Abstract
Objectives
Although most currently available synthetic meshes are lightweight, macroporous polypropylene, they differ in regard to pore size, knit pattern and surface characteristics, all of which may impact behavior. In this study, we compare the biomechanical properties of 4 commonly used prolapse meshes relative to Gynecare PS ™, using a tensile testing protocol.
Methods
Full length meshes [Gynecare PS™ (Ethicon), Pelvitex ™ (Bard), Popmesh™, Timesh ™ (Caldera), and Polyform (Boston Scientific)] were divided into 15 × 5 cm samples. Light microscopy was used to define pore size. For tensile testing, each mesh was either loaded to failure (n=5 per group) or cyclically loaded (n=3 per group). Data abstracted from the largely bilinear load-elongation curves included the low and high stiffness, the point of transition between them (inflection point), the load at failure, and the relative elongation.
Results
Microscopic analysis demonstrated that the pore size for all of the meshes were distinct. With the exception of Popmesh which displayed linear behavior, all prolapse meshes were characterized by a very low initial stiffness increasing by an order of magnitude into the high stiffness region. The newer meshes were 70%-90% less stiff than Gynecare (p< 0.05) and more readily deformed in response to cyclical loading (greater permanent elongation; p < 0.001). There was a significant positive correlation between mesh weight and load at failure, (p< 0.001).
Conclusions
Newer meshes are significantly less stiff than Gynecare PS. The significant amount of permanent elongation of these meshes may be important to consider, especially during the early post-operative period when tissue in growth has yet to take place.
Introduction
Synthetic meshes are commonly used in most urogynecologic procedures including sacrocolpopexy, sub-urethral slings, colposuspension and anterior and posterior repairs. A recent web-based survey sent to the American Urogynecological Society revealed that 93% of respondents use synthetic mesh when performing a sacrocolpopexy and 58% when performing vaginal reconstructive surgery [1]. There is consistent and robust evidence supporting use of synthetic mesh in the apical compartment in an abdominal approach with sacrocolpopexy [2]. There is however, a paucity of data supporting the use of synthetic material in vaginal reconstructive surgery. Despite this lack of evidence, the use of synthetic mesh has increased. Although many pelvic reconstructive surgeons have incorporated the use of synthetic mesh in prolapse repair, the rationale for this decision and the choice of mesh material generally lacks a scientific basis.
The rationale for using mesh is to improve the permanency and success of reconstructive procedures that currently have a failure rate in excess of 30% [3]. In addition, there is data and clinical experience to suggest that the endogenous tissues of women with prolapse may be inherently weaker [4, 5] and therefore, prone to failure in the absence of augmentation. Currently, there is a wide-range of biomaterials available to clinicians for incontinence and prolapse procedures with little quantitative data on which to base the selection of a particular material. Although limited data suggests that in terms of anatomical [6] and biomechanical [7] outcomes synthetic polypropylene meshes are superior to biologic meshes, there is significant evidence that the complications associated with synthetic meshes can cause significant morbidity including infection, exposure, and dyspareunia [8].
Synthetic graft materials differ by composition (monofilament vs. multifilament), flexibility [4], pore size, surface properties (coated vs. noncoated), and architecture (knit vs. woven). Importantly, a graft should not interfere with vaginal function, not cause pain and be compatible with the properties of the tissue it is replacing. There are multiple graft materials available, but to date, none meet all of these criteria [9, 10].
Synthetic mesh can provide the necessary support to strengthen and improve the durability of prolapse repair. Unfortunately, the vaginal tissue to be augmented is often structurally compromised, atrophic and devascularized. Such poor tissue quality increases the risk of poor tissue incorporation into the mesh resulting in suboptimal healing and mesh exposure. In addition, there is some evidence that meshes shrink in vivo leading to increased stiffness, pain and poor restoration of the normal properties of the vagina. In this way, the choice of mesh for surgical augmentation is extremely important.
