Introduction
Frailty is a common clinical syndrome in older adults that carries an increased risk for poor health outcomes including falls, incident disability, hospitalization, and mortality (1-5). Elucidating its etiology and natural history is therefore critical for identifying high risk subsets and new arenas for frailty prevention and treatment.
In an attempt to standardize and operationalize the definition of frailty, Fried and colleagues proposed a clinical phenotype of frailty as a well-defined syndrome with biological underpinnings (2). They hypothesized that the clinical manifestations of frailty are related in a mutually exacerbating cycle of negative energy balance, sarcopenia, and diminished strength and tolerance for exertion. Building on this conceptual framework, preliminary evidence has now been obtained on the natural history of the clinical phenotype of frailty (3, 6). This paper reviews the current state of knowledge regarding the epidemiology of frailty by focusing in six specific areas: (i) clinical definitions of frailty, (ii) evidence of frailty as a medical syndrome, (iii) prevalence and incidence of frailty by age, gender, race, and ethnicity, (iv) transitions between discrete frailty states, (v) natural history of manifestations of frailty criteria, and (vi) behavior modifications as precursors to the development of clinical frailty.
Definition of Frailty
Frailty is theoretically defined as a clinically recognizable state of increased vulnerability resulting from aging-associated decline in reserve and function across multiple physiologic systems such that the ability to cope with everyday or acute stressors is comprised. In the absence of a gold standard, frailty has been operationally defined by Fried et al. as meeting three out of five phenotypic criteria indicating compromised energetics: low grip strength, low energy, slowed waking speed, low physical activity, and/or unintentional weight loss (2) (Table 1). A pre-frail stage, in which one or two criteria are present, indentifies a subset at high risk of progressing to frailty. Various adaptations of Fried’s clinical phenotype have emerged in the literature, which were often motivated by available measures in specific studies rather than meaningful conceptual differences.
Table 1.
Frailty-defining criteria: Women’s Health and Aging Studies (WHAS) and Cardiovascular Health Study (CHS)
| Characteristics | CHS | WHAS |
|---|---|---|
| 1. Weight loss |
Baseline: Lost > 10 pounds unintentionally in last year Follow-up: (weight in previous year-current weight)/(weight in previous year)≥0.05 and the loss was unintentional |
Baseline: Either of:
Follow-up: Either of :
|
| 2. Exhaustion | Self report of either of:
|
Self report of any of: |
| 3.Low Physical Activity |
Women: Kcal < 270 on activity scale (18 items) Men: Kcal < 383 on activity scale (18 items) |
Women: Kcal < 90 on activity scale (6 items) Men: Kcal< 128 on activity scale (6 items) |
| 4. Slowness | walking 15 feet (4.57m) at usual pace Women: time >= 7 s for height <= 159 cm time >= 6 s for height > 159 cm Men: time >= 7 s for height <= 173 cm time >= 6 s for height > 173 cm |
walking 4m at usual pace Women: speed <= 4.57/7 m/s for height <= 159 cm speed <= 4.57/6 m/s for height > 159 cm Men: speed <= 4.57/7 m/s for height <= 173 cm speed <= 4.57/6 m/s for height > 173 cm |
| 5. Weakness | Grip strength Women: <= 17 kg for BMI <= 23 <=17.3 kg for BMI 23.1 - 26 <= 18 kg for BMI 26.1 - 29 <= 21 kg for BMI > 29 Men: <= 29 kg for BMI <= 24 <= 30 kg for BMI 24.1 - 26 <= 30 kg for BMI 26.1 - 28 <= 32 kg for BMI > 28 |
Grip strenth: Same as in CHS |
Rated on 0-10 scale, where 0 = “no energy” and 10 = “the most energy that you have ever had.”
If yes, there followed questioning “how much of the time” the feeling persisted; responses “most” or “all” of the time were considered indicative of exhaustion.
Alternatively, frailty has been operationalized as a risk index by counting the number of deficits accumulated over time (termed “frailty index (FI)”) including disability, diseases, physical and cognitive impairments, psychosocial risk factors, and geriatric syndromes (e.g. falls, delirium, and urinary incontinence) (44). It was argued that, compared to Fried’s frailty phenotype, the FI is a more sensitive predictor of adverse health outcomes due to its finer graded risk scale, and its robustness in clinical inferences with regard to the number and actual composition of the items in the FI (46).
