Abstract
Purpose
Wilms' tumors arise from arrested differentiation of renal progenitor cells. CITED1 is a transcriptional regulator that blocks the metanephric mesenchymal-to-epithelial conversion and is expressed in the blastema of both the developing kidney and Wilms' tumors. We hypothesized that alterations of CITED1-dependent signaling promote persistence of blastema and thereby subject these pluripotent cells to future oncogenic events.
Methods
We used a retroviral delivery system to overexpress the full-length CITED1 (F/L) protein and 2 deletion mutants lacking either of its known functional domains, ΔSID (Smad-4 Interacting Domain) and ΔCR2 (Conserved Region 2; the CITED1 transactivation domain), in a human Wilms' tumor cell line that endogenously expresses CITED1. In vitro effects on cellular proliferation and apoptosis were assayed. In vivo effects on tumorigenesis, growth, proliferation, and apoptosis were determined after heterotransplantation into immunodeficient mice (n = 15 per cell line).
Results
In vitro, overexpression of CITED1-F/L significantly increased, whereas overexpression of the functionally inactivating mutant, CITED1-ΔCR2, significantly reduced cellular proliferation relative to the other lines ( P <.0001). In vivo, Wilms' tumor incidence was significantly reduced in animals injected with cells overexpressing the mutant CITED1-ΔCR2 (7%) compared with CITED1-F/L (40%, P = .03) and CITED1-ΔSID (60%, P < .002). Similarly, mean tumor volume was least in the CITED1-ΔCR2 animals when compared with CITED1-F/L ( P = .03) and CITED1-ΔSID animals ( P <.005). Furthermore, the CITED1-ΔCR2 tumor showed the least cellular proliferation. Misexpression of CITED1 did not affect apoptosis either in vitro or in vivo.
Conclusions
Overexpression of CITED1 in a human Wilms' tumor cell line significantly increases proliferation in vitro, whereas mutation of its functionally critical transactivation domain (ΔCR2) significantly reduces proliferation. This mutation further perturbs tumorigenesis and tumor growth after heterotransplantation into immunodeficient mice. We speculate that overexpression of CITED1 promotes expansion of a rapidly proliferating population of blastema and thereby induces an unstable environment highly susceptible to future oncogenic events.
Keywords: Wilms' tumor, CITED1, Melanocyte-specific gene 1, Mesenchymal-to-epithelial conversion
Wilms' tumor, or nephroblastoma, is the most common renal malignancy of childhood, and roughly 460 new cases are diagnosed each year in the United States. Although overall 4-year survival rates now exceed 90%, the most aggressive Wilms' tumors often are composed predominantly of an undifferentiated blastemal cell population, and in 10% of cases, display unfavorable histology (anaplasia) [1-3]. Unfortunately, poorly differentiated, unfavorable histology is an ominous finding that accounts for over half of deaths related to nephroblastomas [4-6].
Wilms' tumors appear to caricature early nephrogenesis and are thought to arise from epithelial progenitor cells of the fetal kidney known as the metanephric mesenchyme or blastema. Abnormal regulation of the mesenchymal-to-epithelial transition of the metanephric blastema is thought to underlie the expansion of undifferentiated epithelial precursors in the development of these tumors. Most commonly, Wilms' tumors are composed of 3 cell types histologically reminiscent of those found in the early developing kidney: the undifferentiated blastema, and the more differentiated epithelia and stroma [7,8]. Intermixed with the undifferentiated blastemal compartment, many of these tumors contain primitively differentiated structures that resemble simple embryonic tubules, and glomerular elements yet show no organized tissue architecture. Clues to the development of this embryonal tumor therefore lie in the mechanisms that regulate normal mesenchymal-to-epithelial conversion and renal morphogenesis, but that must fail in Wilms' tumorigenesis. As a result, these tumors provide an excellent paradigm to study embryonal tumorigenesis.
In a screen to identify factors that regulate the mesenchymal-to-epithelial conversion, our laboratory has shown that the transcriptional co-factor, CITED1 (formerly melanocyte-specific gene 1 [msg1]), is expressed strongly in freshly isolated rat metanephric mesenchyme, but is downregulated after differentiation into epithelia [9-11]. We showed further in these studies that overexpression of CITED1 blocks epithelial morphogenesis of cultured metanephric mesenchymes and that CITED1 may regulate early nephronic patterning [11]. Interestingly, we have also observed, using immunohistochemical techniques, that CITED1 expression is restricted to the blastemal compartments of both the developing kidney and Wilms' tumors.