Here, we contend that prior to determining the behavior of a mesh in vivo, one must have a comprehensive understanding of its tensile properties ex vivo. By improving our understanding of the differences between different meshes prior to host incorporation, we will have a more durable foundation upon which to base differences in the behavior of these slings in vivo. To date, the primary shortcoming of synthetic mesh is host tolerability. Inability for the host to tolerate mesh manifests as exposure, erosion into hollow viscous, chronic inflammation, pain and infection. Many manufacturers have modified polypropylene mesh in attempt to improve host tolerability. Popmesh ™ is manufactured as a light-weight (19 g/m2) polypropylene with increased flexibility for increased patient comfort. Timesh ™ is composed of titanized polypropylene for less foreign body reaction, decreased shrinkage, and increased biocompatibility; it is available in various densities including a light (35 g/m2) and an extra light (16 g/m2) mesh. Pelvitex ™ is marketed with a hydrophilic porcine collagen I coat for enhanced healing, increased strength and durability. Polyform ™ is an uncoated polypropylene mesh that is marketed as a softer, thinner mesh and is said to be 40% less stiff than the currently available prolapse meshes. All of the currently available meshes are likely modifications of Gynecare PS ™, which was the first polypropylene mesh created exclusively for prolapse repair (Table 1).
Table 1. Properties of Prolapse meshes provided by manufacturer.
| Mesh Type | Ethicon (Gynecare PS) | Boston Scientific (Polyform) | Caldera (Popmesh) | Caldera (Timesh) | Bard (Pelvitex) |
| Material | polypropylene | polypropylene | polypropylene | polypropylene | polypropylene |
| Structure | monofilament knit | monofilament knit | monofilament knit | monofilament knit | monofilament knit |
| Thickness (mm) | 0.1 | 0.1 | .22 | .24 | .26 |
| Weight (g/m2) | 44 | 40 | 19 | 38 | 16 |
| Features | * | Thin PP | Lightweight | Titanized PP | Porcine coated PP |
Not applicable
Mechanical behavior of a specific mesh is dependent on its composition, fiber type, weave, and pore size. Thus, in this study we seek to quantitate these differences in comparison to Gynecare PS™utilizing a tensile testing protocol. Each mesh is loaded along its longitudinal axis and load at failure and relative elongation is obtained. Slopes in the low and high stiffness region of the curves will be assessed and compared to Gynecare.
Materials/Methods
Prolapse mesh manufacturers have suggested that changes in the newer of meshes may result in superior host tolerability and decreased complications. These novel characteristics are thought to result in improved mesh tolerance due to decreased inflammation and increased comfort. The properties have been delineated in Table 1.
The methods for tensile testing have previously been described [13]. Briefly, sterile samples of five full-length meshes [Gynecare PS™ (Ethicon), Pelvitex ™ (Bard), Popmesh™, Timesh ™ (Caldera), and Polyform (Boston Scientific)] were obtained. Samples were removed from its sterile packing and divided into 15 × 5 cm sections, these dimensions chosen to simulate the size of mesh implanted surgically in an abdominal prolapse repair. A small 1.0 cm section was removed from the end of each mesh for imaging. A total of five samples of each mesh were obtained and tested separately (N=5) under a load to failure protocol.
Imaging
Samples of each mesh were obtained and imaged with transmission electron microscopy. Pore size was calculated as the average area over all pores within the sample.
Tensile Testing Protocol
Mesh samples were attached to a custom clamps to form a clamp-mesh-clamp construct. Clamp to clamp distances were measured. To ensure that samples were loaded uniformly, an aspect ratio of at least five was maintained for all samples.
Once the meshes were properly affixed in the clamps, each sample was placed in a 37-degree saline bath and given ten minutes to equilibrate. One clamp was rigidly affixed to the base of the Instron ™ 4502 screw driven testing apparatus, and the other fixed to a load cell, which is attached to the crosshead of the testing apparatus. A preload of 0.1 N was applied using an elongation rate of 10 mm/min. Measurement of the clamp to clamp distance was obtained for calculations of relative elongation. After preloading, the samples were loaded to failure along the longitudinal axis at a rate of 50 mm/min. The load at failure in Newton's (N) and the elongation in millimeters (mm) was obtained. The relative elongation of the samples was calculated by dividing the elongation by the initial pre-test clamp to clamp distance.