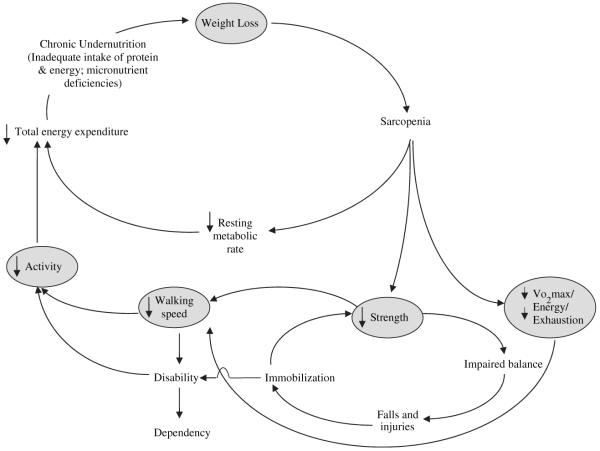
However, our discussion of the epidemiology of frailty in this chapter focuses on Fried’s phenotypic definition of frailty for a number of reasons. First, there is increasing consensus that frailty is a definable clinical state involving multiple signs and symptoms. Second, the clinical manifestations of frailty, in theory, may be organized into a self-perpetuating cycle of naturally progressing events (Figure 1) (2, 7) consistent with clinical observations. Third, converging lines of evidence suggest that these manifestations exhibit associations (8-13) that are consistent with a syndromal presentation(1). Fourth, all the above provides a priori theoretical framework that facilitates the investigation of mechanisms underlying the development of frailty (14). Lastly, we would argue that the 5-component phenotype is more appealing for use in a clinical setting compared to the FI that typically contains 30-70 items.
Fig. 1.
Cycle of frailty. (Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci 2008;63(9):984–90, by permission of the Gerontological Society of America.)
Natural History of Manifestations of Frailty Criteria
Understanding points of onset of frailty is vital to early identification of at-risk individuals and intervention on those components that are first affected, when reversal may be most possible. Preclinical detection of early manifestations leading to the frailty syndrome requires understanding of the natural history of frailty development. We suggest two potential hypotheses as to the natural history of frailty initiation and progression. We hypothesized that the cycle of frailty could be initiated via any of the clinical manifestations, which could then precipitate a “vicious cycle” culminating in an aggregate syndrome; and different initial manifestations may lead to differential rates of progression to frailty. Based on a 7.5-year longitudinal study of 420 WHAS II participants who were defined as non-frail using Fried’s phenotype at baseline, we found initial evidence of a partially hierarchical order in the onset of frailty manifestations over time (6). Although there was notable heterogeneity in the initial manifestations of frailty, weakness was the most common first manifestation, and occurrence of weakness, slowness, and low physical activity preceded exhaustion and weight loss in 76% of the women who were non-frail at baseline.
That weakness should presage frailty onset is consistent with earlier reports that loss of muscle strength begins in midlife (19-21). Decline in strength has been attributed to the loss of muscle mass and muscle quality referred to as sarcopenia, resulting from anatomic and biochemical changes in the aging muscle (22). The causal mechanisms underlying sarcopenia are many, including oxidative stress, dysregulation of inflammatory cytokines and hormones, malnutrition, physical inactivity, and muscle apoptosis (23, 24), all of which have been hypothesized to contribute to frailty through interactive pathways at multiple temporal and spatial scales(14).
The finding of heterogeneity in initial criteria is consistent with the hypothesis that the cycle of frailty may be initiated by insults at many points in a hypothesized cycle of dysregulated energetics (2, 7). Notably, it was not the number of early manifestations (i.e., 1 or 2) but the specific manifestations initially present that distinguished the risk and rate of onset of frailty. Specifically, women with exhaustion or weight loss as initial presenting symptoms were 3–5 times more likely to become frail than were women without any criterion, after adjusting for baseline age, race, education, and comorbidity. Weakness was moderately predictive of frailty onset (hazard ratio (HR) = 2.6). Notably, neither slow walking speed nor low activity at baseline was significantly associated with incident frailty. It remains to be determined whether the different patterns of initial accumulation of frailty criteria represent different etiologic pathways with different rates of progression to frailty, either organ-specific or representing systemic physiologic dysregulations of aging. Alternatively, certain of our criterion measures may be more sensitive than others to changes associated with “normal aging,” for instance performance-based as opposed to self-reported criteria.
