Abstract
We present a clinical case of a 63-year old Caucasian man with Ehlers-Danlos syndrome who was admitted with atrial fibrillation and arterial hypertension. We present this not as a cardiological case but instead address the key questions of differential diagnosis, diagnosis criteria, management and improving the patient’s quality of life.
BACKGROUND
Ehlers-Danlos syndrome (EDS) is an interesting academic rare disease, with many manifestations, about which the doctors and medical students must be informed about and not be intimidated by.
CASE PRESENTATION
A 63-year-old Caucasian male patient was admitted in February 2009 with chief complaints of retrosternal, constricting pain on moderate physical activity, palpitations, general weakness, dyspnoea on minimal physical activity and occipital headache.
Past history
Arterial hypertension for approximately 10 years varies between 105/70–220/105 mmHg. He has had palpitations from 2005. He regularly receives aspirin 75 mg/day, enalapril 10 mg twice daily and neutral protamine hagedorn (NPH) insulin (16 U+16 U). Connective tissue disorder was diagnosed in childhood; he started walking at 4 years of age; had anosmia from birth; could not complete university studies due to the “inability to memorise”; repeated painful left shoulder subluxations, which he himself reduced; collapsed plantar arches—bilateral flat feet; early osteoarthritis; left ear deafness at the age of 50 years; hypermetropy OU +3.5; early cataract; osteoporosis. There was no history of hepatitis or tuberculosis. He has had type 2 diabetes mellitus for about 10 years and received insulin treatment for the last 2 years. There is a history of anaphylactic shock with lidocaine. He is allergic to penicillins. He has had bilateral inguinal hernia, perforation of the muscles of the abdominal wall, apendicectomy and surgical removal of hematomas on both legs.
Cardiovascular risk factors
There is no family history of cardiovascular disease; he smoked for 20 years in the past; occasionally drinks alcohol; dyslipidaemia (hypertrigliceridaemia); body mass index 25.6.
Physical examination
On a general exam, the patient’s facial skin looked 10–20 older than his age. He had hyper-extensible skin. He had minute granules under the skin of the thorax, hyperpigmentated shins, multiple “cigarette-paper like” scars (fig 1), visible superficial veins, collateral circulation visible on the abdomen, rubin spots, fibro-fatty nodules under the skin (fig 2), no palpable lymph nodes, no peripheral oedema and varicose veins in the lower limbs.
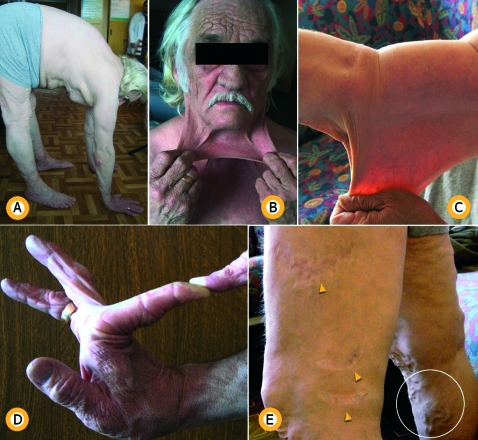
Figure 1.
(A) Spinal hypermobility with knees locked straight, bends and lays palms on the ground. (B, C) Hyperextensibility of the skin. (D) Hypermobility of joints. (E) Yellow arrows indicate “cigarette paper scars”; white ring marker indicates varicose veins.
Figure 2.
(A) Moluscoid fibro-fatty nodules. (B) Discolouration (hyperpigmentated) of shins. (C) Minute granules under the skin of the thorax.
He had vesicular breathing and reduced breath sounds in the basal region bilaterally. His respiratory rate was 16/min.
His heart borders were within normal limits on percussion. Cardiac sounds clear, arrhythmic, no murmurs. Heart rate was 120 bpm and blood pressure 160/95 mmHg. His abdomen was soft and painless on palpation.
Liver and spleen were not palpable.
Intestinal transit was normal, painless and free micturation. Giordano’s sign was negative.