Load versus relative elongation curves were plotted and analyzed. Curves were bilinear, with an initial region of low stiffness that transitioned into a high stiffness region at the point of inflection (Figure 2). The low stiffness region(N/mm) was defined as the minimum slope over a 15% interval of relative elongation. The high stiffness region (N/mm) was defined as the maximum slope over a 30% interval of relative elongation. The inflection point was defined as the intercept of the two tangent lines fit in these two regions. The load (N) and relative elongation (%) at failure were also recorded.
Figure 2.
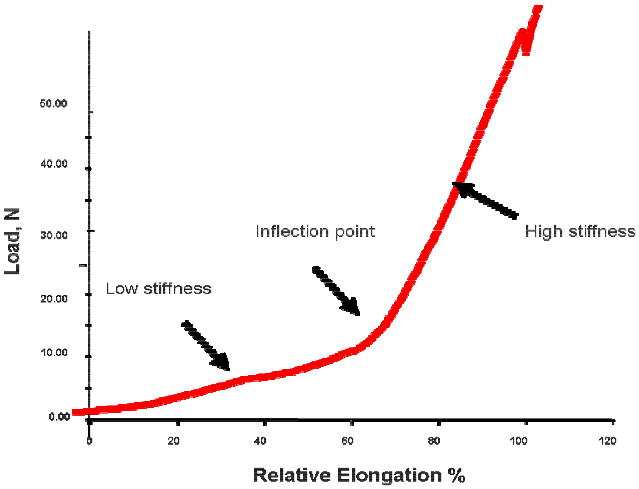
Typical Load - Relative Elongation Curve demonstrating the non-linear behavior of the prolapse meshes.
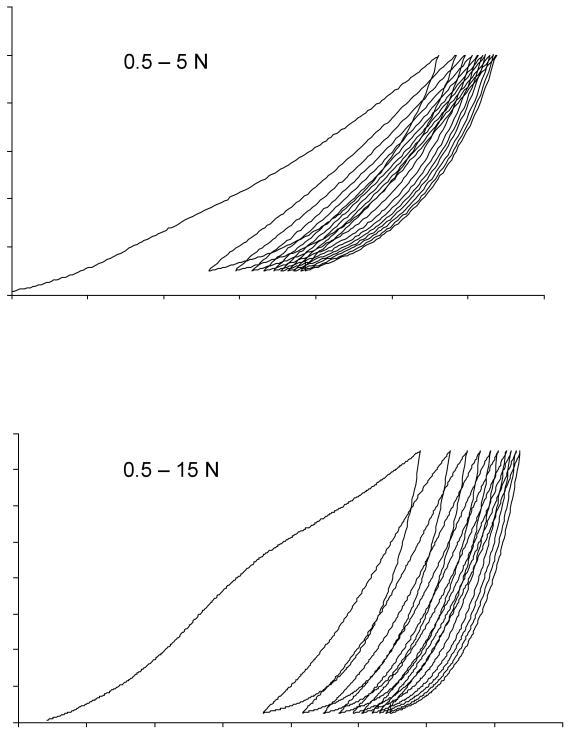
An additional three samples per mesh type (N=3) were cyclically tested using three protocols (C1-C3) in order to assess permanent elongation of the mesh. Samples were testing using a cyclical loading protocol to simulate repetitive loading in-vivo. This protocol has been described previously [10]. Cyclical testing was performed under the assumption that the prolapse meshes will undergo consecutive loads in-vivo as a result of activities resulting in brief increases in intrabdominal pressure. Briefly, samples were preloaded to 0.1 N at a rate of 10 mm/min and the cross-head position was set to zero. The clamp-to-clamp distance was measured; meshes were then cycled from 0.5-5 N (C1), 0.5-15 N (C2), and finally 0.5-5 N (C3), each for ten cycles. The relative elongation after each portion of the cyclical loading protocol (C1-C3) was again measured by applying a 0.1 N preload and measuring the difference between the current crosshead position from its initial position. This represented the permanent deformation of the mesh in response to cyclical loading.
