Abstract
Hepatocyte nuclear factor 4α (HNF4α) controls the expression of many critical metabolic pathways, and the Mediator complex occupies a central role in recruiting RNA polymerase II (Pol II) to these gene promoters. An impaired transcriptional HNF4α network in human liver is responsible for many pathological conditions, such as altered drug metabolism, fatty liver, and diabetes. Here, we report that Med25, an associated member of the Mediator complex, is required for the association of HNF4α with Mediator, its several cofactors, and RNA Pol II. Further, increases and decreases in endogenous Med25 levels are reflected in the composition of the transcriptional complex, Pol II recruitment, and the expression of HNF4α-bound target genes. A novel feature of Med25 is that it imparts “selectivity.” Med25 affects only a significant subset of HNF4α target genes that selectively regulate drug and lipid metabolism. These results define a role for Med25 and the Mediator complex in the regulation of xenobiotic metabolism and lipid homeostasis.
The liver is the central organ that controls/regulates such metabolic processes as lipid metabolism (lipogenesis and fatty acid β-oxidation [FAO]), energy homeostasis (glycolysis and gluconeogenesis), bile acid synthesis, and drug metabolism (4, 48, 49, 57). Metabolism in the liver is governed by a highly dynamic transcriptional regulatory network whose key receptors may be classical (glucocorticoid), adopted (peroxisome proliferator activated receptor, farnesoid X receptor, liver specific X receptor, and constitutively active receptor/pregnane X receptor), or orphan (hepatocyte nuclear factor 4α [HNF4α]) (6). HNF4α, also known as NR2A1, is an important member of the orphan nuclear receptor superfamily (51, 53). When HNF4α binds to DNA, it regulates the expression of both constitutive genes, such as apolipoprotein genes (37), CYP7A1 and CYP8B1 (25, 27), the FXII and XIIIB genes (26), the proline oxidase gene (30), CYP2D6 (5), CYP2A6 (42), UGT1A9 (1), SULTA1 (31), and the glucose-6-phosphatase (G6PC) and Pck1 genes (47), and also xenobiotic inducible genes, such as CYP3A4 (56), CYP2C9 (8), and CYP2B6 (24). Malfunctions in the regulation of genes by HNF4α are responsible for alterations in drug metabolism and metabolic disorders, such as atherosclerosis, diabetes, hemophilia, hypoxia, medium-chain acyl-coenzyme A (CoA) dehydrogenase (MCAD) deficiency, and ornithine transcarbamylase (OTC) deficiency (16, 22).
Like most nuclear receptors, HNF4α displays a typical DNA binding domain (DBD), including a double zinc finger motif, a ligand binding domain (LBD), and two activation domains, AF1 and AF2, located in the N- and C-terminal regions, respectively (21, 53). The DNA binding domain binds to a cis-acting element which optimally consists of a direct repeat of AGGTCA with a 1- or 2-nucleotide spacer (DR1/DR2) to which HNF4α binds as a homodimer (52). The ligand binding domain is structurally homologous to other receptors of the retinoid receptor family, with a well-defined hydrophobic pocket capable of binding fatty acids (11, 21). Fatty acids have been long believed to act as ligands for many orphan receptors (23, 61); recently, Yuan and coworkers (64) showed that HNF4α is selectively occupied by linoleic acid (LA; C18: omega6) in mammalian cells, although ligand occupancy was not necessary for transcriptional activity of HNF4α, as shown by genome-wide profiling.
The AF2 motif of HNF4α is responsible for binding to several groups of cofactors, such as the CBP/p300 class (10, 38), to members of the SRC family (59), including the histone acetyltransferase complex (62), to the NCOA6/PRIP-anchored ASCOM complex (17, 54), to the Med1-anchored Mediator complex (33, 38, 43), and to the energy sensor PGC-1α (47). The diverse nature of these coactivators suggests that a variable functional complex not only controls the constitutive expression of genes but also directs specialized functions of the liver, such as fatty acid metabolism, drug metabolism, and energy homeostasis. These hepatic pathways are affected by the hormonal and environmental signals that induce metabolic syndrome, a combination of disorders that increase the risk of diabetes and cardiovascular diseases (13, 14, 19, 45, 65).
In this report, we identify a new coactivator of HNF4α, Med25. We have demonstrated here that Med25 plays a vital role in modulating HNF4α transcriptional activity by (i) converting the HNF4α-bound transcriptional complex from the inactive to the active state, (ii) facilitating the recruitment of RNA polymerase II (Pol II) to the promoter site, and (iii) giving HNF4α an exquisite specificity in controlling the genes that are responsible for lipid and drug metabolism, viz., the cytochrome P-450s.
MATERIALS AND METHODS
Cell lines and cell culture.
HepG2 cells were cultured in Eagle's minimal essential medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 1 mM sodium pyruvate, and penicillin-streptomycin at 37°C under 5% CO2. HEK293 cells were grown in Dulbecco's modified Eagle medium supplemented with 10% FBS. Primary human hepatocytes were obtained from CellzDirect (Invitrogen, Durham, NC) and maintained in William's E medium supplemented with ITS (insulin, human transferrin, sodium selenite, bovine serum albumin, and linoleic acid in Earle's balanced salt solution; Sigma), HEPES, l-glutamine, and 100 nM dexamethasone.
Plasmids and adenovirus (Ad) construction.
Full-length Med25 was cloned from a Mammalian Gene Collection (MGC) clone, BCO65297, into the pcDNA3.1, p3XFLAG-CMV-7.1, and pEGFP-N1 vectors. Deletion constructs of Med25 were generated in the pGEX4T1 and pACT vectors. Mutation constructs were prepared using a site-directed mutagenesis kit (Stratagene) from the wild-type constructs. All constructs were sequence verified.
For adenoviral production, Flag-Med25, HNF4α, and CAR were cloned in a pShuttle vector and recombined with an AdEasy vector backbone. Short hairpin RNAs (shRNAs) were identified using Genescript's target finder and cloned into pRNAT-H1.1/Adeno vector. The viruses were produced and amplified in AD293 cells using standard procedures. Viral particles were purified on a CsCl gradient and stored in sucrose buffer.
Yeast and mammalian two-hybrid screening and assay.
ProNet Technologies automated two-hybrid screening was performed by Myraid Genetics (Salt Lake City, UT) as described previously (15). A liver cDNA library was used to screen proteins that interact with HNF4α. To map the interaction domains between Med25 and HNF4α, deletion derivatives of Med25 were fused to VP16 AD in a pACT vector. The ligand binding domain of HNF4α was fused to the GAL4 DBD in a pBind vector. The promoter luciferase reporter 9XGAL4 UAS was employed to assay the interaction in HepG2 cells. Promega's Dual-Glo System was used to measure the luciferase activity.
GST pulldown assays.
Glutathione S-transferase (GST) fusion proteins of nuclear receptors (CAR, PXR, and HNF4α) were expressed in Escherichia coli BL-21, and Med25 fragments were purified on glutathione (GSH)-Sepharose beads (Amersham). It should be noted that the endogenous ligand of HNF4α (linoleic acid) is not present in bacteria, but crystallographic studies of recombinant HNF4α indicate that bacterial fatty acids occupy the ligand binding pocket (11). The indicated full-length proteins were translated in vitro in rabbit reticulocyte lysate (TNT Quick coupled transcription and translation system; Promega) with radiolabeled [35S]methionine (MP Biomedicals). Expression and purification of recombinant GST fusion proteins and interaction assays were described previously (54).
Nuclear extract preparation, immunoblot analysis, and protein identification by LC-MS-MS.
Nuclear extracts were prepared from HepG2 cells and primary human hepatocytes with reduced or increased Med25 as described before (12). Nuclear extracts from both of the preparations were subjected to immunoprecipitation (IP) with rabbit anti-HNF4α antibody (Ab) (sc8987)-coupled agarose beads using a coimmunoprecipitation kit (Pierce). The bound proteins were eluted and silver stained using a SilverQuest silver-staining kit (Invitrogen) for visualization. Proteins were immunoblotted with the indicated antibodies. For mass spectrometry (MS) analysis, the protein bands from the Coomassie blue-stained gels were cut and digested with trypsin as described by Choi et al. (9). The resulting peptide digests were then analyzed by nano-liquid chromatography (nano-LC) electrospray ionization (ESI)-MS and tandem mass spectrometry (MS-MS) on an Agilent XCT Ultra ion trap mass spectrometer. The data were processed and searched against the NCBI nonredundant database as previously described (9).
Transfections and confocal microscopy.
