be useful to treat severe forms of either resistant or malignant hypertension. It is even conceivable that vaccination might be employed to prevent hypertension in the future.
Acknowledgements
We appreciate editorial comments of Dr. William Lewis, Department of Pathology, Emory University.
Sources of Funding: This work was supported by NIH R01HL039006, P01HL058000, P01HL095070. Drs. Marvar and Madhur were supported by NIH F32 post-doctoral fellowship grants. Drs. Thabet, Lob and Vinh were supported by post-doctoral fellowships from the Amercian Heart Association. Dr. Guzik was supported by the European Molecular Biology Organization Young Investigator Program and the Polish Ministry of Science and Technology.
Footnotes
Disclosure of Conflict of Interest: The authors have no conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Vinay DS, Kwon BS. TNF superfamily: costimulation and clinical applications. Cell Biol Int. 2009;33:453–465. doi: 10.1016/j.cellbi.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shilling RA, Bandukwala HS, Sperling AI. Regulation of T:B cell interactions by the inducible costimulator molecule: does ICOS “induce” disease? Clin Immunol. 2006;121:13–18. doi: 10.1016/j.clim.2006.04.574. [DOI] [PubMed] [Google Scholar]
- 3.Gelin C, Sloma I, Charron D, Mooney N. Regulation of MHC II and CD1 antigen presentation: from ubiquity to security. J Leukoc Biol. 2009;85:215–224. doi: 10.1189/jlb.0308206. [DOI] [PubMed] [Google Scholar]
- 4.Pober JS, Kluger MS, Schechner JS. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann N Y Acad Sci. 2001;941:12–25. doi: 10.1111/j.1749-6632.2001.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 5.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 6.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas A, Lichtman A. Cellular and molecular immunology. Elsevier Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 8.Murphy KM, Travers P, Walport M. Janeway’s Immunobiology. Garland Science, Taylor and Francis Group; London, England: 2007. [Google Scholar]
- 9.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 11.Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 12.Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 13.Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock HW, Weber PC. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb. 1994;14:1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- 14.Dhawan S, Singh S, Aggarwal BB. Induction of endothelial cell surface adhesion molecules by tumor necrosis factor is blocked by protein tyrosine phosphatase inhibitors: role of the nuclear transcription factor NF-kappa B. Eur J Immunol. 1997;27:2172–2179. doi: 10.1002/eji.1830270909. [DOI] [PubMed] [Google Scholar]
- 15.Moriuchi H, Moriuchi M, Fauci AS. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 16.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335:191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkbacka H. Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr Opin Lipidol. 2006;17:527–533. doi: 10.1097/01.mol.0000245258.25387.ec. [DOI] [PubMed] [Google Scholar]
- 18.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 19.Cuzzocrea S, McDonald MC, Filipe HM, Costantino G, Mazzon E, Santagati S, Caputi AP, Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur J Pharmacol. 2000;390:209–222. doi: 10.1016/s0014-2999(99)00910-3. [DOI] [PubMed] [Google Scholar]
- 20.Zacharowski K, Olbrich A, Cuzzocrea S, Foster SJ, Thiemermann C. Membrane-permeable radical scavenger, tempol, reduces multiple organ injury in a rodent model of gram-positive shock. Crit Care Med. 2000;28:1953–1961. doi: 10.1097/00003246-200006000-00044. [DOI] [PubMed] [Google Scholar]
- 21.Di Paola R, Mazzon E, Zito D, Maiere D, Britti D, Genovese T, Cuzzocrea S. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J Clin Periodontol. 2005;32:1062–1068. doi: 10.1111/j.1600-051X.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Jubeh TT, Nadler-Milbauer M, Barenholz Y, Rubinstein A. Local treatment of experimental colitis in the rat by negatively charged liposomes of catalase, TMN and SOD. J Drug Target. 2006;14:155–163. doi: 10.1080/10611860600648429. [DOI] [PubMed] [Google Scholar]