Statistical Analysis
Sample Size calculations were based on initial data from a previous study [13] from testing Gynecare TVT™ slings. Five samples per group were needed to detect minimum of 100% difference in low stiffness, 15% difference in the inflection point, and 75% difference in permanent elongation between Gynecare and other brands with 80% power to detect the difference. One way Analysis of variance was used to assess differences between groups. Post hoc comparison was performed doing Dunnett's multiple comparison procedure.
Results
Ultrastructural analysis by transmission electron microscopy revealed that knit patterns for all the meshes were distinct as shown in Figure 1. All meshes were knitted polypropylene. Gynecare PS ™ had the largest pore size at 5.82 mm2 and Timesh was the smallest at 1.16 mm2.
Figure 1.
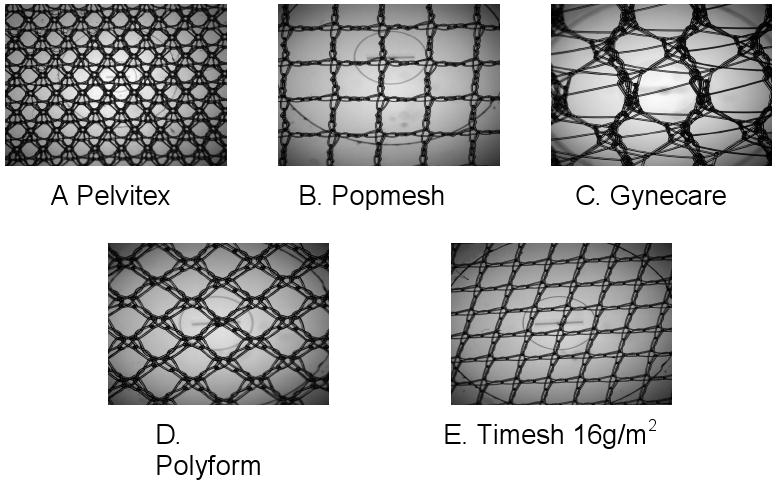
Macroscopic analysis of meshes revealed that knit pattern and pore sizes were unique for each mesh type. A. Pelvitex (1.23 mm2), B. Popmesh (2.98 mm2), C. Gynecare (5.82 mm2), D. Polyform (1.63 mm2), and E. Timesh 16 (1.16 mm2).
The results of the mechanical loading protocol revealed that the general shape of Load -Relative Elongation curves for Gynecare, Pelvitex, Timesh and Polyform were similar (Figure 2). Specifically, the curves were bilinear for all the meshes with the exception of Popmesh, which displayed a distinctly linear behavior (Figure 3). In this way, for Popmesh only a single stiffness value is reported. In contrast, the curves from all remaining meshes were defined by two distinct regions each characterized by a stiffness value. Initially, the curves displayed a region of low stiffness in which the meshes easily deformed under the application of a small load. This was followed by a transition into a region of high stiffness in which the mesh became more resistant to deformation. We referred to the inflection point between the two regions as the transition point. The low stiffness region was significantly higher for Gynecare (0.27± 0.09 N/mm) when compared to Pelvitex, Polyform and Timesh at, 0.07± 0.03, 0.05±0.01 and 0.02± 0.01 N/mm, respectively. Popmesh displayed linear behavior on its resultant load elongation curve with a single stiffness 036 ±0.09 N/mm.
Figure 3.
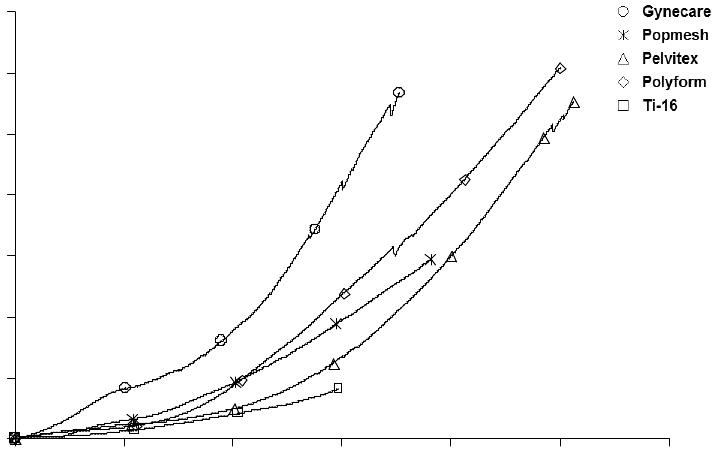
Load - Relative Elongation Curve demonstrating the Bi-linear behavior of the prolapse meshes, namely Gynecare PS ™, Pelvitex, Polyform, and Timesh. The inflection point represents the transition between low and high stiffness. Note the linear behavior of Popmesh through the load-elongation curve.