CITED1 is a non–DNA-binding transcriptional co-factor having 2 known functional domains, Smad-4 Interacting Domain (SID) and Conserved Region 2 (CR2) [12-16]. At its carboxy-terminal domain, CR2, the CITED1 protein binds directly to the CBP/p300 transcriptional integrators [12-14]. Binding of the CITED1-CR2 domain to the CBP/p300 complex is required to promote its trans-activating properties and specific transcriptional responses [13]. CITED1 has been shown further to activate transforming growth factor β (TGF-β)–dependent transcription mediated through its second known functional domain, the SID [13,16]. Functionally, CITED1 is known both to stimulate Smad4-dependent transcriptional responses of the TGF-β superfamily while simultaneously repressing Wnt/β-catenin signaling [11]. CITED1 may regulate specific cellular functions of the metanephric mesenchyme by providing a transcriptional “switch” that coordinates mesenchymal survival or epithelial differentiation in the developing kidney. Alterations in the normal interaction of CITED1, CREB binding protein (CBP)/p300, and Smad-4 therefore may modify its ultimate transcriptional responses.
Taken together, these observations suggest a molecular mechanism whereby CITED1 expression promotes persistence of the metanephric blastema, and its downregulation signals differentiation of the metanephric blastema. On this basis, we hypothesize that alterations in CITED1 expression may promote persistence of the metanephric mesenchyme and therefore subject highly susceptible cells to future oncogenic events.
1. Methods
1.1. Cell culture and CITED1 detection
To characterize the functional role of CITED1 in Wilms' tumorigenesis, we chose to study the blastemal-predominant human Wilms' tumor cell line, SK-NEP-1 (American Type Culture Collection, Manassas, Va). Wild-type SK-NEP-1 cells were maintained in McCoy's 5a medium (InvitrogenGibco, Carlsbad, Calif) containing 15% fetal calf serum (Gibco) and 1% penicillin-streptomycin (Gibco) at 37°C in a humidified atmosphere of 5% CO2.
SK-NEP-1 cells were lysed in buffer containing 1% Triton X-100, 100 mmol NaCl, 25 mmol Hepes (pH 7.5), 5 mmol EDTA, and 10% glycerol in the presence of phosphatase and protease inhibitors to detect endogenous CITED1 protein expression. Lysates were normalized for protein concentration using the Bradford assay (Bio-Rad, Hercules, Calif) and immunoprecipitated using an affinity-purified rabbit polyclonal antibody, J72220K, raised against a specific C-terminal peptide, residues 178 to 193 of CITED1 [12,17]. Rabbit immunoglobulin G was used as a negative control for immunoprecipitation. Antibody-protein complexes were next eluted using agarose A/G beads according to company protocol (Santa Cruz Biotechnology, Santa Cruz, Calif). Immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% Tris-HCl gels and probed by Western blot using CITED1-specific mouse monoclonal antibody, 2H6 [12,17]. To detect endogenous CITED1 messenger RNA expression, RNA was extracted from cells using Trizol reagent (Invitrogen), and reverse transcriptase-polymerase chain reaction (PCR) was performed using CITED1- and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primer sets designed to cross intron-exon boundaries (CITED1-F: GGCCTGCACTGGATGTCAAG, CITED1-R: GGAAGCTCATTGGCTCGGTC; GAPDH-F: CCCTTCATTGACCTCAACTACATGG, GAPDH-R: CTTGCCCACAGCCTTGGC). Polymerase chain reaction products were resolved by gel electrophoresis and detected under UV light by ethidium bromide staining.
1.2. CITED1 misexpression studies
To determine the tumorigenic potential of CITED1 in human Wilms' tumor, we used the retroviral LZRS delivery system (Gary Nolan, Stanford University, Palo Alto, Calif) to misexpress CITED1 in the SK-NEP-1 cell line [18]. We subcloned 3 different N-terminus FLAG-tagged CITED1 constructs into a modified LZRS retroviral construct that includes the multiple cloning site, internal ribosomal entry sequence, and the green fluorescent protein (GFP) to enable selection. The Phoenix packaging cell line was transfected using a calcium chloride technique [18]. The supernatants were harvested and added to subconfluent SK-NEP-1 cultures for 4 hours at 33°C in the presence of polybrene (4 μg/mL), as described. Infections were repeated using fresh supernatant on 4 consecutive days to promote efficient infection. After 72 hours of growth, each cell line was sorted and enriched by flow cytometry for GFP-expressing cells, and expanded. Four lines of cells were generated: (i) control SK-LZRS, infected with the empty virus; (ii) SK–full length (F/L), overexpressing CITED1-F/L; (iii) SK-ΔSID, overexpressing a CITED1 deletion mutant that lacks the SID; and (iv) SK-ΔCR2, overexpressing a CITED1 deletion mutant that lacks the functionally critical C-terminus transactivation domain.