Transfections in HepG2 and HEK293 cells were performed with the indicated plasmids using Lipofectamine 2000 (Invitrogen). For primary human hepatocytes, transfections were performed on freshly isolated human hepatocytes without overlay using Effectene transfection reagent (Qiagen) according to the manufacturer's procedures.
For colocalization studies, HEK293 cells were transfected on chamber slides with enhanced green fluorescent protein (EGFP)-Med25 and the indicated cDNA constructs. The slides were processed for immunostaining, and the proteins were localized with a Zeiss LSM-510 UV confocal microscope.
Promoter assay and gene induction.
HepG2 cells or primary human hepatocytes were seeded in 24-well plates at a density of 2.5 × 105 cells per well. After 24 h, the cells were transiently transfected and infected sequentially. Forty-eight hours after transfection, cells were harvested and assayed with a Dual-Glo luciferase assay system (Promega) using a LUMIstat OPTIMA. Gene expression analyses were performed by TaqMan assay with an ABI 7900 while the RNA was isolated using standard Qiagen procedures. The relative quantities were calculated by the 2−ΔΔCT method.
EMSAs and ChIP analysis.
Electrophoretic mobility shift assays (EMSAs) were performed as described before, using a double-stranded 32P-labeled oligonucleotide containing the bp −150 HNF4α response element of the CYP2C9 promoter (8). Conventional chromatin immunoprecipitation (ChIP) analysis was performed on HepG2 cells treated with the indicated adenovirus as described before (54), while for primary human hepatocytes, ChIP was performed according to the Magnify ChIP procedure (Invitrogen, CA). Quantitative PCR (qPCR) was performed using Power SYBR green master mix (Applied Biosystems).
Fatty acid oxidation assay.
Primary human hepatocyte homogenates were prepared in a homogenization buffer containing 300 mM mannitol, 0.1 mM EGTA, and 10 mM HEPES (pH 7.2). The homogenates were centrifuged at 1,000 × g for 1 min, and supernatants were used for the acyl-CoA oxidase activity as described previously (41).
RESULTS
Identification of Med25 as a novel interactor of hepatocyte nuclear factor 4α.
We identified Med25 as an HNF4α ligand binding domain interacting protein in a yeast two-hybrid screen with a human liver cDNA library. Med25 is also known as p78, PTOV2, ACID1, or ARC92 (2, 3, 39, 58, 63). Two clones of Med25 that code for amino acids 583 to 747 and 624 to 702, respectively, were isolated; both clones include the NR binding motif, LXXLL. The function of Med25 is indicated by the conserved domain database search described earlier (32), showing a distinct VWA domain, a PTOV domain between two SD domains, and an NR-interacting domain. It appears that Med25 functions to efficiently coordinate the NR-based transcriptional activation. Only one other Mediator complex protein, Med1, has been reported to bind to HNF4α (38).
To confirm the interaction between Med25 and HNF4α, we tested the interaction between Med25 and HNF4α as well as that between CAR and PXR, another pair of key regulators of hepatic xenobiotic metabolism. Our data showed that Med25 exhibited a strong interaction with HNF4α but failed to interact with CAR and PXR (Fig. 1A, top). PGC-1α, a known cofactor for the HNF4α ligand binding domain, interacted with both CAR and HNF4α (Fig. 1A, bottom).
FIG. 1.
Med25 interacts with HNF4α and cofactors in vitro. (A) Med25 interacts with HNF4α in vitro. Nuclear receptors CAR, PXR, and HNF4α were expressed as GST fusion proteins and incubated with full-length in vitro-translated 35S-labeled Med25 (top panel) and PGC-1α (lower panel). CAR was incubated with Med25 and PGC-1α in the presence of 100 nM CAR ligand CITCO, and PXR was incubated with Med25 and PGC-1α in the presence of 10 μM PXR ligand rifampin. HNF4α was incubated with Med25 and PGC-1α without exogenous ligand. DMSO, dimethyl sulfoxide. (B) Schematic representation of Med25 along with generated domains for interaction mapping. The numbers indicate the amino acid (aa) residues, and the length of the recombinant protein fragment, and LXXLL denotes the NR-interacting motif. (C) Med25 interacts with HNF4α through the LXXLL domain. GST-Med25 domains were incubated with the indicated in vitro-translated 35S-labeled nuclear receptors in the presence and absence of ligands (for CAR, CITCO; for PXR, rifampin; for RAR, trans-retinoic acid; and for HNF4α, no exogenous ligand). (D to F) In vivo interaction of HNF4α with Med25. (D) Mammalian two-hybrid analysis. In vivo interaction analysis on 9XGal4 luciferase promoter expressed in HepG2 cells with the Gal4-HNF4α ligand binding domain (pBind fusion) and the VP-16 Med25 domains (pACT fusion) confirms the interaction through the LXXLL motif in domain III. Values represent means ± standard errors (SE) of results from triplicate analyses (*, significantly greater than the level for the control at P values of <0.001). The interaction is abolished by mutating the LXXLL motif to LXXAA. #, significantly lower than the level for domain III (P < 0.001). (E) Immunoprecipitation of HNF4α with Med25 and vice versa. HNF4α and Flag-Med25, which are absent in HEK293 cells, were expressed ectopically individually and in combination. The nuclear extracts were immunoprecipitated with Flag Ab for Med25 and probed with HNF4α Ab (top panel); reciprocally, the extracts were immunoprecipitated with HNF4α Ab and probed with Flag Ab (lower panel). The inputs were from HNF4α- and Med25-expressed extracts. WB, Western blot. (F) Confocal microscopy. Nuclear receptor HNF4α and the indicated cofactors are colocalized in the nucleus with GFP-tagged Med25 in HEK293 cells. Endogenous RNA polymerase II is also colocalized with Med25, whereas NCOA6 does not colocalize with Med25 in the nucleus. DAPI, 4′,6-diamidino-2-phenylindole.
To identify the interacting region between Med25 and HNF4α, we generated a series of constructs (Fig. 1B) expressing Med25 domains as GST fusion proteins. Med25 specifically interacted with HNF4α, and the interaction was mediated through the LXXLL motif (Fig. 1C). The interaction was lost when the LXXLL motif was mutated to LXXAA. HNF4α-Med25 interactions were assayed with no additional ligand, but Med25 domains showed a classical ligand-dependent interaction with retinoic acid receptor (RAR) (Fig. 1C), in agreement with an earlier report (32). In addition, the partial domains failed to interact with CAR and PXR in the presence or absence of their respective ligands.
To further assess the interaction strength and specificity between Med25 and HNF4α in vivo, we employed a mammalian two-hybrid assay, in which Med25 domains representing the N-terminal (VWA region), the middle (PTOV region), and the C-terminal (NR box) regions were assayed with the ligand binding domain of HNF4α. Med25 domain III specifically interacted with the HNF4α LBD as shown (Fig. 1D). The mutated construct expressing LXXAA instead of LXXLL did not show any appreciable interaction. Consistent with the GST pulldown assay, no interactions were found when Med25 domains were assayed with the CAR or PXR xenobiotic sensing nuclear receptor (data not shown). These results confirm that Med25 associates with HNF4α but not with CAR or PXR.
HNF4α binds with Med25 and other coactivators in vivo and in vitro.
To confirm the interaction between HNF4α and Med25 in vivo, we performed a reciprocal immunoprecipitation assay with HEK293 cells. Since the expression levels of HNF4α and Med25 are negligible in HEK293 cells, HNF4α and Flag-tagged Med25 were ectopically expressed. Med25 was indeed shown to interact with HNF4α in vivo via IP with Flag antibody and subsequent immunoblotting of the immunoprecipitates with HNF4α antibody (Fig. 1E, top) and via a reciprocal basis IP with HNF4α antibody and immunoblotting with anti-Flag antibody (Fig. 1E, bottom).
To identify HNF4α binding proteins, we extracted nuclear proteins from HepG2 cells that had been infected with adenovirus coding for HNF4α and Flag-tagged Med25. Although HNF4α is expressed in HepG2 cells, ectopic expression of HNF4α further induces HNF4α target genes, e.g., the CYP2C genes (29). Because a commercial antibody for Med25 was not available, we tracked the expression of Med25 by a Flag-tagged recombinant protein. The HepG2 nuclear extract was coimmunoprecipitated with IgG or anti-HNF4α antibody. When the bound proteins were separated on a 4 to 20% SDS-PAGE gel (see Fig. S2A at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf), silver stained, and subjected to mass spectral identification, a variety of cofactors were immunoprecipitated with anti-HNF4α antibody and very few proteins with IgG (control). Some of the notable proteins identified by mass spectrometry and confirmed by immunoblotting (see Fig. S2B at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf) include Med1, Med8, CBP, and NCOA6. The entire list of proteins identified is given in Table S-I at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf. Interestingly, the proteins that were coimmunoprecipitated with HNF4α represent the majority of the known subcomplexes, i.e., the chromatin-modifying group (CBP and SRC family), the Mediator group (Med1 and Med8), the ASCOM complex (NCOA6), the RNA polymerase II (Pol II) complex, and associated proteins such as PGC-1α, in addition to HNF4α and Med25. The identification of proteins representing all the subcomplexes suggests that HNF4α binds to these proteins in a functional transcriptional complex such as the preinitiation complex (PIC).