On average, the inflection point corresponded to approximately 10 N of applied load or roughly 2.2 lbs. At the inflection point Gynecare, Polyform, Pelvitex, Timesh elongated an average of 20% of their initial length, with relative elongation values of 33.33 ± 9.62%, 25.4 4± 7.09 %, 41.28 ± 19.23%, and 18.03 ± 9.10 %, (p=.043) respectively. As stated previously, no value is reported for Popmesh which displayed a single stiffness with increasing loads.
In the high stiffness region, the loads applied to the mesh exceed was is expected in normal physiologic conditions and at this point the meshes had elongated by 20% of their initial length. Overall, the meshes behaved similarly in this region of the Load Relative Elongation curve. Gynecare was the stiffest mesh with a slope of 1.25± 0.21 N/mm, p<0.05. The newer meshes were significantly less stiff, 0.36 ± 0.09, 0.69 ± 0.13, 0.87 ± 0.07, and 0.17 ± 0.013 N/mm for Popmesh, Polyform, Pelvitex, and Timesh respectively.
There was a strong positive correlation between mesh weight and load at failure (r= 0.938, p<0.001). Mesh weight compromised tensile strength with the lighter meshes failing at the lowest loads. Timesh and Popmesh failed at 9.62± 1.21 N, 21.40 ± 6.13 N respectively. Corresponding to 61.66 ± 4.52 % and 60.95 ± 9.96 % relative elongation. The remaining meshes had similar failure behavior. Gynecare, Polyform and Popmesh failed at 68.34 ±12.45 N, 51.67± 8.53 N, and 55.35 ± 6.99 N, respectively, having reached 71.50 ± 2.97, 60.95 ± 9.96, 92.25± 16.70, and 100.65 ± 8.62 % of their initial length. As previously observed, all meshes tended to fail near the clamp [13]. As a result, failure behavior is likely underestimating the true failure values. Never-the-less, these parameters; load at failure and relative elongation, are magnitudes beyond those that would be experienced in vivo.
The cyclical loading protocol was developed to simulate chronic intermittent increases in intra abdominal pressure. When cycled between .5 and 15N (C2 protocol), Timesh failed as predicted from the tensile testing (average failure load of TiMesh was 9.62 N). This magnitude was exceeded in C2 as the protocol required cycling from 0.5-15 N. Typical curves for cyclical loading of Gynecare PS are shown in Figure 4 and results are provided in Table 3.
Figure 4.
Typical curves for cyclical loading of Gynecare PS ™ following cyclical loading at C1 (10 cycles from, 0.5-5 N) and C2 (10 cycles from 0.5-15 N) Note with each cycle the peaks and valleys of the curve move towards the right indicating permanent elongation of the mesh.
Table 3. Cyclical Loading of Polypropylene Prolapse meshes relative to those of Gynecare PS.
| Mesh | Percent elongation after C1 (%) | Percent elongation after C2 (%) | Percent elongation after C3 (%) |
|---|---|---|---|
| Gynecare (n=3) | 3.0 ± 1.5 | 19.6 ± 1.8 | 20.3 ± 3.0 |
| Popmesh (n=3) | 9.2 ± 0.7 | 31.6 ± 4.3 | 32.1 ± 4.0 |
| Polyform (n=3) | 11.3 ± 2.6 | 28.7 ± 2.4 | 29.6 ± 1.7 |
| Pelvitex (n=3) | 19.9 ± 1.4 | 37.5 ± 2.8 | 39.5 ± 1.2 |
| TiMesh (n=3) | 23.6 ± 1.0 | Not Done | Not Done |
| Overall P* | < 0.001 | 0.001 | < 0.001 |
| Gynecare vs. Pop mesh† | 0.002 | 0.003 | 0.002 |
| Gynecare vs. Polyform† | < 0.001 | 0.013 | 0.008 |
| Gynecare vs. Pelvitex† | < 0.001 | < 0.001 | < 0.001 |
| Gynecare vs. TiMesh† | < 0.001 | Not Done |
Overall P-values from one-way analysis of variance
P-values from Dunnett's multiple comparison's procedure comparing Gynecare to the other brands.