Despite heterogeneous entry points into the cycle of frailty, 80% of transitions to frailty involved adding exhaustion and/or weight loss. This finding raises the possibility that decreased energy production or increased utilization, as in wasting conditions, may be involved in the threshold transition in a final common pathway toward frailty. That weight loss and exhaustion rarely developed alone, but rather co-occurred with other manifestations, is consistent with the reliability theory (25) whereby an emergent aggregation of multiple frailty manifestations would result from depletion of system redundancy or compensatory mechanisms, such that any new deficit leads to failure of the whole organism (26-29). Then, early detection of subclinical changes or deficits at the molecular, cellular, and/or physiologic level would be key to preventing or delaying the development of frailty.
The clinical utility of these findings lies in the fact that weakness was the most common initial manifestation of the frailty phenotype. It evidenced only moderate predictive validity for incident frailty; however, by our conceptualization the development of frailty is progressive and multisystemic, and any one specific criterion alone, especially at an early stage in the process as in the case of weakness, may be neither sufficient nor specific for frailty prediction. Given that the criterion defining thresholds for grip strength are known to be associated with meaningfully greater risk of adverse outcomes including disability and mortality (30), weakness may nevertheless be a clinically meaningful indicator of increasing vulnerability at a relatively early stage of the frailty process, when preventive intervention could be easiest to implement and theoretically most effective. Although the subsequent or “concurrent” onset of weight loss or exhaustion with the other criteria may better predict frailty onset, by the time someone experiences weight loss or exhaustion, it may be too late to implement frailty interventions. Therefore, consideration should be given to the possible tradeoff between risk prediction and potential for benefits in deciding the proper timing and targets of interventions.
Evidence of Frailty as a Medical Syndrome
A medical syndrome is “a group of signs and symptoms that occur together and characterize a particular abnormality”. To formally evaluate the degree to which the frailty phenotype conforms to the definition of a medical syndrome, Bandeen-Roche et al. analyzed patterns of co-occurrence of the five frailty-defining criteria based on data from a combined sample of women aged 70-79 from the Women’s Health and Aging Studies (WHAS) I and II (1). Patterns of criteria co-occurrence that would support the syndrome definition are (a) manifestation in a critical mass; and (b) aggregation in a hierarchical order, as would occur in a cycle in which dysregulation in a sentinel system may trigger a cascade of alterations across other systems. Propensity for criteria to co-occur in distinct subgroups would suggest the effects of distinct biologic processes rather than a syndrome. Using latent class analysis (LCA) (15), they identified three population subsets (also termed “classes”) with similar profiles of frailty criteria co-occurrence; and each criterion’s prevalence increased progressively across the population subsets indicating increase in frailty severity. These findings supported the internal validity of the frailty criteria vis a vis stated theory characterizing frailty as a medical syndrome and provided justification to the current counting strategy for defining frailty categories (i.e. non-frail, pre-frail, frail).
Prevalence and Incidence of Frailty
Based on frailty criteria developed in CHS, the overall prevalence of frailty in community-dwelling older adults aged 65 or older in the United States ranges 7-12%. In the CHS, prevalence of frailty increased with age from 3.9% in the 65-74 age group to 25% in the 85+ group and was greater in women than men (8% vs. 5%) (2). African Americans were more than twice as likely to be frail than Caucasians in CHS (13% vs. 6%) and WHAS (16% vs. 10%). The estimate for the 1996 Mexican Americans from the Hispanic Established Populations for Epidemiologic Studies of the Elderly was 7.8%, similar to those of Caucasians (4).
Similar age trends and gender differences have been reported for older adult populations in European and Latin American countries (Table 2). A recent survey of 7,510 community-dwelling older adults in 10 European countries found that prevalence of frailty ranged from 5.8% in Switzerland to 27% in Spain with an overall prevalence of 17%, and was higher in southern than in northern Europe consistent with an unexplained north-south health risk gradient previously reported in the same population (16, 17). The geographic variation in frailty prevalence among these European countries persisted after adjusting for age and gender, which led the authors to speculate that there may be differences in cultural characteristics influencing the perception of health and/or interpretation of the frailty questions (16). According to a survey of 7,334 older adults aged 60 or older living in five large Latin American and Caribbean cities including Bridgetown, Barbados (n=1446); Sao Paulo, Brazil (n=1879); Santiago, Chile (n=1220); Havana, Cuba (n=1726); and Mexico City, Mexico (n=1063), prevalence of frailty varied from 30% to 48% in women and from 21% to 35% in men, which were much higher than their USA and European counterparts (45).