As the conserved-domain database search revealed four different domains in Med25, we hypothesized that these domains could play a role in different cofactor associations. When we examined fragments of Med25 containing single domains, we found that Med25 interacted with HNF4α through the LXXLL motif in fragments IV and VI (see Fig. S2C at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). PGC-1α, one of the previously known cofactors, bound to fragment IV, fragment V, and fragment VII, but not to fragment VI, suggesting that PGC-1α interacts in the C-terminal region of Med25 but that binding is not dependent on the LXXLL motif. SRC-1/NCOA1 and GRIP-1/SRC-2/NCOA2 (which are part of the CBP binding complex involved in chromatin modifications and HAT-containing enzymes) were found to bind exclusively with Med25 fragment I, which codes for the VWA domain. Interestingly, although NCOA6 coimmunoprecipitated with HNF4α and is known to be an interacting coactivator of this receptor, it did not interact with Med25 directly (data not shown). Among other proteins that bound to Med25, CBP was found to bind very strongly with Med25 fragment III (synapsin domain-1) and moderately with fragments IV, V, and VII but not with fragment VI, which codes for the LXXLL motif. The mutation of the LXXLL motif had no effect on CBP interaction, suggesting that Med25 interacts with CBP mainly through the SD-1 and PTOV/ACID regions. Med1/PBP/Trap220 bound exclusively with Med25 fragment 1, coding for the VWA motif.
To summarize, Med25 interacts with the nuclear receptors HNF4α and RAR via the C-terminal region that encodes the LXXLL motif. Med1, SRC-1, and GRIP-1 interact with Med25 in the N-terminal region. PGC-1α interacts with Med25 via the C-terminal region but not through the LXXLL motif, while CBP interacts with Med25 through the SD-1 and PTOV domains. To further augment the in vitro interaction data, we analyzed these interactions by coexpressing the proteins to determine their localization in HEK293 cells. Med25 was expressed as a green fluorescent protein (GFP) fusion protein and coexpressed with HNF4α, PGC-1α, CBP, SRC-1, GRIP-1, and Med1, all of which colocalized in the nucleus, as judged by confocal microscopy (Fig. 1F). NCOA6, shown here as a negative control, did not colocalize with Med25.
Med25 regulates HNF4α activation.
To elucidate the role of Med25 in HNF4α signaling of the drug-metabolizing enzymes CYP2C9 and CYP3A4, we employed primary human hepatocytes and HepG2 cells (a human liver cell line) and investigated the connection between Med25 and the transactivation of HNF4α with luciferase assays. HepG2 cells were transfected with a 1.9-kb CYP2C9 promoter construct in the pGL3 reporter vector along with the nuclear receptors CAR, HNF4α, and Med25 in the combinations shown in Fig. 2A, followed by treatment with the CAR ligand CITCO {6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime} (100 nM) or vehicle. We have previously shown that both CAR and HNF4α activate the CYP2C9 promoter and that there is a synergistic activation when both receptors are present, which is enhanced in the presence of CITCO (54). As expected, Med25 did not activate the CYP2C9 promoter, nor did it augment the activation by CAR. However, the activation of the CYP2C9 promoter by HNF4α (7) and the synergistic activation by CAR and HNF4α were markedly enhanced by ectopic expression of Med25. In analogous experiments with CYP3A4 (Fig. 2C), CYP3A4 was activated by CAR in the presence and absence of CITCO, but coexpression of Med25 had no further effect. However, HNF4α also activated the CYP3A4 promoter, and coexpression of Med25 enhanced this activation. CYP3A4 was also synergistically activated by CAR and HNF4α in the presence or absence of CITCO, and the presence of Med25 clearly augmented this activation. Taken together, Med25 enhances HNF4α-mediated gene activation whether the gene is activated by HNF4α alone or synergistically with CAR and HNF4α.
FIG. 2.
Med25 regulates HNF4α-dependent activation. (A) Med25 promoted HNF4α-dependent CYP2C9 promoter activation. HepG2 cells were cotransfected with luciferase promoter constructs, Med25, and nuclear receptors (CAR and HNF4α individually and in combination). Med25 stimulates transcriptional activation by HNF4α but not CAR. Med25 enhances HNF4α-dependent CYP2C9 promoter activity by 3-fold and increases the synergistic activation of CYP2C9 by CAR and HNF4α in the presence or absence of the CAR ligand CITCO. Data represent means ± SE (n = 3). *, statistically significant increase between groups indicated by bars (P < 0.05). (B) Med25 enhances HNF4α-induced CYP2C9 mRNA expression in HepG2 cells as measured by qPCR. CYP2C9 mRNA is induced by coexpression of Med25 with nuclear receptors (HNF4α alone or in combination with CAR). Data represent means ± SE (n = 3). *, statistically significant increase between groups indicated by bars (P < 0.05). (C) Med25 stimulates HNF4α-dependent CYP3A4 promoter activation. HepG2 cells were transfected with a CYP3A4 luciferase promoter construct along with Med25 and nuclear receptors CAR and HNF4α. Med25 specifically promotes HNF4α-dependent but not CAR-dependent CYP3A4 activation. The synergistic activation of CYP3A4 by CAR and HNF4α is also enhanced by Med25. Data represent means ± SE (n = 3). *, statistically significant increase between groups indicated by bars (P < 0.05). (D) Med25 enhances HNF4α-induced CYP3A4 gene expression in HepG2 cells. CYP3A4 mRNA is induced by expression of HNF4α individually or in combination with CAR. This induction is enhanced by coexpression of Med25. Data represent means ± SE (n = 3). *, statistically significant increase between groups indicated by bars (P < 0.05). (E) Mutating the HNF4α binding sites in the CYP2C9 promoter abrogates activation by HNF4α and Med25. The three known proximal HNF4α response elements in the CYP2C9 promoter were mutated as previously described (44). HepG2 cells were transfected with the wild-type CYP2C9 promoter. Expression of HNF4α significantly transactivates the wild-type CYP2C9 promoter (*, P < 0.05), and cotransfection of Med25 further enhances this transactivation (†, P < 0.05). Conversely, there was no statistically significant effect of coexpression of HNF4α and Med25 on the mutant promoter. #, the level of transactivation of the mutated promoter was significantly lower than that of the wild-type promoter (P < 0.05). Data represent means ± SE, n = 3. (F) Silencing Med25 affects CYP2C9 promoter activation. HepG2 cells were transfected with a CYP2C9 luciferase promoter construct along with nuclear receptors (CAR and HNF4α). Med25 was silenced using AdshMed25. Silencing Med25 had no effect on CAR-mediated CYP2C9 activation but significantly reduced HNF4α-dependent activation. The synergistic activation of CYP2C9 was also significantly reduced proportional to the HNF4α-mediated activation. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (G) Silencing Med25 decreases CYP2C9 mRNA induction. HepG2 cells were infected with adenovirus containing CAR and HNF4α individually and in combination for induction of CYP2C9 mRNA. Silencing Med25 levels does not alter induction by CAR but alters induction by HNF4α and dramatically reduces the synergistic induction of CYP2C9 by CAR and HNF4α. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (H) Silencing Med25 decreases CYP3A4 promoter activation by HNF4α. Reduction of Med25 had no effect on CAR-mediated CYP3A4 activation but significantly reduces HNF4α-dependent activation and the synergistic activation of CYP3A4 by HNF4α and CAR. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (I) Silencing Med25 decreases CYP3A4 mRNA induction by HNF4α. Silencing Med25 had no effect on CYP3A4 mRNA induction by CAR but decreases induction by HNF4α and the synergistic induction by CAR and HNF4α. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (J) Reduction of Med25 in human primary hepatocytes reduces HNF4α-dependent CYP2C9 promoter activation as well as the synergistic activation by HNF4α and CAR. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (K) Silencing Med25 in human primary hepatocytes reduces CYP2C9 mRNA induction by HNF4α (individually and in combination with CAR) in the presence or absence of the CAR ligand CITCO. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (L) Silencing of Med25 in primary human hepatocytes also decreases CYP3A4 mRNA induction by HNF4α alone or in combination with CAR in the presence or absence of the CAR ligand CITCO. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05).