To confirm expression of the respective epitope-tagged proteins, overexpressed CITED1 and its 2 mutant forms were detected as above after immunoprecipitation and Western blot analysis of cell lysates with anti-CITED1 antibody, 2H6, and anti-FLAG M5 monoclonal antibodies (Sigma-Aldrich, St Louis, MO). Overexpressed CITED1 and its 2 deletion mutants were probed with the anti-CITED1 antibody, 2h6, (mouse antihuman monoclonal raised against the GST-cited1 fusion protein), the anti-SID domain antibody, E623312M [12,17], and the M5 anti-FLAG antibody.
1.3. In vitro studies: proliferation and apoptosis assays
Proliferation and apoptosis assays were conducted to determine the mechanisms by which alterations in CITED1 expression promote cell survival and expansion. In vitro effects on cellular proliferation were assayed by thymidine incorporation. The 4 misexpressing SK-cell lines were plated separately at a density of 3 × 104 cells in 450 μL of starvation medium in 6 wells each of a 24-well plate (BD Falcon, San Jose, Calif) and allowed to grow for 48 hours. Studies were completed in triplicate. On day 3, 25 μL of H3 thymidine in McCoy's 5a medium with 0.2% fetal bovine serum (FBS) (0.5μCi per well) were added to each well and incubated for 2 hours at 37°C. Cells were then harvested, washed in phosphate-buffered saline for 3 cycles, and suspended in 500 μL of 1% SDS for 5 minutes. Each aggregate of cells was added separately to 10 mL of Aquasol and counted by scintigraphy (Beckman Coulter, Fullerton, Calif).
In vitro effects on apoptosis were assayed by annexin V–PE (phycoerythrin-labeled) and caspase 3 kits in triplicate according to manufacturer protocols (BD Pharmingen, San Diego, Calif) to determine whether the thymidine incorporation scintillation counts accurately reflected an increase in cell volume related to proliferation or to greater programmed cell death. Cells were maintained identically to those conditions as for the thymidine incorporation studies, and fluorescence-activated cell sorting (FACS) was used to determine percentage of dead cells.
1.4. In vivo studies: animals and heterotransplantation
To test the effect of CITED1 misexpression on Wilms' tumorigenesis in vivo, we manipulated our previously published heterotransplant model [19]. Female severe combined immunodeficient-beige mice (C.B17 SCID-beige; Charles River Laboratories, Wilmington, Mass), 4 to 6 weeks of age, were housed in groups of 4 under pathogen-free conditions in the Animal Care Facility of the Vanderbilt University School of Medicine (Nashville, Tenn) and were fed food and water ad libitum. The Vanderbilt University Institutional Animal Care and Use Committee approved all experimental protocols, which maintained accordance with guidelines as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immediately before in vivo injections, each of the 4 cell lines was detached from culture plates using trypsin-EDTA and resuspended in phosphate-buffered saline (pH 7.4) at a final concentration of 5 × 106 cells/mL. Under pathogen-free conditions and isoflurane (Baxter, Deerfield, Ill) general anesthesia, a 500-μL aliquot of this cell suspension was injected subcutaneously beneath the panniculus carnosus into the left flank of each mouse (total n = 60, n = 15 per cell line); heterotopic injection of this concentration of wild-type SKNEP-1 cells yields consistent tumor development, growth, and biology in more than 90% of animals [19]. Animals were observed subsequently for a period of 5 weeks and then killed, and a full necropsy was performed to determine extent of disease. At necropsy, tumor incidence was determined for each cell line, and tumor volume was estimated using digital calipers (tumor volume = L × W × H × π/6) [19]. The tumor-host interface was harvested and fixed overnight in 4% paraformaldehyde to determine tumor histology and local invasion after hematoxylin-eosin staining. Tissue sections were also analyzed by immunofluorescence using the anti-FLAG antibody, M2 (Sigma-Aldrich), to ensure expression and detection of exogenous protein.
Sections were also immunostained with Ki67 and terminal transferase dUTP nick end labeling (TUNEL) antibodies to determine in vivo alterations in cellular proliferation and programmed cell death, respectively. To highlight actively replicating cells, tissue sections were incubated with rabbit anti-human Ki-67 (NovaCastra Laboratories, Newcastle, UK) at a working dilution of 1:1000. The Dako Envision+ horseradish peroxidase and 3,3-diaminobenzidine (HRP/DAB) System (DakoCytomation, Carpinteria, Calif) was used to produce localized, visible staining. To detect fragmented DNA of apoptotic cells, tissue sections were interrogated with the DeadEnd Colorimetric TUNEL System (Promega, Madison, Wis). Sections were treated subsequently with equilibration buffer (Promega), and biotinylated nucleotides using terminal deoxynucleotidyl transferase were incorporated into apoptotic cells. Applications of streptavidin/HRP and DAB produced apoptotic-specific visible nuclear staining.