Table 2.
| Source | Country | n | Frailty Prevalence (%) | Frailty Criteria | |
|---|---|---|---|---|---|
| Fried et al. 2001 | USA | 5317 | Age | CHS criteria (see Table 1) | |
| 65-74 | 3.9 | ||||
| 75-84 | 11.6 | ||||
| 85+ | 25.0 | ||||
| Sex | |||||
| Female | 8.2 | ||||
| Male | 5.2 | ||||
| Race | |||||
| Caucasian | 5.9 | ||||
| African American | 12.9 | ||||
|
| |||||
| Bandeen-Roche et al. 2006 | USA | 786 | Age | WHAS criteria (see Table 1) | |
| 70-79 | 11.3 | ||||
| Race | |||||
| Caucasian | 9.8 | ||||
| African American | 15.8 | ||||
|
| |||||
| Santos-Eggimann et al. 2009 | 10 European countries: Sweden, Denmark, Netherlands, Germany, Austria, Switzerland, France, Italy, Spain, Greece |
7510 | Age 65+ | 17.0 | Three or more of the following five criteria: Weight loss: self-report of a “diminution in desire for food” in response to the question “What has your appetite been like” Exhaustion: responding yes to the question “In the last month, have you had too little energy to do things you wanted to do.” Weakness: same as in CHS Slowness: Self-report of either having “difficulty [expected to last more than 3 months] walking 100 meters” or “climbing one flight of stairs without resting” due to health reasons. Low activity: responding “one to three times a month” or “hardly ever or never to the question “How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or going for a walk?.” |
| 873 | 8.6 | ||||
| 635 | 12.4 | ||||
| 830 | 11.3 | ||||
| 933 | 12.1 | ||||
| 707 | 10.8 | ||||
| 412 | 5.8 | ||||
| 687 | 15.0 | ||||
| 833 | 23.0 | ||||
| 816 | 27.3 | ||||
| 784 | 14.7 | ||||
|
| |||||
| Graham et al. 2008 | USA | 1996 | Age 65+ | Three or more of the following five criteria: Weight loss: unintentional weight loss ≥4.5 kg in last year Exhaustion: same as in CHS Weakness by Grip Strength: weakest 20% for men: ≤21 kg for BMI ≤24.2 ≤24.5 kg for BMI 24.3–26.8 ≤25.4 kg for BMI 26.9–29.5 ≤25.5 kg for BMI >29.5 weakest 20% for women: ≤13.5 kg for BMI ≤24.7 ≤14.2 kg for BMI 24.8–28.3 ≤15.0 kg for BMI 28.4–32.1 ≤15.0 kg for BMI >32.1 Slowness by 4.9-meter timed walk at fast pace slowest 20% for men: ≥11.2 s for height ≤168 cm ≥9.7 s for height >168 cm slowest 20% for women: ≥12.0 s for height ≤154 cm ≥11.2 s for height >154 cm Low activity: lowest 20th percentile by gender based on Physical Activity Scale for the Elderly (PASE). lowest 20% for men ≤30 lowest 20% for women ≤27.5 |
|
| Race | 7.8 | ||||
| Mexican American | |||||
|
| |||||
| Alvarado et al. 2008 | Age 60+ | Three or more of the following five criteria: Weight loss: self-report of loss > 10 pounds unintentionally during the previous 3 months Exhaustion: Responding “No” to the question “Do you have lots of energy” and/or responding “Yes” to the question “Have your dropped many of your activities or interests” Weakness: same as in CHS Slowness: self-report of difficulty walking 100 yards and/or climbing one flight of stairs Low activity: Responding “No” to the question “In the last twelve months, have your exercised regularly or participated in vigorous physical activity such as playing a sport, dancing or doing heavy housework 3 or more times a week?” |
|||
| Barbados | 1446 | Female | 30.0 | ||
| Male | 21.5 | ||||
| Cuba | 1726 | Female | 46.7 | ||
| Male | 30.4 | ||||
| Mexico | 1063 | Female | 45.5 | ||
| Male | 30.4 | ||||
| Chile | 1220 | Female | 48.2 | ||
| Male | 31.7 | ||||
| Brazil | 1879 | Female | 44.1 | ||
| Male | 35.4 | ||||
| Avila-Funes et al. 2009 | France | 6030 | Age 65+ | 7.0 | Three or more of the following five criteria: Weight loss: self-report of recent loss ≥ 3 kg unintentionally or BMI <21 kg/m2 Exhaustion: same as in CHS Weakness: responding “Yes” to the question “Do you have difficulty rising from a chair?” Slowness: gender and height adjusted lowest quantile on a timed 6-meter walking test at usual pace Low activity: denied doing daily leisure activities such as walking or gardening or participating in athletic activity at least once a week |