To confirm the activation studies at the gene expression level, we infected HepG2 cells with adenovirus coding for the LacZ, Med25, CAR, and HNF4α proteins as shown and then analyzed the mRNA levels of CYP2C9 and CYP3A4 (Fig. 2B and D). Consistent with the luciferase assay results, CAR and HNF4α modestly induced the CYP2C9 mRNA individually. Med25 alone had no effect on CYP2C9 gene expression, but it enhanced induction by HNF4α. CAR and HNF4α synergistically induced CYP2C9 mRNA, and the induction was increased by the presence of CITCO. Med25 further enhanced this synergistic induction. As shown in Fig. 2D, Med25 alone or in combination with CAR did not induce CYP3A4 mRNA. However, Med25 increased HNF4α-dependent CYP3A4 induction 2-fold and also increased the induction of CYP3A4 mRNA by CAR and HNF4α in combination.
To further confirm that Med25 promotes the expression of CYP2C9 through HNF4α binding on the promoter, we generated mutation constructs in the HNF4α binding sites. HepG2 cells were transfected with either the wild-type promoter construct or a construct containing mutations in the three known HNF4α binding sites, as shown in Fig. 2E. The wild-type CYP2C9 promoter construct was activated significantly by expression of HNF4α, and Med25 clearly enhanced this effect. On the other hand, when the HNF4α response elements in the CYP2C9 promoter construct were mutated, ectopic expression of HNF4α had no statistically significant increase in promoter activity. Moreover, the ectopic coexpression of Med25 and HNF4α failed to activate the mutated promoter, showing that the HNF4α response elements are necessary for the effect of Med25. Consistent with these observations, the wild-type CYP2C9 promoter was not activated by an HNF4α mutant lacking the AF2 domain, nor was HNF4α activation increased by coexpression with a Med25 construct containing a mutation in the LXXLL domain (see Fig. S3A and B at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). These observations demonstrate that Med25 modulates the expression of xenobiotic gene expression through interaction with a functional HNF4α protein.
To further examine the regulatory role of Med25 in HNF4α signaling, we developed adenovirus shRNA constructs to specifically knock down Med25. Out of the three constructs screened, short hairpin Med25 (shMed25) construct II reduced Med25 mRNA expression by more than 60% in HepG2 cells (see Fig. S1A at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). Reduction of Med25 levels with adenovirus expressing shRNA (AdshRNA) did not affect the endogenous levels of HNF4α protein (see Fig. S1B, panel 2, at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf); unfortunately, no antibody which will detect endogenous Med25 protein by immunoblotting is available; only ectopic expression of Med25 could be detected by the Flag antibody (see Fig. S1B, panel 1, at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). As shown in Fig. 2F, silencing Med25 did not affect CYP2C9 promoter activity or its activation by CAR. However, it greatly diminished the transactivation of the CYP2C9 promoter by HNF4α and the synergistic activation by CAR and HNF4α in the presence or absence of CITCO. These results confirm that Med25 is a critical component required for HNF4α-dependent CYP2C9 transactivation. Consistent with these observations, when HepG2 cells were infected with (Ad) CAR and/or (Ad) HNF4α, silencing Med25 diminished HNF4α-dependent CYP2C9 mRNA induction and abolished the synergistic induction seen with CAR and HNF4α, while it did not affect CAR-mediated CYP2C9 mRNA induction (Fig. 2G).
Silencing Med25 also affected the activation of the CYP3A4 promoter construct (Fig. 2H). CAR and HNF4α moderately transactivated CYP3A4 in the presence and absence of the ligand CITCO. Silencing Med25 had no effect on CAR-mediated transactivation, while HNF4α-mediated transactivation was diminished. Silencing Med25 also abolished the synergistic transactivation seen with coexpression of CAR-HNF4α. These luciferase assay results are consistent with CYP3A4 mRNA induction studies (Fig. 2I), i.e., silencing Med25 affected induction by HNF4α and the synergistic induction by CAR-HNF4α but had no effect on induction by CAR.
To extend our observations beyond liver cell lines, we transfected primary human hepatocytes with the CYP2C9 promoter construct with AdCAR or HNF4α, individually or in combination (Fig. 2J). CYP2C9 promoter activity was increased by CITCO. Ectopic expression of CAR slightly enhanced this activation. HNF4α moderately transactivated CYP2C9 promoter activity, and the addition of the CAR ligand CITCO enhanced this activation severalfold. This effect of CITCO reflects the translocation of CAR to the nucleus by CITCO (34) and a subsequent synergistic activation between CAR and HNF4α. Silencing Med25 downregulates the transcriptional activation by HNF4α as seen with HepG2 cells. Ectopic coexpression of CAR and HNF4α synergistically activates promoter activity, which is enhanced by CITCO. Even this ectopic nuclear receptor transcriptional activation is abolished by silencing Med25, suggesting that Med25 has a vital status in the formation of the transcriptional complex responsible for promoter activation.
To determine whether this promoter activation is translated into gene expression, primary hepatocytes were infected with Ad scrambled, CAR, HNF4α, and shMed25 (Fig. 2K), and CYP2C9 mRNA levels were quantified with qPCR. CYP2C9 mRNA was induced when cells were treated with CAR and HNF4α alone or in combination. Treatment with CITCO enhanced induction of CYP2C9 mRNA by HNF4α. Coexpression of CAR and HNF4α further induced CYP2C9 mRNA; ligand produced no further increase. Silencing Med25 downregulated CYP2C9 mRNA induction by HNF4α as well as the CAR-HNF4α synergistic induction. The induction of CYP3A4 mRNA mirrored that of CYP2C9, although the magnitude of the induction differed (Fig. 2L). To conclude, Med25 plays an important role in the regulation of CYP2C9 and CYP3A4 in primary human hepatocytes as well as HepG2 cells, and the reduction of Med25 levels greatly diminishes this regulation.
Med25 is critical for HNF4α-mediated active transcriptional complex formation.
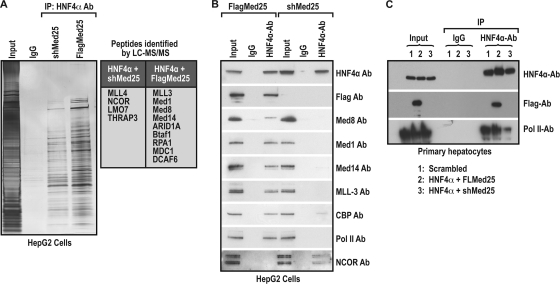
To investigate the molecular mechanisms involved with Med25 in HNF4α-dependent CYP gene regulation, we sought to identify the differential binding of nuclear factors to HNF4α when Med25 was present or diminished by silencing. HepG2 cells were infected with HNF4α-Med25 (AdHNF4α and AdFlag-Med25) or HNF4α-shMed25 (AdHNF4α and AdshMed25), and nuclear extracts were prepared. These nuclear extracts were immunoprecipitated with anti-HNF4α antibody or IgG controls, and the bound proteins were eluted, separated on a 4 to 20% SDS-PAGE gel, and visualized by silver staining as shown in Fig. 3A. Mass spectral analysis of excised gel slices by LC-MS-MS showed that the pattern of transcriptionally important proteins bound to HNF4α when Med25 was present differed from that found when Med25 was silenced (Fig. 3A, right panel). The complete list of proteins found in both lanes is given in Table S-I at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf. Notably, important proteins that were bound to HNF4α when Med25 was reduced belonged principally to the transcriptional repression complex anchored by NCOR and MLL4. On the other hand, Med25 expression transformed the HNF4α complex into a transcriptional activation complex that consists of Med1, Med14, Med8, MLL3, and RNA polymerase II subunits. This suggests that Med25 may play an important role in the association of HNF4α with the Mediator complex proteins, several other cofactors, and RNA Pol II. To date and to our knowledge, this is the first instance where a single cofactor association has altered the nature of the transcriptional complex of a nuclear receptor.
FIG. 3.