- 23.Tsuhako MH, Augusto O, Linares E, Chadi G, Giorgio S, Pereira CA. Tempol ameliorates murine viral encephalomyelitis by preserving the blood-brain barrier, reducing viral load, and lessening inflammation. Free Radic Biol Med. 2010;48:704–712. doi: 10.1016/j.freeradbiomed.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H Oxidase Mediates Angiotensin II-Induced Vascular Macrophage Infiltration and Medial Hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 25.Roson MI, Penna SL Della, Cao G, Gorzalczany S, Pandolfo M, Toblli JE, Fernandez BE. Different protective actions of losartan and tempol on the renal inflammatory response to acute sodium overload. J Cell Physiol. 2010;224:41–48. doi: 10.1002/jcp.22087. [DOI] [PubMed] [Google Scholar]
- 26.Zettl UK, Mix E, Zielasek J, Stangel M, Hartung HP, Gold R. Apoptosis of myelin-reactive T cells induced by reactive oxygen and nitrogen intermediates in vitro. Cell Immunol. 1997;178:1–8. doi: 10.1006/cimm.1997.1113. [DOI] [PubMed] [Google Scholar]
- 27.Pahlavani MA, Harris MD. Effect of in vitro generation of oxygen free radicals on T cell function in young and old rats. Free Radic Biol Med. 1998;25:903–913. doi: 10.1016/s0891-5849(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 28.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huebner RJ, Casey MJ, Chanock RM, Schell K. Tumors induced in hamsters by a strain of adenovirus type 3: sharing of tumor antigens and “neoantigens” with those produced by adenovirus type 7 tumors. Proc Natl Acad Sci U S A. 1965;54:381–388. doi: 10.1073/pnas.54.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeCarvalho S. Detection of neoantigens in the serum of patients with active neoplastic diseases by the absorption-immunodiffusion method. Oncology. 1973;27:193–234. doi: 10.1159/000224734. [DOI] [PubMed] [Google Scholar]
- 31.Kenna JG, Neuberger J, Williams R. Evidence for expression in human liver of halothane-induced neoantigens recognized by antibodies in sera from patients with halothane hepatitis. Hepatology. 1988;8:1635–1641. doi: 10.1002/hep.1840080627. [DOI] [PubMed] [Google Scholar]
- 32.Strife CF, Prada AL, Clardy CW, Jackson E, Forristal J. Autoantibody to complement neoantigens in membranoproliferative glomerulonephritis. J Pediatr. 1990;116:S98–102. doi: 10.1016/s0022-3476(05)82710-6. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Xiang Y, Nakamura H, Nishioka K. Neoantigens in osteoarthritic cartilage. Curr Opin Rheumatol. 2004;16:604–608. doi: 10.1097/01.bor.0000133661.52599.bf. [DOI] [PubMed] [Google Scholar]
- 34.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 37.Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- 38.Hamet P, Malo D, Tremblay J. Increased transcription of a major stress gene in spontaneously hypertensive mice. Hypertension. 1990;15:904–908. doi: 10.1161/01.hyp.15.6.904. [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, Li DG, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995;92:1223–1229. doi: 10.1161/01.cir.92.5.1223. [DOI] [PubMed] [Google Scholar]
- 40.Kunes J, Poirier M, Tremblay J, Hamet P. Expression of hsp70 gene in lymphocytes from normotensive and hypertensive humans. Acta Physiol Scand. 1992;146:307–311. doi: 10.1111/j.1748-1716.1992.tb09424.x. [DOI] [PubMed] [Google Scholar]
- 41.White FN, Grollman A. Autoimmune Factors Associated with Infarction of the Kidney. Nephron. 1964;204:93–102. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 42.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–264. [PubMed] [Google Scholar]
- 43.Takeichi N, Hamada J, Takimoto M, Fujiwara K, Kobayashi H. Depression of T cell-mediated immunity and enhancement of autoantibody production by natural infection with microorganisms in spontaneously hypertensive rats (SHR) Microbiol Immunol. 1988;32:1235–1244. doi: 10.1111/j.1348-0421.1988.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 44.Takeichi N, Boone CW. Spontaneous rosette formation of rat thymus cells with guinea pig erythrocytes. Cell Immunol. 1976;27:52–59. doi: 10.1016/0008-8749(76)90153-2. [DOI] [PubMed] [Google Scholar]
- 45.Purcell ES, Wood GW, Gattone VH., 2nd Immune system of the spontaneously hypertensive rat: II. Morphology and function. Anat Rec. 1993;237:236–242. doi: 10.1002/ar.1092370211. [DOI] [PubMed] [Google Scholar]
- 46.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 47.Purcell ES, Gattone VH., 2nd Immune system of the spontaneously hypertensive rat. I. Sympathetic innervation. Exp Neurol. 1992;117:44–50. doi: 10.1016/0014-4886(92)90109-4. [DOI] [PubMed] [Google Scholar]
- 48.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun. 1981;99:600–607. doi: 10.1016/0006-291x(81)91787-3. [DOI] [PubMed] [Google Scholar]