Furthermore, the remaining portions of each tumor were snap frozen in liquid nitrogen for subsequent tissue homogenization, protein extraction, and Western blotting. Tissue was homogenized using an ultrasonic homogenizer in lysis buffer. Lysates were handled and probed similarly as above to confirm integration and persistent misexpression of the retroviral constructs.
1.5. Statistical analysis
Analyses of study results focused on estimating the association between the expression level of CITED1 and the study end points, such as proliferation level, tumor incidence rate, and tumor volume. The Fisher's Exact test was applied for the end point of the tumor incidence rate. The Kruskal-Wallis test and the general linear model were applied for the end points of tumor volume and the proliferation assay studies. All tests of significance were 2-sided, and differences were considered statistically significant when P values were .05 or less. All data were expressed as mean ± SD. SAS version 9 (SAS Institute, Cary, NC) was used for all analyses.
2. Results
2.1. Endogenous CITED1 expression in SK-NEP-1 cells
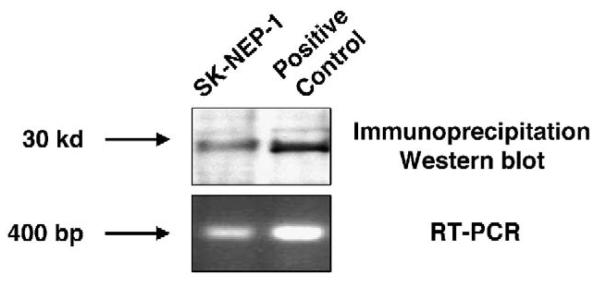
CITED1 is detected by immunoprecipitation and Western blot, as well as reverse transcriptase-PCR, in wild-type SKNEP-1 cells (Fig. 1).
Fig. 1.
The endogenous CITED1 protein is detected by Western blot after immunoprecipitation of wild-type SK-NEP-1 cell lysates. CITED messenger RNA is detected by PCR. The positive control is the human embryonic kidney (HEK-293+) cell line that has been manipulated to overexpress CITED1.
2.2. Overexpression of CITED1 and its deletion mutant proteins in SK-NEP-1 cells
Full-length CITED1 (SK-F/L) and its 2 deletion mutant proteins, SK-ΔSID and SK-ΔCR2, are detected in cultured cells by immunoblot for CITED1 and the FLAG constituent, confirming stable integration, transcription, and translation of the misexpressing DNA vectors (Fig. 2).
Fig. 2.
Western blot confirms overexpression of CITED1 (F/L) and its 2 mutant constructs, CITED1-ΔSID and CITED1-ΔCR2, in the human Wilms' tumor cell line, SK-NEP-1. The upper panel confirms specific protein detection using anti-CITED1 antibody, whereas the lower panel shows detection of constructs by probing with anti-FLAG antibody. 293+ indicates positive control cell line that stably overexpresses FLAG-tagged CITED1-F/L; WT, wild-type SK-NEP-1 cells. In this figure, CITED1 is not visible by Western blot alone in either wild-type or control LZRS SK-NEP-1 cells, but is detected after initial immunoprecipitation, as shown in Fig. 1. The CITED1-ΔCR2+ cell line was used for study purposes.
2.3. In vitro studies: proliferation and apoptosis
Overexpression of CITED1-F/L showed a marked increase in SK-NEP-1 cell proliferation relative to control cells (SK-LZRS, P < .0001) and the 2 deletion mutant cell lines (SK-ΔSID, P = .0002; SK-ΔCR2, P < .0001; Fig. 3). Interestingly, overexpression of the ΔCR2 mutant protein significantly reduced proliferation in SK-NEP-1 cells relative to all other cell lines (SK-LZRS, P = .0174; SK-F/L, P < .0001; SK-ΔSID, P < .0001; Fig. 3).
Fig. 3.
Results of the thymidine incorporation assay used to assess the in vitro effect on SK-NEP-1 cellular proliferation in this model system. Overexpressing CITED1-F/L significantly increased proliferation relative to all other cell lines (asterisk). Overexpressing CITED1-ΔSID significantly increased cellular proliferation relative to the control SK-LZRS and dominant-negative SK-ΔCR2 cell lines (asterisk). Overexpressing CITED1-ΔCR2 in SK-NEP-1 cells significantly reduced cellular proliferation (number sign).