Silencing Med25 alters the HNF4α binding complex. (A) Med25 is critical for the HNF4α binding complex. HepG2 cells were infected with adenoviral vectors expressing the indicated proteins either to increase Med25 (HNF4α-Flag-MED25) or to reduce Med25 (HNF4α-shMed25). Nuclear extracts were immunoprecipitated with IgG and HNF4α antibodies, and the bound proteins were visualized by silver staining. The lanes were excised and subjected to LC-MS-MS identification. Transcriptionally important proteins identified by LC-MS-MS are shown in the table. (B) Immunoblotting of HNF4α-bound proteins. The above-mentioned nuclear extracts were subjected to immunoprecipitation with HNF4α and IgG antibodies and probed with the indicated antibodies. The nuclear repressor complex anchored by NCOR is present when Med25 is silenced, while Mediator components, CBP, MLL-3, and RNA polymerase II are identified when Med25 is coexpressed with HNF4α. (C) Med25 is critical for Pol II association with HNF4α primary human hepatocytes. Nuclear extracts from primary hepatocytes infected with adenoviral constructs expressing the indicated protein scrambled shRNA (lane 1), HNF4α-Flag-Med25 (lane 2), and HNF4α-shMed25 (lane 3) were immunoprecipitated with IgG and HNF4α antibodies (input shown on the left). The precipitates were probed with the indicated antibodies. RNA polymerase II is found in association with HNF4α in the presence of Med25 and greatly diminished when Med25 is silenced.
To confirm the mass spectral data, we immunoblotted the HNF4α-bound eluates from nuclear extracts in which Med25 was ectopically expressed or silenced (Fig. 3B). IgG was used as the control. HNF4α was pulled down with HNF4α antibody from the nuclear extracts of HNF4α-Med25 and HNF4α-shMed25. When the immunoprecipitates were probed with anti-Flag antibody, Med25 was detected only in the HNF4α-Med25 pulldowns, as expected. But when the immunoprecipitates were probed with antibodies for Med8, Med14, MLL3, and RNA polymerase II, these proteins were detected only when the immunoprecipitates were from nuclear extracts of cells infected with HNF4α-Med25 and not from those infected with HNF4α-shMed25. Conversely, NCOR was detected only in the eluates from immunoprecipitates from cells infected with HNF4α-shMed25, not in those from cells infected with HNF4α-Med25.
To further extend our observations to primary hepatocytes, these cells were infected with scrambled, Flag-Med25, and shMed25 adenovirus and immunoprecipitated with IgG and anti-HNF4α antibodies as described above. As shown in Fig. 3C, when the eluates were probed with anti-HNF4α antibodies, HNF4α was immunoprecipitated by the HNF4α antibody but not by IgG. As expected, Flag was detected only in the immunoprecipitates where Flag-Med25 was ectopically expressed, not in those where Med25 was silenced. Importantly, when the immunoprecipitates were probed with antibody to Pol II, RNA Pol II was detected in association with HNF4α when Med25 was ectopically expressed, and its association with HNF4α was highly reduced when Med25 was silenced in primary hepatocytes (Fig. 3C). These proteins were not detected in the IgG controls. Our results suggest that Med25 is critical in controlling the association of the RNA polymerase II complex with the HNF4α binding complex, irrespective of the cell line, suggesting that this phenomenon is specific to HNF4α.
Med25 is required for the recruitment of RNA Pol II to the promoter.
As described above, we demonstrated with immunoprecipitation that Flag-Med25 associates with HNF4α and RNA Pol II. In order to demonstrate that this interaction occurs on the promoter, we first employed a modified electromobility supershift assay. Primary human hepatocytes were infected with adenovirus expressing or silencing the indicated proteins. As shown in Fig. 4A, immunoblots of the nuclear extracts confirmed the relative expression levels of HNF4α, Flag-Med25, GFP, and RNA Pol II. After ectopic expression, the level of HNF4α was only slightly higher than that observed in the cells infected with the scrambled controls. Reduced levels of Med25 did not change the expression of HNF4α, GFP, or RNA Pol II.
FIG. 4.
Med25 is required for recruitment of Pol II to the CYP2C9 promoter in HepG2 cells and primary human hepatocytes. (A) Relative levels of expression of HNF4α, Flag-Med25, and Pol II in primary human hepatocytes. Hepatocytes were infected with adenoviruses containing scrambled shRNA, HNF4α, Flag-Med25, and shMed25 as indicated. The expression levels of the indicated proteins were detected by immunoblot analysis. (B to E) Electrophoretic mobility shift analysis (EMSA) of HNF4α binding to the bp −150 HNF4α response element of the CYP2C9 promoter in primary human hepatocytes. (B) The indicated nuclear extracts were incubated with a 32P-labeled oligonucleotide containing the proximal HNF4α response element (at bp −150) of the CYP2C9 gene in the presence of rabbit IgG. The mixture was separated on a 5% PAGE gel using 0.25× Tris-borate-EDTA (TBE). (C to E) The indicated nuclear extracts were incubated with the HNF4α binding site as before in the presence of antibody to HNF4α (C), antibody to Flag (D), or antibody to RNA Pol II (E). (F) ChIP analysis of HNF4α, Med25, and Pol II on the proximal HNF4α response elements of the CYP2C9 promoter in HepG2 cells. ChIPs with IgG as a control, HNF4α, Flag, and Pol II antibodies show the accumulation of the indicated proteins on the HNF4α binding sites when infected with adenoviruses containing LacZ, HNF4, Flag-Med25, HNF4α-Flag-Med25, HNF4α-shMed25, or HNF4α-shMed25mut as shown. Silencing Med25 does not prevent recruitment of HNF4α protein to the HNF4α site but prevents recruitment of RNA polymerase II to this site. An ineffective shRNA construct (shMed25mut) that fails to reduce the expression of Med25 does not prevent recruitment of RNA polymerase II to the HNF4α binding site. Flag antibody detects the accumulation of Med25 to this site when Flag-Med25 and HNF4α are ectopically expressed. (G) Accumulation of HNF4α, Med25, and RNA Pol II on the CYP2C9 promoter in primary human hepatocytes. Chromatin extracts from primary human hepatocytes infected with adenovirus expressing either LacZ, HNF4α, Med25, HNF4α-Med25, or HNF4α-shMed25 were subjected to ChIP analysis with anti-HNF4α (a), Flag (b), and Pol II (c) antibodies and IgG as a control. The immunoprecipitates were analyzed by qPCR with primers flanking the HNF4α binding region in the promoter of CYP2C9. Data shown are representative of three independent experiments. *, significant enrichment of HNF4α on the CYP2C9 promoter indicated by bars (P < 0.05); #, enrichment of HNF4α on the CYP2C9 promoter is significantly decreased when Med25 is silenced (HNF4α versus HNF4α-shMed25; P < 0.05). (H) Accumulation of HNF4α, Med25, and RNA Pol II on an HNF4α site at kb −1.9 of the CYP3A4 promoter in primary human hepatocytes. Chromatin extracts from primary human hepatocytes infected with adenovirus expressing either LacZ, HNF4α, Med25, HNF4α-Med25, or HNF4α-shMed25 were subjected to ChIP analysis with anti-HNF4α (a), Flag (b), and Pol II (c) antibodies and IgG as control. The immunoprecipitates processed as before were analyzed by qPCR with primers flanking the HNF4α binding region (1.9 kb) in the promoter of CYP3A4. Data shown are representative of three independent experiments. *, significant enrichment of HNF4α on the CYP3A4 promoter indicated by bars (P < 0.05); #, enrichment of HNF4α is significantly decreased when Med25 is silenced (HNF4α versus HNF4α-shMed25; P < 0.05).
Nuclear extracts were then incubated with radiolabeled double-stranded oligonucleotides spanning the bp −150 HNF4α response element of CYP2C9 in the presence of IgG as the control. HNF4α from the nuclear extracts bound to the probe and retarded the mobility of the complex in the gel compared to the level for the free probe. When HNF4α was ectopically coexpressed with Flag-Med25, the mobility of the complex was slower than that observed for HNF4α alone (Fig. 4B). The same nuclear extracts were incubated with the anti-HNF4α antibodies in the presence of labeled oligonucleotide probe (Fig. 4C). HNF4α antibody supershifted the probe compared to IgG in the samples in which HNF4α was expressed, i.e., scrambled, HNF4α, and HNF4α-shMed25. The complexes seen with Flag-Med25 and HNF4α-Flag-Med25 were shifted higher than those seen with HNF4α alone due to their direct interaction. When nuclear extracts were incubated with anti-Flag antibody as shown in Fig. 4D, those containing Flag-Med25 and HNF4α-Flag-Med25 were supershifted compared to the complexes from scrambled shRNA, HNF4α, and HNF4α-shMed25 nuclear extracts. Interestingly, antibody to RNA Pol II (interacting with the endogenous protein) supershifted the labeled probe from the nuclear extracts of scrambled and HNF4α-treated primary hepatocytes as well as those from Flag-Med25 and HNF4α-Flag-Med25 hepatocytes. However, when the level of Med25 was reduced (HNF4α-shMed25) in the nuclear extract, the RNA Pol II antibody did not supershift the complex (Fig. 4E), clearly proving that RNA Pol II does not directly interact with HNF4α. Rather, its presence in the HNF4α binding complex is due to its interaction with Med25, which interacts directly with HNF4α. Med25 is thus responsible for the recruitment or association of RNA Pol II to the HNF4α binding site in vitro or in vivo. The interaction of HNF4α-Med25 and its association with RNA Pol II in co-IP and EMSA suggests that Med25 may facilitate the recruitment of Pol II on the CYP2C9 promoter in vivo.