Skeletal examination found scoliosis, bony prominences bilaterally at the mandibular angle, Heberden nodes in the digits of the upper limbs, pain on palpation of the thoracic and lumbar vertebrae, wrists and metacarpophalangeal joints. He had hypermobile joints, but due development of osteoarthritis joint movements are being restricted compared with when he was younger.
Evaluation for the EDS scores: the hypermobility score in our patient (as per Beighton Hypermobility Score)1 was 9, the hyperextensibility score was 5, the scarring score was 4 and the bruising score was 2.
INVESTIGATIONS
Full blood count within normal limits, erythrocyte sedimentation rate (ESR) =10 (2–10) and urine analysis was normal. Fasting glucose 6.7mmol/l (120.6 mg/dl); pre-prandial glucose profile 8.6mmol/l (154.8 mg/dl), 8.9 mmol/l (160.2 mg/dl) and 12.7 mmol/l (228.6 mg/dl); other tests of coagulation time, bilirubin, alanine transaminase (serum glutamic pyruvic transaminase), aspartate aminotransferase (serum glutamic oxaloacetic transaminase), creatine kinase-MB, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, creatinine and urea were normal. Triglycerides were 2.72 mmol/l (105 mg/dl). Electrocardiogram found atrial fibrillation heart rate 120 bpm and sinus rhythm heart rate 86 bpm; normal results were obtained on spirometry. Chest x ray (fig 3) revealed a relaxed diaphragm.
Figure 3.
Chest x ray. (A) Postero-anterior view and (B) left lateral view (taken February 2009) with relaxation of diaphragm. (C) Postero-anterior view (taken November 2007) revealed bilateral diaphragmal relaxation (white arrows). Costodiaphragmal sinus is also visible (black arrow).
Ultrasound of internal organs: chronic pancreatitis, chronic acalculus cholecystitis, renal cysts (left kidney: 1 cyst of 1 cm; right kidney: 2 cysts of 2.8 and 3.1 cm), prostatic adenoma. Echocardiography: moderate dilatation of left and right atria. Global contractile function of the left ventricle myocardium sufficient, ejection fraction 60%. Doppler echocardiography: mitral valve regurgitation gr.II, tricuspid valve regurgitation gr. I–II, affected relaxation of the left ventricle. Ophthalmological consult: early bilateral opacification of the lens. Optic nerve papilla: pink-pale, clear margins, dilated veins, narrowing and sclerosis of arteries. Conclusion: retinal angiosclerosis and incipient cataract.
DIFFERENTIAL DIAGNOSIS
Among the major differentials in EDS are Marfans syndrome,2 osteogenesis imperfecta,3 Menkes syndrome (similar to type 9 EDS, copper metabolism defect),4 Alport syndrome, Lowe syndrome, pseudoxanthoma elasticum and cutis laxa syndromes,3 babies with Down syndrome, all the diseases presenting with myotonic dystonia in infants, hypothyroidism and fragile-X syndrome.3
The eye in EDS provides a vast differential diagnosis too, with features such as (1) cataracts (diabetic—snowflake cataract, myotonic dystrophy—christmas tree cataract and so forth); (2) ectopia lentis (marfan—suprotemporal dislocation, with high IQ, EDS—normal IQ); (3) blue sclera 7 (types I, II osteogenesis imperfect); (4) lenticonus—Alport syndrome (anterior), Lowe’s syndrome (posterior); (5) high myopia; (6) keratoconus; (7) epicanthal folds; (8) angiod streaks; (9) dry eyes; (10) glaucoma (with pachymetry for accuracy).
TREATMENT
Aspirin 75 mg/day orally, metoprolol 25 mg twice daily orally, enalapril 10 mg twice daily orally, omega 3 fatty acids 1 three times daily orally, NPH insulin (16 U + 16 U), warfarin 3 mg/day (target index normalised ratio 2–2.5) and vitamin C. We discharged the patient with advice aiming to improve his quality of life: bracing to stabilise joints; ear, nose, throat (ENT) consult for probable need of hearing aid; orthodontics consult to verify if dental prosthesis is possible; ophthalmological consult to treat cataract; family and relatives genetic counselling. We advised him to get a thyroid, parathyroid hormonal assay and a dual energy x ray absorptiometry (DEXA) scan to appreciate his bone mineral density and evaluate the need for bisphosphonates. He was also prescribed anti-inflammatories to reduce his joint pains
DISCUSSION
EDS is a heterogeneous group of inherited connective tissue disorders characterised by joint hypermobility, cutaneous fragility and hyperextensibility. The collagen defect has been identified in only six types of EDS.4 Crossover symptoms for all types are prevalent.