The permanent elongation after C1 (cycling between, 0.5 and 5 N, 10 cycles) for the Gynecare mesh was different from all other samples tested. Gynecare samples elongated to 3.0% of its initial length, which was significantly lower than all other meshes Popmesh (9.2 %± 0.7, p=.002), Polyform (11.3% ± 2.6, p<0.001), Pelvitex (19.9% ± 1.4, p<0.001), and Timesh (23.6 % ± 1.0, p<0.001).
The permanent elongation after C2 (cycling between, 0.5 and 15 N, 10 cycles) of the Gynecare mesh was also distinct from all other samples. After C2, Gynecare samples elongated to 20% of its initial length which was significantly lower than all other meshes Popmesh (31.6% ± 4.3, p=0.003), Polyform (28.7% ± 2.4, p=0.013), Pelvitex (37.5 ± 2.8, p<0.001). Timesh failed during C2.
Permanent elongation remained significant after C3, this cycle repeated the loading conditions of C1. Gynecare samples elongated to 20% of the initial length which was significantly lower than all other meshes. Popmesh (32.1%± 4.0, p<0.001), Polyform (29.6% ± 1.7, p=0.002), and Pelvitex (39.5% ± 1.2, p<0.001).
Discussion
We performed ex vivo uniaxial tensile testing of synthetic prolapse meshes to distinguish mesh mechanical behavior in comparison to Gynecare PS ™. Gynecare is a widely used prolapse mesh during clinical repairs, and it is important to compare this standard to the structural properties and design of meshes that are now available. We have shown that newer prolapse meshes have distinct tensile behavior when compared to Gynecare. Gynecare had a significantly higher stiffness value when compared to each of the described meshes. This was true for all meshes in the low and high stiffness regions, with the exception of Popmesh which displayed a linear behavior corresponding to increased stiffness.
Currently, the emphasis in clinical outcomes following a mesh procedure has been the restoration of anatomy. Most studies are limited by small sample size; a lack of a consensus on what defines an anatomic cure, and a failure to consistently report adverse outcomes. Huebner et al. [11] reviewed the available literature on the use of graft materials in anterior, posterior, and apical compartments. They found anatomic success rates of 42-100%, erosion rates of 0-12.9%, and 5-20% of cases were complicated by de novo dyspareunia. Other complications associated with synthetic meshes include de novo urgency, de novo urge or stress incontinence, and urinary retention. Clinically, and particularly problematic, is the fact that on exam following surgery, synthetic meshes significantly change the texture of the vaginal wall and can be palpated as a stiff material below the surface of the vaginal epithelium. Moreover, the sites of graft placement have poor distensibility and flexibility indicating properties that are not compatible with those of the non-grafted vagina clinically manifesting as pain, dypareunia and pelvic discomfort.
What we do know is that a Type I, large pore, monofilament is the preferable synthetic material [10-12]. There are however, additional properties of the mesh that are left to the discretion of the surgeon including mesh thickness, stiffness/elasticity, coating, and pore size (within the macroporous spectrum).
Therefore it is important to understand the stiffness of each of these meshes and the impact it may have in vivo. In the low stiffness region, which corresponds to more physiologic loads experienced by the mesh in vivo, meshes deformed an average of 30% of their initial length. At failure, meshes were roughly 80% longer (10 cm) than their initial length. However, loads seen at the high stiffness region and failure are supraphysiologic and would not likely be experience in vivo, thus making values in the low stiffness region more clinically relevant.