We further analyzed the accumulation of HNF4α and Med25 on the HNF4α response element using chromatin immunoprecipitation assays performed with HepG2 cells in a steady state. Although some HNF4α is expressed endogenously in HepG2 cells, the stringency of our pulldown and washing conditions was such that only ectopically expressed HNF4α was detected on the HNF4 binding site of CYP2C9 compared to the levels for the LacZ controls (Fig. 4F). Flag-Med25 was also recruited to the HNF4α binding site when Flag-Med25 was coexpressed with HNF4α. Interestingly, a robust recruitment of Pol II was shown on the HNF4α binding site when HNF4α and Flag-Med25 were coexpressed. Silencing Med25 prevented the recruitment of RNA Pol II on the promoter. Importantly, a nonfunctional shRNA (shMed25 mut) against Med25 did not affect recruitment of HNF4α or RNA Pol II to the binding site on the CYP2C9 promoter, verifying that the association of RNA Pol II to the HNF4α binding site on the CYP2C9 promoter is dependent on Med25 expression.
To demonstrate the recruitment of HNF4α, Med25, and RNA Pol II on the CYP2C9 promoter in chromatin of primary human hepatocytes, we used quantitative ChIP assays. We infected hepatocytes with adenoviruses expressing LacZ, HNF4α, Flag-Med25, HNF4α-Med25, or HNF4α-shMed25. Chromatin extracts immunoprecipitated with the indicated antibodies were quantified with PCR using SYBR green as shown in Fig. 4G. HNF4α was recruited to its binding site in all the samples, including endogenous levels as indicated by the LacZ controls. Its recruitment level was slightly higher when HNF4α was ectopically expressed. Flag antibody exclusively pulled down the HNF4α binding site when Flag-Med25 was expressed ectopically. RNA Pol II was recruited to the HNF4α binding site when Med25 was endogenously present or ectopically expressed; however, its recruitment was significantly diminished when Med25 was silenced. A similar recruitment pattern for HNF4α, Med25, and RNA Pol II was also observed at an HNF4α site at position −1925 of the CYP3A4 promoter (Fig. 4H). Taken together, our results show that RNA Pol II recruitment to HNF4α sites of the promoter region of CYP2C9 or CYP3A4 is dependent on the association of Med25 with HNF4α.
Downregulation of Med25 impairs a specific set of HNF4α target genes, the cytochrome P-450s, in primary human hepatocytes.
To elucidate the regulatory role of Med25 in HNF4α signaling in the liver, we infected primary human hepatocytes (Fig. 5A to G) and HepG2 cells (see Fig. S4A to F at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf) with adenoviruses for the ectopic expression of CAR and HNF4α and simultaneous knockdown of Med25; we then treated the cells with the CAR ligand CITCO. As shown in Fig. 5, CYP2C9, CYP2B6, CYP1A2, and CYP2D6 mRNAs were upregulated by HNF4α in primary hepatocytes and silencing Med25 decreased this upregulation. CYP2C9 and CYP2B6 were further induced by coexpression of CAR, and CYP2C9 induction was increased by the presence of CITCO. Furthermore, when Med25 was silenced, the induction of the CYP2C9 and CYP2B6 genes by HNF4α or HNF4α plus CAR was significantly decreased. In contrast, HNF4α target genes involved in glucose homeostasis (glucose-6-phosphatase [G6PC]), phosphoenolpyruvate carboxykinase 1 (PCK1), and fatty acid binding protein (FABP4; ap2) were not affected by silencing the Med25, as shown in Fig. 5E to G. Similar results were seen in HepG2 cells, as shown at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf.
FIG. 5.
Med25 regulates expression of specific xenobiotic metabolizing enzymes in primary human hepatocytes. Primary hepatocytes were infected with adenovirus coding for ectopic expression of CAR, HNF4α, and Med25, and expression was reduced with adenoviral shRNA. (A) Silencing Med25 downregulates the induction of CYP2C9 mRNA by HNF4α or HNF4-CAR in primary hepatocytes. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (B) The induction of CYP2B6 mRNA by HNF4 or HNF4α and CAR is downregulated by silencing Med25. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (C) Silencing Med25 inhibits CYP1A2 mRNA induction by HNF4α. CYP1A2 is induced by HNF4α but is not activated by CAR. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). (D) Silencing Med25 affects CYP2D6 mRNA induction by HNF4α. The upregulation of CYP2D6 by HNF4α is reduced by silencing Med25. Data represent means ± SE (n = 3). #, significant decrease between groups indicated by bars (P < 0.05). The classical targets of HNF4α which are involved in glucose homeostasis and fatty acid binding are not affected by the loss of function of Med25. (E) G6PC, a direct HNF4α target in human liver, is not affected by silencing Med25. Data represent means ± SE (n = 3). #, shMed25 modestly but significantly reduces HNF4α induction (P < 0.05). (F) PCK1 is also a direct target of HNF4α in human liver but is not affected by silencing Med25. (G) FABP4 (ap2) is not induced by the expression of HNF4α in primary hepatocytes and is not affected by the loss of Med25 function. CAR downregulates FABP4 gene expression.
Overall, these results suggest that the association of Med25 does not affect all HNF4α targets but that a specific subset of target genes is upregulated by ectopic expression of Med25 and downregulated by silencing Med25. To our knowledge, this is the first report of a cofactor that specifically regulates the expression of a subset of genes, in this case the cytochrome P-450s.
Reduction of Med25 alters specific HNF4α-signaling pathways.
HNF4α is an important regulator of various metabolic parameters in human liver. We have shown herein that Med25 has a vital link as a coactivator of HNF4α. To examine the functions of Med25 in various gene partnerships, we reduced the expression of Med25 in primary human hepatocytes by adenoviral shRNA targeted against Med25. For comparison, we reduced HNF4α by adenoviral shRNA and ectopically overexpressed HNF4α. Reduction of endogenous Med25 had no effect on primary hepatocyte viability or the expression of HNF4α mRNA (see Fig. S5 at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). Microarray analysis and ingenuity pathway analysis (IPA) identified two main pathways that were affected by the reduction of endogenous Med25: (i) the xenobiotic signaling pathway, affecting drug metabolism (Fig. 6A) (P = 3.43 × 10−4), and (ii) the fatty acid oxidation pathway, affecting lipid metabolism (see Fig. S6A at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf) (P = 4.25 × 10−6). Since HNF4α target genes are found under a variety of pathophysiological conditions, we focused on all the CYP genes, the majority of which are regulated by HNF4α and involved in xenobiotic metabolism. Reduced levels of Med25 were found to downregulate 37 of the total 52 CYP genes, including ∼CYP2C9, CYP3A4, and CYP2B6, while 15 genes were found to be upregulated by the reduced expression of Med25 (Fig. 6B; see also Table S-II at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). In comparison, the majority of the CYP genes that were downregulated by silencing Med25 in primary hepatocytes were also upregulated ectopic expression of HNF4α. Ingenuity pathway analysis showed that the HNF4α-driven network involving cellular assembly, organization, function maintenance, and transport is the topmost network affected by the reduced levels of Med25 (Fig. 6C).
FIG. 6.
Downregulation of Med25 impairs a specific set of HNF4α target genes in human primary hepatocytes. (A) Microarray analysis of CYP genes from scrambled-virus (control) and Med25 knockdown in primary human hepatocytes. The log ratios of shMed25/scrambled, shHNF4α/scrambled, and HNF4α/scrambled are presented in the heat map. (B) Venn diagram representation of the subset of CYP genes involved in drug metabolism that were reduced in the primary human hepatocytes treated with shMed25. (C) Schematic diagram of the HNF4α network that was significantly affected by the reduced levels of Med25 in primary human hepatocytes as identified in IPA analysis. Green denotes downregulated genes, and pink and red denote upregulated genes. (D to K) qPCR analysis of mRNA of selected genes from fatty acid oxidation pathways from total RNA of primary human hepatocytes infected with the indicated adenovirus. Data represent means ± SE (n = 3). #, significantly decreased mRNA expression compared to the level for the control (scrambled shRNA) (P < 0.05); †, significantly decreased mRNA expression in HNF4α-shMed25 samples compared to the level for HNF4α (P < 0.05); *, shMed25 significantly increased mRNA expression compared to the level for the control (scrambled) (P < 0.05); ‡, the level of mRNA expression was significantly higher in cells infected with HNF4α-shMed25 than in those expressing ectopic HNF4α alone (P < 0.05).