The patient presents with anosmia, high relaxed diaphragm5 and renal cysts—features that have not been described as typical manifestation of EDS as such in the literature reviewed by us. Our opinion is renal cysts in this patient is an isolated event not related to EDS. Anosmia and high relaxed diaphragm could have some association with EDS.
Mutations in the genes causing EDS are COL1A1, COL1A2, COL3A1, COL5A1 and COL5A2,2–4,6 which provide instructions for making proteins that are used to assemble different types of collagen; and ADAMTS2, PLOD1 and TNXB,2,7 which provide instructions for making proteins that process or interact with collagen. Types 1, 2, 3, 4, 7A and 7B are autosomal dominant; type 5 and 9 are X-linked recessive; type 6 and 7C are tenascin-X deficient type autosomal recessive.2,6 Some patients inherit the mutation from one affected parent while some other cases result from new (sporadic) gene mutations. If the patient has autosomal recessive EDS the parents were carriers and did not show signs and symptoms. When the gene is non-penetrant it appears that the gene has skipped a generation and variation of gene expression is seen.2 Both phenomena are seen with autosomal dominant cases. Prenatal testing should be considered in high-risk pregnancies.6 Parents of our patient did not exhibit any symptoms of EDS and neither did his daughter. However, his granddaughter did present some symptoms of EDS at the age of 10 years. Diagnosis in infants and small children is impossible as abnormal joint hypermobility and skin elasticity are difficult to recognise; however, babies may present as floppy infants. In children, joint hypermobility and hypotonia may cause delayed motor development, problems with walking and delayed milestones like in our patient here.
LEARNING POINTS
Beighton score8 is used to calculate hypermobility score (a score ⩾4 for hypermobility), which is also a part of the Brighton criteria used to diagnose benign hypermobility.
Our patient came in with atrial fibrillation and arterial hypertension diabetes mellitus, which we treated. The present problem should not be overshadowed by the rare disease of the patient.
Not every symptom of the patient could be explained by the syndrome, but nevertheless could be related—for example, anosmia in our patient.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Beighton PH, Horan F. Orthopedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg [Br] 1969; 51: 444–53 [PubMed] [Google Scholar]
- 2.Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. GeneReviews [online]. University of Washington; http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=eds (accessed 10 July 2009) [Google Scholar]
- 3.Louise JT, Elizabeth JE, Craig M, et al. The differential diagnosis of children with joint hypermobility: a review of the literature. Pediatric Rheumatology 2009; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceccolini E, Schwartz RA. Ehlers-Danlos syndrome. eMedicine Journal [online]. http://emedicine.medscape.com/article/1114004-overview (accessed 10 July 2009) [Google Scholar]
- 5.Royce MP, Steinmann BU. Connective tissue and its heritable disorders: molecular, genetic, and medical aspects. 2nd ed. Wiley-Liss, Inc; 2002: 442–3 [Google Scholar]
- 6.Reed EP. Ehlers-Danlos syndrome. N Engl J Med 2000; 342: 730–2 [DOI] [PubMed] [Google Scholar]
- 7.German Society of Human Genetics Indication criteria for genetic testing, Indication criteria for the disease: Ehlers-Danlos syndrome types I-VII. http://www.eshg.org/documents/indications/Indication%20criteria%20-%20EDS.pdf (accessed 10 July 2009) [Google Scholar]
- 8.Beighton P, De Paepe A, Steinmann B, et al. Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK). Am J Med Genet 1998; 77: 31–7 [DOI] [PubMed] [Google Scholar]