With cyclical loading, we see that there is a significant mesh deformation that is permanent. This finding might be of considerable importance in the early post-operative period prior to tissue in-growth when the mesh may possibly move more freeing between host tissues. With continued consecutive increases in intrabdominal pressure, meshes may undergo significant permanent alteration in length that could translate into early post operative failures.
Currently, to the authors knowledge, this is the first study designed to compare Gynecare and the new generation of meshes based on the structural properties. Other studies have noted the complications of these meshes without examining truly trying to understand the difference between these meshes that may give rise to the different results observed clinically. An editorial written by Ison-Batz and Zimmerman illustrates the lack of information concerning these meshes and its possible impact on creating ineffective treatment for patients seeking corrective surgery for pelvic organ prolapse [14]. Characterizing each of these meshes structural properties may help clinicians gain insight on the mechanism of mesh and poor surgical outcome.
Previously mesh erosion has been correlated to the stiffness of the mesh [11]. A material with a higher stiffness value will deform less with increasing loads. Meshes for prolapse repair can have an erosion occur in up to 12.0% of patients when placed abdominally [15]. Performing this simple tensile test may provide an understanding of why these rates occur and with future studies help determine which type of mesh may lead to a lower rate erosion. Although this was an ex vivo study, currently standardized testing of these meshes has not been reported which leaves clinicians with little information on determining which mesh type would provide the best outcome for their patients. Pore size of each of these was also found to be considerably different from Gynecare. Pore size is known to affect the biological response that could lead to infection, erosion, and inflammation of the tissue interacting with the mesh.
The major weakness of this paper was the use of ex vivo tensile testing, therefore the biological response to these meshes, their mechanical properties, pore size, and coating could not be determined. It is clear that such testing must be completed after the graft has been well incorporated into host tissue, as their will be considerable load sharing between the mesh and the newly incorporated host tissue. However, understanding the mechanical behavior and design differences (i.e. pore size) of each prolapse meshes ex vivo is a fundamental first step in allowing clinicians to reach a more educated decision for patient graft choice. Future studies will involve the use of an animal model to explore mesh behavior after periods of healing and host incorporation, enabling us to define parameters most relevant to clinicians in selection of prolapse meshes.
Table 2. Tensile properties of Polypropylene Prolapse meshes relative to those of Gynecare PS.
| Mesh | Weight ‡ | Low Stiffness (N/mm) | High Stiffness (N/mm) | Relative Elongation at Inflection Point (%) | Load at failure (N) | Relative Elongation at failure (%) |
|---|---|---|---|---|---|---|
| Gynecare (n=5) | 44 | 0.27 ± 0.09 | 1.25 ± 0.21 | 33.33 ± 9.62 | 68.34 ± 12.45 | 71.50 ± 2.97 |
| Popmesh (n=5) | 19 | N/A | 0.36 ± 0.09 | N/A | 21.40 ± 6.13 | 60.95 ± 9.96 |
| Polyform (n=5) | 40 | 0.05 ± 0.01 | 0.69 ± 0.13 | 25.44 ± 7.09 | 51.67 ± 8.53 | 92.25 ± 16.70 |
| Pelvitex (n=6) | 38 | 0.07 ± .03 | 0.87 ± 0.07 | 41.28 ± 19.23 | 55.35 ± 6.99 | 100.65 ± 8.62 |
| TiMesh (n=5) | 16 | 0.02 ± .01 | 0.17 ± 0.03 | 18.03 ± 9.10 | 9.62 ± 1.21 | 61.66 ± 4.52 |
| Overall P* | < 0.001 | < 0.001 | 0.043 | < 0.001 | < 0.001 | |
| Gynecare vs. Popmesh† | N/A | < 0.001 | N/A | < 0.001 | 0.282 | |
| Gynecare vs. Polyform† | < 0.001 | < 0.001 | 0.643 | 0.011 | 0.010 | |
| Gynecare vs. Pelvitex† | < 0.001 | < 0.001 | 0.609 | 0.043 | < 0.001 | |
| Gynecare vs. TiMesh† | < 0.001 | < 0.001 | 0.174 | < 0.001 | 0.338 |
Overall P-values from one-way analysis of variance
P-values from Dunnett's multiple comparison's procedure comparing Gynecare to the other brands.