IPA analysis identified PPARα signaling as one of the major pathways affected by the reduced levels of Med25. Genes involved in fatty acid metabolism (such as those involved in oxidation, ketone body synthesis, fatty acid binding, transport, and activation) were analyzed; microarray analysis revealed that 17 of these genes were downregulated by reduced expression of Med25. One of the noted genes, the PPARα gene, was downregulated, which drives fatty acid metabolism (see Fig. S6A and B and Table S-III at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). qPCR confirmed the decreased expression of PPARα and several of its target genes, including those encoding acyl-CoA carboxylase (Acox), liver bifunctional enzyme (Ehhadh), medium-chain acyl-CoA dehydrogenase (MCAD), SPOT14, and CYP4AF2, while CYP4A11 and the fatty acid transporter (CD36) were upregulated by reduced Med25 levels (Fig. 6D to K).
Reduced levels of Med25 impair drug metabolism in primary human hepatocytes.
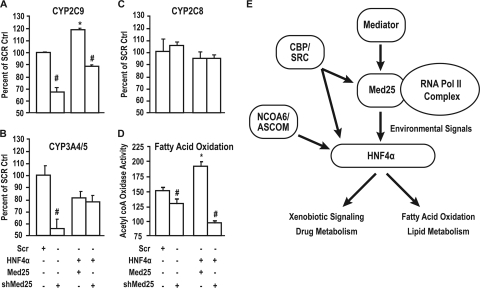
Activation of the xenobiotic sensing nuclear receptors (CAR/PXR) induces drug metabolism in the liver. The regulation of HNF4α by Med25 suggests that reduced availability of Med25 could impair xenobiotic signaling and the metabolism of drugs in vivo. Indeed, in primary human hepatocytes, when Med25 was blunted with shRNA (targeted against Med25) (Fig. 7A), basal levels of CYP2C9-mediated metabolism were decreased by 30% compared to the level for the control (primary human hepatocytes infected with LacZ), and CYP3A4/5 metabolism was reduced by 50%. Metabolism of a CYP2C8 substrate, on the other hand, was not affected by reduced availability of Med25. When HNF4α levels were elevated by ectopic expression of HNF4α and Med25 expression was reduced by shRNA, CYP2C9-mediated metabolism was reduced by 30% compared to the level for hepatocytes with only elevated HNF4α. Metabolism of a CYP2C8-specific substrate remained unchanged. Metabolism of testosterone, a substrate for the CYP3A4/CYP3A5 enzymes, was surprisingly decreased by ectopic expression of HNF4α and unchanged by simultaneously silencing Med25. However, testosterone is a shared substrate for the two enzymes, which complicates the interpretation of this data. Our data suggest that CYP2C9 expression and substrate metabolism are tightly regulated by Med25 and provide evidence for the involvement of Med25 in CYP3A4 expression. CYP2C8, on the other hand, is regulated by HNF4α, but Med25 appears to have no role in the expression and substrate metabolism of CYP2C8.
FIG. 7.
Reduction of Med25 in primary human hepatocytes alters drug metabolism. (A to C) Regulation of the metabolism of diclofenac (CYP2C9); paclitaxel (CYP2C8), and testosterone (CYP3A4/5) in microsomes from primary human hepatocytes treated with the indicated adenoviruses. Data represent means ± SE (n = 3). *, silencing Med25 significantly increased enzymatic activity compared to the level for the control (scrambled) (P < 0.05); #, significantly decreased enzymatic activity compared to the level for the control (scrambled) (P < 0.05). (D) Reduction in Med25 levels affects fatty acid oxidation. The oxidation rate of palmitic acid in primary human hepatocytes was reduced significantly when Med25 levels were reduced compared to the levels for primary human hepatocytes treated with scrambled virus. Data represent means ± SE (n = 3). *, significantly increased enzymatic activity compared to the level for the control (scrambled) (P < 0.05); #, significantly decreased enzymatic activity compared to the level for the control (scrambled) (P < 0.05). (E) Schematic model of the Med25 regulation of HNF4α target genes in drug and lipid metabolism and their associated subcomplexes, which interact directly with either the promoter or Med25 to execute the finely tuned gene regulation on specific environmental clues.
DISCUSSION
The liver is the primary organ that responds to a variety of stimuli to regulate drug and lipid metabolism, including detoxification, storage, transport, and elimination (46). Hepatocyte nuclear factor 4α is implicated in regulating the genes involved in metabolic processes (22). Dysfunctional signaling in the HNF4α network is linked to several metabolic disorders, including maturity-onset diabetes of the young (MODY) (20), diabetes, and atherosclerosis, and to altered drug metabolism and clearance (6, 13, 16). Several coactivators belonging to different subcomplexes have been discovered and implicated in gene regulation controlling the various metabolic processes, either directly interacting with HNF4α (47) or interacting in tandem with other nuclear receptors (CAR, GR, and C/EBP) (40, 59, 60). While many of these cofactors were found to bind directly or indirectly to nuclear receptors to form the transcriptional complex, how they are targeted to specific loci on chromatin to give the regio- and genospecificity remains poorly understood. In the present study, we have identified Med25 as an interacting partner of HNF4α that transforms the inactive transcriptional complex to an active transcriptional complex and uncovered a role for Med25 in drug and lipid metabolism in the human liver. Med25 is variably associated with the Mediator complex (36, 50). Ectopic expression of HNF4α and Med25 stimulates many drug metabolism pathways, while the reduction of Med25 decreases them, clearly linking Med25 to the HNF4α-signaling network.
Mediators are a complex of coactivators anchored by Med1 (28) that are able to synchronize gene expression to a variety of environmental signals regulating diverse biological processes (55). However, evidence suggests that not all NR-mediated transcriptional activity is dependent on Med1 (35), and transcriptional complexes could be formed in the absence of Med1 (18, 32). In our quest to identify other cofactors that regulate HNF4α signaling, we have identified Med25. Interestingly, although many cofactors bind with all nuclear receptors, Med25 is an exception since it fails to bind with two xenobiotic sensing receptors, CAR and PXR, or the vitamin D receptor (32).
Several known coactivators have been identified in the immunoprecipitates of HNF4α belonging to the CBP-HAT complex (CBP and SRC-1), the Mediator complex (Med8), and the ASCOM complex (NCOA6), in addition to PGC-1α and RNA Pol II. We have now identified Flag-Med25 and Med8 as additional coactivators. Interestingly, most of these coactivators were found to bind directly with Med25 in GST pulldown in vitro interaction assays, but the sites of interaction on Med25 are quite different. HNF4α binds to Med25 through the LXXLL motif, whereas chromatin modifiers such as CBP interact with the middle of the Med25-spanning PTOV domain. PGC-1α interacts with the C-terminal region but not through the LXXLL motif. Med1, SRC-1, and GRIP interact in the N-terminal region (see Fig. S2C, diagram at bottom, at http://www.niehs.nih.gov/research/atniehs/labs/ltp/human/docs/med25supplemental-data.pdf). Although NCOA6 was identified in the HNF4α complex, it does not bind with Med25 directly. HNF4α and the majority of these coactivators colocalize with Med25 in the nucleus, whereas NCOA6 does not colocalize, suggesting that although these two proteins belong to the HNF4α-bound complex, they may exist as separate subcomplexes or be recruited in a sequential order.
Med25 enhances the transactivation potential of HNF4α. However, the synergistic activation of two target genes, CYP2C9 and CYP3A4, by CAR and HNF4α is further boosted by expression of Med25. The CAR ligand CITCO enhanced the synergistic transcriptional effect, suggesting that Med25 might be the final piece of the puzzle explaining the transcriptional activation of these target genes by xenobiotics.
The effect of Med25 appears to be solely mediated through a functional HNF4α protein, and reduction of Med25 levels inhibited the HNF4α-dependent activation of CYP2C9 but not CAR-mediated transcriptional activity. The synergistic activity of CAR and HNF4α was also blunted when endogenous Med25 levels were reduced, as seen from CYP2C9 and CYP3A4 promoter activation assays and mRNA induction assays. Our studies show that Med25 not only is essential for mediating HNF4α-dependent upregulation of target genes but also dramatically influences CAR-dependent upregulation through HNF4α (Fig. 2). These transcriptional effects were seen in HepG2 cells and in primary hepatocytes, suggesting that the Med25 effects are not cell line specific and are thus applicable to human liver drug metabolism.