Frailty Transitions
Epidemiological data on transitions between frailty states (i.e. non-frail, pre-frail, and frail) were first reported by Gill et al. in a 4.5-year longitudinal study of 754 community-living older adults aged 70 years or above (3). Of the 754 participants, 58% had at least one transition between any two of the three frailty states at one of the three follow-up visits 18-month apart during the study; 37%, 22%, and 9% had 1, 2, 3 transitions, respectively. About one-third (35%) of all 18-month transitions were from states of greater frailty to states of less frailty (calculated based on data in Table 3 of Gill et al.). However, the likelihood of transitioning from being frail to non-frail was extremely rare during each of the 18-month intervals.
Table 3.
Numbers and rates of transitions according to follow-up interval.
| Baseline to 18 month | 18 to 36 month | 36 to 72 month | 72 to 90 month | |||||
|---|---|---|---|---|---|---|---|---|
| Transition | No. | Rate, % | No. | (Rate, %) | No. | (Rate, %) | No. | (Rate, %) |
| Non-frail to | N=244 | N=222 | N=147 | N=129 | ||||
| Non-frail | 179 | 73.4 | 132 | 59.5 | 93 | 63.3 | 66 | 51.2 |
| Pre-frail | 61 | 25.0 | 86 | 38.7 | 40 | 27.2 | 58 | 45.0 |
| Frail | 3 | 1.2 | 4 | 1.8 | 6 | 4.1 | 2 | 1.6 |
| Death | 1 | 0.4 | 0 | 0 | 8 | 5.4 | 3 | 2.3 |
| Pre-frail | N=137 | N=130 | N=161 | N=130 | ||||
| Non-frail | 48 | 35.0 | 26 | 20.0 | 36 | 22.4 | 22 | 16.9 |
| Pre-frail | 75 | 54.7 | 89 | 68.5 | 92 | 57.1 | 85 | 65.4 |
| Frail | 9 | 6.6 | 11 | 8.5 | 15 | 9.3 | 15 | 11.5 |
| Death | 5 | 3.7 | 4 | 3.1 | 18 | 11.2 | 8 | 6.2 |
| Frail | N=12 | N=13 | N=14 | N=28 | ||||
| Non-frail | 2 | 16.7 | 1 | 0.8 | 2 | 14.3 | 0 | 0 |
| Pre-frail | 7 | 58.3 | 10 | 76.9 | 5 | 35.7 | 10 | 35.7 |
| Frail | 2 | 16.7 | 2 | 15.4 | 7 | 50.0 | 13 | 46.4 |
| Death | 1 | 8.3 | 0 | 0 | 0 | 0 | 5 | 17.9 |
In WHAS II, frailty status of 405 women representing two-thirds least disabled community-dwelling women aged 70-79 was repeatedly assessed at baseline and at least one of 4 follow-up visits spanning 7.5 years (approximately 18 months apart except for the interval between the third and the fourth exam, which was, on average, 3 years). Seventy-two percent of the 405 women had at least one transition between frailty states over 7.5 years; 37%, 24%, 16%, and 2% had 1, 2, 3, and 4 transitions, respectively. Consistent with Gill et al.’s finding, most of the transitions occurred between adjacent frailty status; one-third (34%) of all 18-month transitions were from states of greater frailty to states of less frailty. In WHAS II, the rate of transition from frail to non-frail was noticeably higher (17%) during the first 18 months than that of the previous study, which could be due to small sample size of the frailty group in our sample (Table 3). We also found that two-thirds of the 24 (n=15) women who were non-frail at baseline and became frail during the course of the study did so slowly and progressively, while one-third (n=9) had rapid onset of frailty without progressing through any identified pre-frail stage. This suggests that the rate at which frailty progresses may vary dramatically among older adults, i.e. more sudden and catastrophic in some people and slowly progressive among others. Similar findings have been reported Gill et al. (3) and for severe mobility disability, with the rate of progression depending on level of comorbidity as well as specific disease types (18). Due to low frailty incidence, we had limited power in detecting factors differentiating the pace of frailty development.