- 49.Dzielak DJ. Immune mechanisms in experimental and essential hypertension. Am J Physiol. 1991;260:R459–467. doi: 10.1152/ajpregu.1991.260.3.R459. [DOI] [PubMed] [Google Scholar]
- 50.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 51.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C. 1980;88:1–5. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 52.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansson GK. Immune and inflammatory mechanisms in the development of atherosclerosis. Br Heart J. 1993;69:S38–41. doi: 10.1136/hrt.69.1_suppl.s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran LT, MacLeod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem. 2009;330:219–228. doi: 10.1007/s11010-009-0136-z. [DOI] [PubMed] [Google Scholar]
- 59.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 63.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr., Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 64.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sturgis LC, Cannon JG, Schreihofer DA, Brands MW. The role of aldosterone in mediating the dependence of angiotensin hypertension on IL-6. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1742–1748. doi: 10.1152/ajpregu.90995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witowski J, Ksiazek K, Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee KA, Kang MH, Lee YS, Kim YJ, Kim DH, Ko HJ, Kang CY. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol. 2008;251:50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 71.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 73.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 74.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 75.Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H938–944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 76.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 78.Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13:693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- 79.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 80.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 81.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 82.Fannon LD, Phillips MI. Chronic ICV infusion of neuropeptides alters lymphocyte populations in experimental rodents. Regul Pept. 1991;34:189–195. doi: 10.1016/0167-0115(91)90178-j. [DOI] [PubMed] [Google Scholar]
- 83.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 84.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 85.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 86.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antonelli LR, Dutra WO, Oliveira RR, Torres KC, Guimaraes LH, Bacellar O, Gollob KJ. Disparate immunoregulatory potentials for double-negative (CD4− CD8−) alpha beta and gamma delta T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006;74:6317–6323. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brody M, Fink G, Buggy J, Haywood J, Gordon F, Knuepfer M, Mow M, Mahoney L, Johnson A. Critical role of the AV3V region in development and maintenance of experimental hypertension. In: Schmitt H, Meyers P, editors. Perspectives in Nephrology and Hypertension. Wiley and Flammarion; New York, NY: 1978. pp. 76–84. [Google Scholar]
- 90.Svendsen UG. The role of thymus for the development and prognosis of hypertension and hypertensive vascular disease in mice following renal infarction. Acta Pathol Microbiol Scand A. 1976;84:235–243. doi: 10.1111/j.1699-0463.1976.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 91.Takeichi N, Suzuki K, Okayasu T, Kobayashi H. Immunological depression in spontaneously hypertensive rats. Clin Exp Immunol. 1980;40:120–126. [PMC free article] [PubMed] [Google Scholar]
- 92.Takeichi N, Ba D, Koga Y, Kobayashi H. Immunologic suppression of carcinogenesis in spontaneously hypertensive rats (SHR) with T cell depression. J Immunol. 1983;130:501–505. [PubMed] [Google Scholar]
- 93.Takeichi N, Ba D, Kobayashi H. Natural cytotoxic autoantibody against thymocytes in spontaneously hypertensive rats. Cell Immunol. 1981;60:181–190. doi: 10.1016/0008-8749(81)90258-6. [DOI] [PubMed] [Google Scholar]
- 94.Norman RA, Jr., Dzielak DJ. Spontaneous hypertension is primarily the result of sympathetic overactivity and immunologic dysfunction. Proc Soc Exp Biol Med. 1986;182:448–453. doi: 10.3181/00379727-182-42364. [DOI] [PubMed] [Google Scholar]