as reported by manufacturer
Bibliography
- 1.Pulliam SJ, Ferzandi TR, Hota LS, Elkadry E, et al. Use of synthetic Mesh in pelvic reconstructive surgery: a survey of attitudes and practice pattern of urogynecologists. Int Urogynecol J. 2007 doi: 10.1007/s00192-007-0360-6. [DOI] [PubMed] [Google Scholar]
- 2.Nygaard IE, MS, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM, Zyczynski H, Pelvic Floor Disorders Network Abdominal Sacrocolpopexy: A Comprehensive Review. Obstetrics & Gynecology. 2004 October;104(4):805–823. doi: 10.1097/01.AOG.0000139514.90897.07. [DOI] [PubMed] [Google Scholar]
- 3.Olsen AL, Smith VJ, Bergstom JO, et al. Epidemiology of Surgically Managed Pelvic Organ Prolapse and Urinary Incontinence. Obstet Gynecol. 1997;89:501–06. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 4.Jameson JS, Chia YW, Kamm MA, et al. Effects of age, sex, and parity on anorectal function. Br J Surg. 1994;81:1689–92. doi: 10.1002/bjs.1800811143. [DOI] [PubMed] [Google Scholar]
- 5.Jones NHJ, Healy JC, King LJ, et al. Pelvic Connective Tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br J Surg. 2003;90:466–72. doi: 10.1002/bjs.4065. [DOI] [PubMed] [Google Scholar]
- 6.Chen CCG, Ridgeway B, Paraiso MF. Biologic grafts and Synthetic meshes in pelvic reconstructive Surgery. Clin Obstet and Gynecol. 2007;50(2):383–411. doi: 10.1097/GRF.0b013e31804b184c. [DOI] [PubMed] [Google Scholar]
- 7.Chandler D, Dimarco D, Zobitz M, Elliot D. Time Dependent variations in Biomechanical properties of Cadaveric Fascia, Porcine Dermis, Porcine small Intestine Submucosa, polypropylene mesh and autologous fascia in the rabbit model: implication for sling surgery. Journal of Urology. 2004;171:1970–1973. doi: 10.1097/01.ju.0000121377.61788.ad. [DOI] [PubMed] [Google Scholar]
- 8.Amrute K, Badlani G. Female Incontinence: a review of biomaterials and minimally invasive techniques. Current Opinion Urol. 2006;16:54–59. doi: 10.1097/01.mou.0000193381.93608.dc. [DOI] [PubMed] [Google Scholar]
- 9.Cosson M, Debodinance P, Boukerrou M, Chauvet MP, Lobry P, Crepin G, Ego A. Mechanical properties of Synthetic implants used in repair of prolapse and urinary incontinence in women: Which is the ideal material? Int Urogynecol J. 2003;14:169–178. doi: 10.1007/s00192-003-1066-z. [DOI] [PubMed] [Google Scholar]
- 10.Fenner DE. New Surgical Mesh. Clin Obstet Gynecol. 2000;43:647–52. doi: 10.1097/00003081-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Huebner M, Fenner DE. The use of graft material in vaginal pelvic floor surgery. Int Jour Obstet Gynecol. 2006;92:279–88. doi: 10.1016/j.ijgo.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Amid PK. Classification of biomaterials and their related complications in abdominal wall surgery. Hernia. 1997;1:15–21. [Google Scholar]
- 13.Moalli PM, Papas N, Menefee S, Abramowitch S. Tensile properties of Six Commonly used Mid-Urethral Slings. doi: 10.1007/s00192-007-0499-1. Accepted 8/2007. [DOI] [PubMed] [Google Scholar]
- 14.ANDREW TO INSERT!!!!
- 15.Kohli N, Walsh PM, Roat TW, Karram MM. Mesh Erosion After Abdominal Sacrocolpopexy. Obstet Gynecol. 1998;92:999–1004. doi: 10.1016/s0029-7844(98)00330-5. [DOI] [PubMed] [Google Scholar]