As some misconstrue frailty as a pre-morbid state defining end-of-life, the findings reported above suggest that frailty is not an irreversible process, certainly not inevitable trajectory to death. Therefore, the development and evaluation of interventions designed to prevent or ameliorate frailty should remain as one of the top priorities in frailty research.
Behavioral Precursors to the Development of Frailty
An overt state of frailty is believed to be preceded by behavioral adaptation made in response to declining physiologic reserve and capacity with which to meet environmental challenges. The causes of this loss of physiologic reserve are likely to be multifactorial, including both environmental challenges (e.g., area deprivation) and intraindividual challenges (e.g., age-related physiologic changes). Observations of early behavioral changes during this preclinical phase in older adults in whom frailty is developing, but as yet undetected, could provide insight into the frailty development process and suggest means for early intervention. More importantly, such changes may not be captured by conventional measures of function such as fixed-distance or fixed-time walking tests for mobility function, which assess one’s functional capacity under hypothetical or experimental conditions rather than enacted function in real world (31). Therefore, assessment of changes in real life may reflect net impact of declining reserve, taking into account the balance between internal physiologic capacity and external challenges older adults experience in daily life.
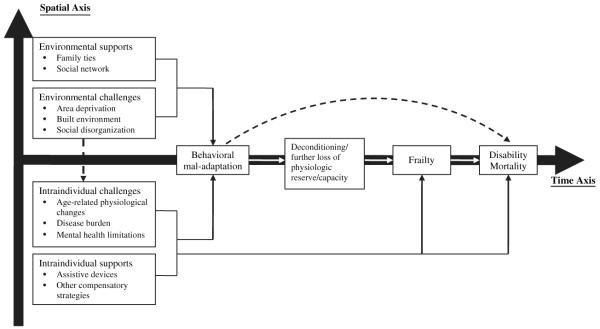
One example of such behavioral precursor is life space – a measure of spatial mobility, defined as the size of the spatial area a person purposely moves through in his/her daily life, as well as the frequency of travel within a specific time frame (32, 33). We analyzed the 3-year cumulative incidence of frailty using the WHAS phenotype in relation to baseline life-space constriction among 599 community-dwelling women aged 65 years or older who were not frail at baseline. Frailty-free mortality (i.e., death prior to observation of frailty) was treated as a competing risk. Multivariate survival models showed that, compared with women who left the neighborhood four or more times per week, those who left the neighborhood less frequently were 1.7 times (95% confidence interval (CI): 1.1, 2.4; p < 0.05) more likely to become frail, and those who never left their homes experienced a threefold increase in frailty-free mortality (95% CI: 1.4, 7.7; p < 0.01), after adjustment for chronic disease, physical disability, and psychosocial factors(34). It is particularly intriguing to find that difficulty with mobility, IADL, and ADL tasks alone did not necessarily lead to a reduction in life space. In fact, 97% of the participants in our study cohort had already reported mobility disability at baseline. Such discordance between functional capacity and actual performance has been reported in a number of other studies (31, 35, 36). To explain the discrepancy, one could argue that some people may compensate for underlying functional decrements by adapting to a modified daily routine (e.g., the use of assistive devices) in order to maintain the same level of performance in real life (i.e., “enacted function”) (37). Although the exact reasons for this discrepancy remain unknown, we hypothesize that the employment of external (e.g., social support) and internal (e.g., using a cane) compensatory strategies (termed “environmental supports” and “intraindividual supports,” respectively, in figure 2) may help to minimize the impact of loss of physiologic reserve and thereby preserve life-space mobility. On the other hand, the ability to compensate effectively for functional limitations may itself be a function of physiologic reserve. It may be the interplay of functional limitations and functional reserve that determines actual function and behavior.