Overexpression of CITED1-F/L and its 2 deletion mutant proteins in SK-NEP-1 cells showed no appreciable effect on apoptosis, as examined by triplicate runs each of annexin V–PE and caspase 3 assays, when subjected to the same conditions as in the thymidine incorporation assay (data not shown).
2.4. In vivo studies: tumorigenicity and volume
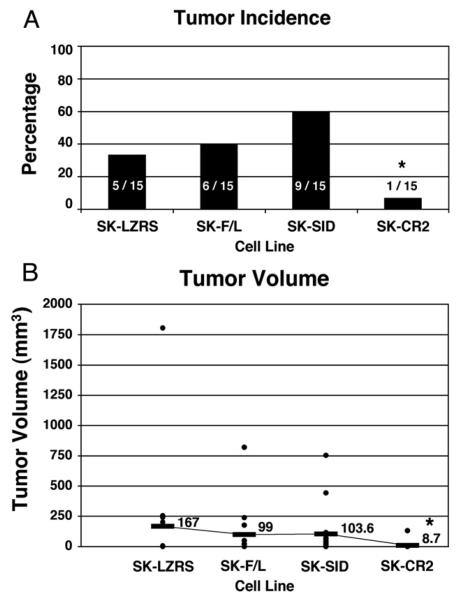
An association was found between tumorigenicity and the 4 cell lines( P =.022, Fig. 4). The ΔCR2 mutation significantly perturbed tumorigenesis (only 7% [1/15 animals] developed a tumor) relative to 2 of the other 3 cell lines in the animal studies (SK-LZRS, 33%, 5/15, P = .06; SK-F/L, 40%, 6/15, P =.03; SK-ΔSID, 60%, 9/15, P < .002; Fig. 4A). Injection of the SKF/L cell line that overexpresses CITED1-F/L showed no statistical difference in tumorigenicity relative to control SKLZRS or SK-ΔSID cell lines (respectively, P =.7 and P =.27). Although the SK-ΔSID cell line showed the highest tumorigenicity relative to each of the other lines, statistical significance was only achieved when compared with SK-ΔCR2.
Fig. 4.
Panel A shows the effect on tumor development in vivo after misexpression of CITED1 in SK-NEP-1 cells. Overexpression of the CITED1-ΔCR2 mutant in this model significantly suppressed Wilms' tumor development in a dominant-negative manner. Overexpression of the CITED1-ΔSID mutant showed the highest tumor incidence relative to the SK-CR2 cells. Panel B similarly shows suppressed tumor growth of the CITED1-ΔCR2 mutant-expressing cells relative to the other 3 cell lines.
Comparison of tumor volumes for each cell line showed a similar pattern to that of tumorigenicity and was predictive of a biological difference between the ΔCR2 mutant and the other 3 cell lines ( P = .05, Fig. 4B). Infection of SK-NEP-1 cells with the ΔCR2 mutant blunted tumor volume relative to the other cell lines and achieved statistical significance for the SK-F/L and SK-ΔSID lines (SK-LZRS, P = .06; SK-F/L, P = .03; SK-ΔSID, P < .005). No significant difference in tumor volume was observed between the SK-LZRS, SK-F/L, and SK-ΔSID lines.
Immediately after necropsy, thin slices of fresh tumor were analyzed under a fluorescent microscope and expression of GFP was confirmed, implying stable integration of the DNA vectors of interest. Furthermore, DNA vector protein products were confirmed in resulting tumor samples by immunoprecipitation and Western blot, and immunofluorescence (data not shown).
2.5. In vivo studies: histology, proliferation, and apoptosis
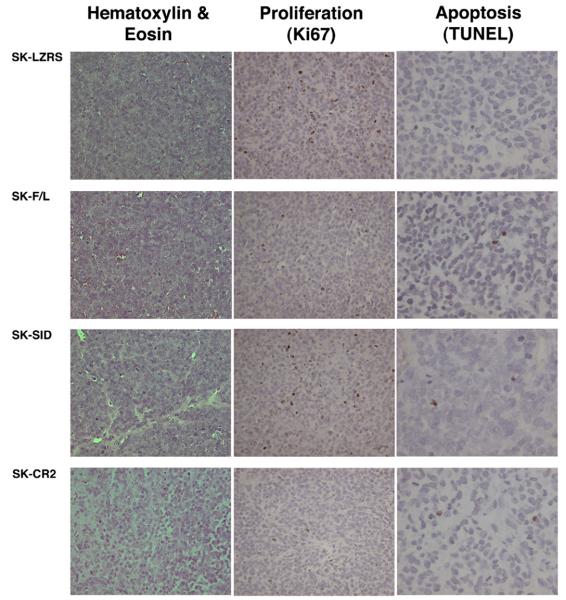
Hematoxylin-eosin staining of SK-LZRS, SK-F/L, and SK-ΔSID histologic specimens showed a similar appearance to wild-type SK-NEP-1 tumors. However, the histology for the SK-ΔCR2 tumor appeared different from that of the other cell lines, showing increased nuclear and cytoplasmic vesiculation, and reduced cellular density (Fig. 5). However, no discrete epithelial structures were identified in this single SK-ΔCR2 tumor. Furthermore, the SK-ΔCR2 tumor showed the least cellular proliferation as examined by Ki67 immunostaining when compared with the other 3 tumor lines. TUNEL immunostaining was no different between tumors, supporting the in vitro data that misexpression of CITED1 does not affect apoptosis (Fig. 5). None of the resulting tumors showed criteria of anaplasia.