Fig. 2.
Theoretical model of the association of life space with the clinical syndrome of frailty. Solid and dashed lines represent direct and indirect effects, respectively; arrows represent causal direction. (Xue QL, Fried LP, Glass TA, et al. Life-space constriction, development of frailty, and the competing risk of mortality: the Women’s Health And Aging Study I. Am J Epidemiol 2008;167(2):240–8, by permission of Oxford University Press.)
Obtaining empirical evidence of this association is the critical first step towards evaluating a broad conceptual framework about the etiology of frailty (Figure 2). In the case of life space, it is theorized that constriction of life space is a marker of declines in physiologic reserve and that constriction of life space itself could lead to decreased physical activity and social engagement, accelerated deconditioning, and exacerbated decline in physiologic reserve, directly contributing—as these processes progress—to the development of clinical frailty and subsequent mortality. Future development of tools for the assessment of physiologic reserve and analysis of their relations to behavioral mal-adaptations could help in delineating the hypothesized causal pathway.
Summary
The recent work on natural history of frailty has advanced our understanding of the aging process and its potential physiological correlates. The ongoing debate on the operational definition of frailty, its subdomains (e.g. physical vs. cognitive), and its relationship with aging, disability, and chronic diseases (38-42) signals that more work is necessary to better define and quantify “reserve” and “resilience” – the hallmarks of frailty (14, 43). Despite this debate, researcher and clinicians have no disagreement on severe impact of frailty on older adults, their care givers, and on society as a whole. While specific treatments for frailty are yet to be developed and tested, the existing clinical measures of frailty provide useful means for identify high risk individuals, therefore could lead to improved treatment decision making and management of care by taking into account individual vulnerabilities and propensity for adverse health outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women’s health and aging studies. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. JGerontolA BiolSciMedSci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Archives of internal medicine. 2006;166(4):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 4.Graham JE, Snih SA, Berges IM, et al. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55(6):644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society. 2009 Mar;57(3):492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. The journals of gerontology. 2008 Sep;63(9):984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Walston J, Hazzard WR, et al. Principles of Geriatric Medicine and Gerontology. McGraw Hill; New York: 1998. Frailty and failre to thrive; pp. 1387–1402. [Google Scholar]
- 8.Tseng BS, Marsh DR, Hamilton MT, et al. Strength and Aerobic Training Attenuate Muscle Wasting and Improve Resistance to the Development of Disability with Aging. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1995;50:113–119. doi: 10.1093/gerona/50a.special_issue.113. [DOI] [PubMed] [Google Scholar]
- 9.Evans WJ. Exercise, Nutrition, and Aging. Clinics in geriatric medicine. 1995;11(4):725. &. [PubMed] [Google Scholar]
- 10.Fleg JL, Lakatta EG. Role of Muscle Loss in the Age-Associated Reduction in Vo2Max. Journal of Applied Physiology. 1988;65(3):1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 11.Buchner DM, Larson EB, Wagner EH, et al. Evidence for a non-linear relationship between leg strength and gait speed. Age and ageing. 1996;25(5):386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 12.Leibel RL. Changes in Energy-Expenditure Resulting from Altered Body-Weight(Vol 332, Pg 621, 1995) New England Journal of Medicine. 1995;333(6):399–399. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE. Anorexia of aging: physiologic and pathologic. American Journal of Clinical Nutrition. 1997;66(4):760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Hadley EC, Walston J, et al. From bedside to bench: research agenda for frailty. Science of Aging Knowledge Environment. 2005;2005(31):24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 15.Goodman LA. Exploratory Latent Structure Analysis Using Both Identifiable and Unidentifiable Models. Biometrika. 1974;61(2):215–231. [Google Scholar]
- 16.Santos-Eggimann B, Cuenoud P, Spagnoli J, et al. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. The journals of gerontology. 2009 Jun;64(6):675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borsch-Supan A, Brugiavini A, Jurges H, et al. First Results From the Survey of Health, Ageing and Retirement in Europe. Mannheim Research Institute for the Economics of Aging; Mannheim, Germany: 2005. pp. 8–27. [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Balfour JL, et al. Progressive versus catastrophic loss of the ability to walk: Implications for the prevention of mobility loss. Journal of the American Geriatrics Society. 2001;49(11):1463–1470. doi: 10.1046/j.1532-5415.2001.4911238.x. [DOI] [PubMed] [Google Scholar]