Fig. 5.
Histologic, and in vivo proliferative and apoptotic analysis of tissue sections. Horizontal rows show representative tumors developing from each misexpressing cell line, and the vertical columns show the basic histology (hematoxylin-eosin stain) and Ki-67 and TUNEL immunostaining. Although we observed no elements of spontaneous differentiation (tubules) by hematoxylin-eosin stain, we did observe in the SK-CR2 tumor reduced cell density and increased nuclear and cytoplasmic vesiculation relative to the other 3 tumor lines, which were composed of densely packed, cytoplasm-poor blastemal cells and were histologically indistinguishable from wild-type SK-NEP-1 tumors. The single SK-CR2 tumor showed minimal Ki-67 staining relative to the other 3 tumor lines, which each showed numerous positive cells. TUNEL staining showed no differences in apoptosis between tumor lines (roughly 2 positive cells per high-power field).
3. Discussion
Our studies link alterations in CITED1-dependent transcription to Wilms' tumorigenesis. In these studies, over-expressing the full-length compliment of CITED1 in the human Wilms' tumor cell line, SK-NEP-1, markedly increased cellular proliferation in vitro, an event that appears specific to proliferation, as no effect on programmed cell death was observed when cells were subjected to identical culture conditions. Furthermore, overexpressing the functionally inactivating mutant protein of CITED1, ΔCR2 (which is missing the critical CBP/p300 binding domain of CITED1), significantly reduced cellular proliferation, and in vivo, the ΔCR2 mutation significantly perturbed tumorigenicity and growth. Taken together, these observations suggest that the CITED1-ΔCR2 mutant may function as a dominant-negative by interfering with endogenous CITED1-dependent cell proliferation and therefore SK-NEP-1 tumor development in vivo.
Our studies further implicate Smad4-dependent TGF-β signaling as a regulatory pathway in Wilms' tumorigenesis. Unlike mutation of the functionally critical CR2 domain, deletion of the CITED1-ΔSID significantly increased cellular proliferation in our model system in vitro, and interestingly, cells overexpressing the mutant protein, CITED1-ΔSID, showed the highest incidence of Wilms' tumor development in vivo. These observations suggest that the prooncogenic effects of CITED1 may be under the counterregulatory control of the transcriptional co-factor and tumor suppressor, Smad4. Of note, Smad4 has been shown definitively to regulate TGF-β tumor suppression of colon and pancreatic adenocarcinomas [20-23]. Consistent with these other tumor suppressor systems, the loss of Smad4 interaction with CITED1 may release suppressive control of specific, CITED1-dependent cellular functions and tumorigenic pathways, as observed in our model.
Embryonal tumors are purported to originate from pluripotent but fated cells that have escaped regulated pathways of cellular differentiation and organogenesis. Knudson and Strong [24,25] initially introduced the concept that embryonal tumors, and specifically Wilms' tumors, arise by a 2-step mutational process that first must signal and promote persistence of embryonal or early progenitor cells of developing organs. Because our laboratory has shown previously that CITED1 overexpression blocks the mesenchymal-to-epithelial conversion and thereby promotes persistence of undifferentiated progenitor cells of the developing kidney [11], we chose to explore the role of CITED1-dependent transcriptional responses in Wilms' tumorigenesis. Further, Oxburgh et al [26] have shown that integrity of TGF-β/Smad4 signaling, a CITED1-associated pathway, is required for normal renal morphogenesis of the metanephric mesenchyme to proceed, as loss of Smad4 in the metanephric mesenchyme results in a failure of epithelial progenitor cells to condense around the ureteric bud tips, and leads to disorganization and ultimate impairment of the mesenchymal-to-epithelial transition. Therefore, in keeping with the Knudson “2-hit” hypothesis of Wilms' tumorigenesis, the initial “hit” might involve altered CITED1 interaction with suppressive Smad4-dependent TGF-β signaling pathways and thus alllowed unabated CITED1-dependent transcription, which may facilitate both persistence and expansion of the early progenitor cell population of the developing kidney.
CITED1 modifies transcription via 2 opposing pathways that are critical for nephrogenesis [11]. CITED1 functions as a transcriptional regulator that both activates bone morphogenetic protein 7/Smad-4–dependent responses, a survival pathway of the metanephric mesenchyme, yet represses Wnt/β-catenin signaling, critical for mesenchymal differentiation [26-33]. These activities are dependent on the integrity of the acidic terminus of CITED1, CR2, because absence of this domain has been shown to modify bone morphogenetic protein 7 and Wnt/β-catenin responses [11]. These observations suggest a molecular mechanism whereby CITED1 expression promotes persistence of the metanephric blastema, and its downregulation signals differentiation of the metanephric blastema by de-repressing Wnt/β-catenin–mediated transcriptional responses and enabling Wnt-dependent epithelial morphogenesis to progress.
Early events that are necessary for persistence and expansion of a cell population include activation of molecular pathways that either signal cellular proliferation or block programmed cell death. Our studies suggest that upregulated CITED1 expression imparts the specific response to enhance cellular proliferation without affecting apoptosis. By increasing proliferation, persistent CITED1 expression may provide an essential mechanism for mesenchymal cellular survival. We speculate that overexpression of CITED1 therefore promotes expansion of a rapidly proliferating population of blastema as the initial hit and thereby induces an unstable environment that renders this cell population highly susceptible to future oncogenic events in a 2-hit model of Wilms' tumorigenesis.
Finally, we showed that mutating the functionally critical CR2 domain significantly reduces cellular proliferation. Wilms' tumorigenesis in our model system also appears to require integrity of the CR2 domain and its interaction with the CBP/p300 co-activators. We speculate that the deletion mutant, CITED1-ΔCR2, may act as a “sink” for some other ”docking” co-factor that too is necessary to activate CITED1-dependent transcription and cellular proliferation. Binding of CITED1-ΔCR2 to this unknown co-factor may consume and render the latter unavailable to coordinate function-dependent binding of endogenous CITED1 to the CBP/p300 co-activators. In a dominant-negative manner, therefore, CITED1-dependent transcription and its specific proliferative responses are blocked.
Taken together, our studies outline a molecular mechanism whereby CITED1 overexpression enhances cellular proliferation and, as a result, may promote persistence and expansion of the metanephric blastema. Our observations further suggest that CITED1 is critical to Wilms' tumor development, as evidenced by the dominant-negative effect of the CITED1-ΔCR2 mutant, and that CITED1 may be under the counterregulatory control of Smad4-dependent TGF-β signaling pathways in Wilms' tumorigenesis. On this basis, we speculate that overexpression of CITED1 promotes expansion of a rapidly proliferating population of blastema and thereby induces an unstable environment highly susceptible to future oncogenic events. These studies warrant a more rigorous investigation of the role of CITED1, including its interactions with the tumor suppressor, Smad4, in Wilms' tumorigenesis.
References
- 1.Green DM, D'Angio GJ, Beckwith JB, et al. Wilms' tumor. Cancer J Clin. 1996;46:46–63. doi: 10.3322/canjclin.46.1.46. [DOI] [PubMed] [Google Scholar]
- 2.Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms' tumor. Med Pediatr Oncol. 1993;21:172–81. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 3.Birch JM, Breslow N. Epidemiologic features of Wilms' tumor. Hematol Oncol Clin. 1995;9:1157–75. [PubMed] [Google Scholar]
- 4.Beckwith JB. The impact of tumor histology on treatment and prognosis of pediatric neoplasms. Semin Pediatr Surg. 1993;2:11–8. [PubMed] [Google Scholar]
- 5.Beckwith JB. New developments in the pathology of Wilms' tumor. Cancer Invest. 1997;15:153–62. doi: 10.3109/07357909709115768. [DOI] [PubMed] [Google Scholar]
- 6.Coppes MJ. The management and biological behavior of Wilms' tumor. Int Rev Exp Pathol. 1994;35:150–76. [PubMed] [Google Scholar]
- 7.Beckwith JB, Zuppan CE, Browning NG, et al. Histological analysis of aggressiveness and responsiveness in Wilms' tumor. Med Pediatr Oncol. 1996;27:422–8. doi: 10.1002/(SICI)1096-911X(199611)27:5<422::AID-MPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt D, Beckwith JB. Histopathology of childhood renal tumors. Hematol Oncol Clin. 1995;9:1179–200. [PubMed] [Google Scholar]