- 19.Nair KS. Muscle Protein-Turnover - Methodological Issues and the Effect of Aging. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1995;50:107–112. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 20.Viitasalo JT, Era P, Leskinen AL, et al. Muscular Strength Profiles and Anthropometry in Random Samples of Men Aged 31-35, 51-55 and 71-75 Years. Ergonomics. 1985;28(11):1563–1574. [Google Scholar]
- 21.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. Journal of Applied Physiology. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 22.Kamel HK. Sarcopenia and aging. Nutrition reviews. 2003;61(5):157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 23.Marcell TJ. Sarcopenia: Causes, consequences, and preventions. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2003;58(10):911–916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 24.Dirks AJ, Hofer T, Marzetti E, et al. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing research reviews. 2006;5(2):179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd DK, Lipow M. Reliability: Management, Methods, and Mathematics. Prentice-Hall, Inc.; Englewood Cliffs, NJ: 1962. [Google Scholar]
- 26.Gavrilov LA, Gavrilova NS. The reliability theory of aging and longevity. JTheorBiol. 2001;213(4):527–545. doi: 10.1006/jtbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- 27.Bortz WM. A conceptual framework of frailty: a review. JGerontolA BiolSciMedSci. 2002;57(5):M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 28.Amaral LA, Diaz-Guilera A, Moreira AA, et al. Emergence of complex dynamics in a simple model of signaling networks. ProcNatlAcadSciUSA. 2004;101(44):15551–15555. doi: 10.1073/pnas.0404843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitano H. Science. 5560. Vol. 295. New York, NY: 2002. Systems biology: a brief overview; pp. 1662–1664. [DOI] [PubMed] [Google Scholar]
- 30.Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. Journal of the American Geriatrics Society. 2003 May;51(5):636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Glass TA. Conjugating the “tenses” of function: Discordance among hypothetical, experimental, and enacted function in older adults. The Gerontologist. 1998;38(1):101–112. doi: 10.1093/geront/38.1.101. [DOI] [PubMed] [Google Scholar]
- 32.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. Journal of the American Geriatrics Society. 2003;51(11):1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 33.May D, Nayak US, Isaacs B. The life-space diary: a measure of mobility in old people at home. IntRehabilMed. 1985;7(4):182–186. doi: 10.3109/03790798509165993. [DOI] [PubMed] [Google Scholar]
- 34.Xue QL, Fried LP, Glass TA, et al. Life-space constriction, development of frailty, and the competing risk of mortality: the Women’s Health And Aging Study I. American journal of epidemiology. 2008 Jan 15;167(2):240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 35.Jette AM. How measurement techniques influence estimates of disability in older populations. SocSciMed. 1994;38(7):937–942. doi: 10.1016/0277-9536(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 36.Cambois E, Robine JM, Romieu I. The influence of functional limitations and various demographic factors on self-reported activity restriction at older ages. DisabilRehabil. 2005;27(15):871–883. doi: 10.1080/09638280500030860. [DOI] [PubMed] [Google Scholar]
- 37.Fried LP, Bandeen-Roche K, Chaves PHM, et al. Preclinical mobility disability predicts incident mobility disability in older women. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2000;55(1):M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 38.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 39.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging clinical and experimental research. 2003 Jun;15(3 Suppl):1–29. [PubMed] [Google Scholar]
- 40.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. The journals of gerontology. 2007 Jul;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. Journal of the American Geriatrics Society. 2009 Mar;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 42.Sarkisian CA, Gruenewald TL, Boscardin W John, et al. Preliminary evidence for subdimensions of geriatric frailty: the MacArthur study of successful aging. Journal of the American Geriatrics Society. 2008 Dec;56(12):2292–2297. doi: 10.1111/j.1532-5415.2008.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varadhan R, Seplaki CL, Xue QL, et al. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mechanisms of ageing and development. 2008 Nov;129(11):666–670. doi: 10.1016/j.mad.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. The Scientific World. 2001 Sep;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarado BE, Zunzunegui MV, Beland F. Life course social and health conditions linked to frailty in Latin American older men and women. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2008;63:1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- 46.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]