- 9.Shioda T, Fenner MH, Isselbacher KJ. MSG1, a novel melanocyte-specific gene, encodes a nuclear protein and is associated with pigmentation. Proc Natl Acad Sci U S A. 1996;93:12298–303. doi: 10.1073/pnas.93.22.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunwoodie SL, Rodriguez TA, Beddington RS. Msg1 and Mrg1, founding members of a gene family, show distinct patterns of gene expression during mouse embryogenesis. Mech Dev. 1998;72:27–40. doi: 10.1016/s0925-4773(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 11.Plisov S, Tsang M, Shi G, et al. Cited1 is a bifunctional transcriptional cofactor that regulates early nephronic patterning. J Am Soc Nephrol. 2005;16:1632–44. doi: 10.1681/ASN.2004060476. [DOI] [PubMed] [Google Scholar]
- 12.Shioda T, Fenner MH, Isselbacher KJ. MSG1 and its related protein MRG1 share a transcription activating domain. Gene. 1997;204:235–41. doi: 10.1016/s0378-1119(97)00551-9. [DOI] [PubMed] [Google Scholar]
- 13.Yahata T, de Caestecker MP, Lechleider RJ, et al. The MSG1 non–DNA-binding transactivator binds to the p300/CBP co-activators, enhancing their functional link to the Smad transcription factors. J Biol Chem. 2000;275(12):8825–34. doi: 10.1074/jbc.275.12.8825. [DOI] [PubMed] [Google Scholar]
- 14.Yahata T, Shao W, Endoh H, et al. Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes Dev. 2001;15:2598–612. doi: 10.1101/gad.906301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shioda T, Lechleider RJ, Dunwoodie SL, et al. Transcriptional activating activity of Smad4: roles of SMAD hetero-oligomerization and enhancement by an associating transactivator. Proc Natl Acad Sci U S A. 1998;95:9785–90. doi: 10.1073/pnas.95.17.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Caestecker MP, Yahata T, Wang D, et al. The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem. 2000;275(3):2115–22. doi: 10.1074/jbc.275.3.2115. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Ahmed NU, Fenner MH, et al. Regulation of expression of MSG1 melanocyte-specific nuclear protein in human melanocytes and melanoma cells. Exp Cell Res. 1998;242:478–86. doi: 10.1006/excr.1998.4123. [DOI] [PubMed] [Google Scholar]
- 18.Lorens JB, Bennett MK, Pearsall DM, et al. Retroviral delivery of peptide modulators of cellular functions. Mol Ther. 2000;1:438–47. doi: 10.1006/mthe.2000.0063. [DOI] [PubMed] [Google Scholar]
- 19.Lovvorn HN, Savani RC, Ruchelli E, et al. Serum hyaluronan and its association with unfavorable histology and aggressiveness of heterotransplanted Wilms' tumor. J Pediatr Surg. 2000;35:1070–8. doi: 10.1053/jpsu.2000.7774. [DOI] [PubMed] [Google Scholar]
- 20.Muller N, Reinacher-Schick A, Baldus S, et al. Smad4 induces the tumor suppressor E-cadherin and P-cadherin in colon carcinoma cells. Oncogene. 2002;21:6049–58. doi: 10.1038/sj.onc.1205766. [DOI] [PubMed] [Google Scholar]
- 21.Duda DG, Sunamura M, Lefter LP, et al. Restoration of Smad4 by gene therapy reverses the invasive phenotype in pancreatic adenocarcinoma cells. Oncogene. 2003;22:6857–64. doi: 10.1038/sj.onc.1206751. [DOI] [PubMed] [Google Scholar]
- 22.Jazag A, Ijichi H, Kanai F, et al. Smad4 silencing in pancreatic cell lines using stable RNA interference and gene expression profiles induced by transforming growth factor–β. Oncogene. 2005;24:662–71. doi: 10.1038/sj.onc.1208102. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Katuri V, Srinivasan R, et al. Transforming growth factor–β suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 2005;65(10):4228–37. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
- 24.Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Nat Acad Sci U S A. 1971;68(4):820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudson AG, Strong LC. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972;48:313–24. [PubMed] [Google Scholar]
- 26.Oxburgh L, Chu GC, Michael SK, et al. TGF-beta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131:4593–605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- 27.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–83. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 28.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 29.Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–13. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 31.Macias-Silva M, Hoodless PA, Tang SJ, et al. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–36. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 32.Luo G, Hofmann C, Bronckers AL, et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 33.Plisov SY, Yoshino K, Dove LF, et al. TGFb2, LIF and FGF2 cooperate to induce nephrogenesis. Development. 2001;128:1045–57. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]