After establishing Med25 as a coactivator of HNF4α, we explored the purpose of Med25 in the transcriptional complex. On immunoprecipitation with HNF4α, nuclear extracts depleted of Med25 bound a different transcriptional complex than when Med25 was ectopically expressed. Mass spectral identification of all the proteins indicated that Med25 depletion resulted in a transcriptional complex, as indicated by the presence of NCOR and MLL4, whereas in the presence of Med25, we observed a transcriptional complex with coactivators such as Med1, Med8, Med14, MLL3, and RPA-1. We suggest that Med25 is essential for converting the NCOR-bound HNF4α complex (inactive) to the Mediator-bound HNF4α transcriptional (active) complex. At the molecular level, although it has been speculated that the Med1-anchored Mediator complex was required for the recruitment of RNA polymerase II, EMSA and ChIP data from HepG2 cells and primary human hepatocytes clearly showed that for a subset of genes typified by CYP2C9 and CYP3A4, it is Med25 and not a Med1-anchored Mediator complex that is essential for Pol II recruitment to the target promoter. Given the fact that Med25 is not part of the Mediator complex but an associated protein, these data clearly show that Med25 is essential for the recruitment. Moreover, from qPCR analysis we found that when Med25 was depleted, PPARα levels were decreased, whereas when HNF4α levels were increased, PPARα levels increased. In addition, PPARα would also require Med25 for transcription regulation of its target genes, namely, those encoding Acox, Ehhadh, etc. These results suggest that PPARα-mediated fatty acid oxidation may be regulated by Med25, leading to alterations in lipid metabolism, one of the two pathways identified in the IPA analysis. The effects of a reduction in Med25 only partially parallel those of a reduction in HNF4α. Clearly, as the Venn diagram indicates, there is only a partial overlap between the genes that are upregulated by overexpressing HNF4α and those that are downregulated by silencing Med25.
Interestingly, the expression of HNF4α target genes belonging to pathways such as Pck1 and G6PC involved in glucose homeostasis was not affected by reduced availability of Med25. These results suggest that Med25 acts in concert with HNF4α in regulating only genes involved in specific pathways. In the future, it will be of interest to investigate why Med25 regulates only some genes containing HNF4α binding sites in the promoter region. For the genes that were selected for further analysis by qPCR, we note that an HNF4α response element was quite close to the transcription start site, whereas in the nonresponsive genes the binding site was far from the start site. In the future, an unbiased genome-wide bioinformatics approach could help identify the mechanism that determines the selectivity of Med25 in regulating only certain HNF4α-dependent pathways.
In summary, we have shown that Med25 plays an important role in the regulation of drug and lipid metabolism in primary human hepatocytes as well as in human liver cell lines. While there are many known coactivators described for modulating a plethora of signaling pathways, we show that Med25 confers selectivity and plays a vital role in the recruitment of various other cofactors through HNF4α binding sites to mediate the effects of HNF4α on metabolism of drugs and lipids.
Acknowledgments
We thank Anton Jetten and Paul Wade for critical reading of the manuscript. We also thank and acknowledge the expertise and assistance of Jason Williams in the Protein Microcharacterization facility, Kevin Gerrish in the Microarray facility, and Jeff Tucker in the confocal microscopy core facility, NIEHS, and Benjamin Fontenot from CellzDirect/Invitrogen.
This study was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, under NIH intramural project number Z01ES02124.
We declare that we have no conflict of interest.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Barbier, O., et al. 2005. Hepatic expression of the UGT1A9 gene is governed by hepatocyte nuclear factor 4alpha. Mol. Pharmacol. 67:241-249. [DOI] [PubMed] [Google Scholar]
- 2.Benedit, P., et al. 2001. PTOV1, a novel protein overexpressed in prostate cancer containing a new class of protein homology blocks. Oncogene 20:1455-1464. [DOI] [PubMed] [Google Scholar]
- 3.Bourbon, H. M., et al. 2004. A unified nomenclature for protein subunits of Mediator complexes linking transcriptional regulators to RNA polymerase II. Mol. Cell 14:553-557. [DOI] [PubMed] [Google Scholar]
- 4.Browning, J. D., and J. D. Horton. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, W., C. A. Smith, A. W. McLaren, and C. R. Wolf. 1996. Characterization of the human cytochrome P4502D6 promoter. A potential role for antagonistic interactions between members of the nuclear receptor family. J. Biol. Chem. 271:25269-25276. [DOI] [PubMed] [Google Scholar]
- 6.Chawla, A., J. J. Repa, R. M. Evans, and D. J. Mangelsdorf. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866-1870. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y., and J. A. Goldstein. 2009. The transcriptional regulation of the human CYP2C genes. Curr. Drug Metab. 10:567-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., G. Kissling, M. Negishi, and J. A. Goldstein. 2005. The nuclear receptors constitutive androstane receptor and pregnane X receptor cross-talk with hepatic nuclear factor 4alpha to synergistically activate the human CYP2C9 promoter. J. Pharmacol. Exp. Ther. 314:1125-1133. [DOI] [PubMed] [Google Scholar]
- 9.Choi, D. S., et al. 2007. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J. Proteome Res. 6:4646-4655. [DOI] [PubMed] [Google Scholar]
- 10.Dell, H., and M. Hadzopoulou-Cladaras. 1999. CREB-binding protein is a transcriptional coactivator for hepatocyte nuclear factor-4 and enhances apolipoprotein gene expression. J. Biol. Chem. 274:9013-9021. [DOI] [PubMed] [Google Scholar]
- 11.Dhe-Paganon, S., K. Duda, M. Iwamoto, Y. I. Chi, and S. E. Shoelson. 2002. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J. Biol. Chem. 277:37973-37976. [DOI] [PubMed] [Google Scholar]
- 12.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel, R. H., S. M. Grundy, and P. Z. Zimmet. 2005. The metabolic syndrome. Lancet 365:1415-1428. [DOI] [PubMed] [Google Scholar]
- 14.Flier, J. S. 2004. Obesity wars: molecular progress confronts an expanding epidemic. Cell 116:337-350. [DOI] [PubMed] [Google Scholar]
- 15.Garrus, J. E., et al. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez, F. J. 2008. Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab. Pharmacokinet. 23:2-7. [DOI] [PubMed] [Google Scholar]
- 17.Goo, Y. H., et al. 2003. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol. Cell. Biol. 23:140-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grontved, L., M. S. Madsen, M. Boergesen, R. G. Roeder, and S. Mandrup. 2010. MED14 tethers Mediator to the N-terminal domain of peroxisome proliferator-activated receptor gamma and is required for full transcriptional activity and adipogenesis. Mol. Cell. Biol. 30:2155-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy, S. M., H. B. Brewer, Jr., J. I. Cleeman, S. C. Smith, Jr., and C. Lenfant. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109:433-438. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, R. K., and K. H. Kaestner. 2004. HNF-4alpha: from MODY to late-onset type 2 diabetes. Trends Mol. Med. 10:521-524. [DOI] [PubMed] [Google Scholar]
- 21.Hadzopoulou-Cladaras, M., et al. 1997. Functional domains of the nuclear receptor hepatocyte nuclear factor 4. J. Biol. Chem. 272:539-550. [DOI] [PubMed] [Google Scholar]
- 22.Hayhurst, G. P., Y. H. Lee, G. Lambert, J. M. Ward, and F. J. Gonzalez. 2001. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21:1393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz, R., J. Magenheim, I. Berman, and J. Bar-Tana. 1998. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature 392:512-516. [DOI] [PubMed] [Google Scholar]
- 24.Inoue, K., and M. Negishi. 2009. Early growth response 1 loops the CYP2B6 promoter for synergistic activation by the distal and proximal nuclear receptors CAR and HNF4alpha. FEBS Lett. 583:2126-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue, Y., G. P. Hayhurst, J. Inoue, M. Mori, and F. J. Gonzalez. 2002. Defective ureagenesis in mice carrying a liver-specific disruption of hepatocyte nuclear factor 4alpha (HNF4alpha). HNF4alpha regulates ornithine transcarbamylase in vivo. J. Biol. Chem. 277:25257-25265. [DOI] [PubMed] [Google Scholar]
- 26.Inoue, Y., L. L. Peters, S. H. Yim, J. Inoue, and F. J. Gonzalez. 2006. Role of hepatocyte nuclear factor 4alpha in control of blood coagulation factor gene expression. J. Mol. Med. 84:334-344. [DOI] [PubMed] [Google Scholar]
- 27.Inoue, Y., et al. 2006. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J. Lipid Res. 47:215-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito, M., et al. 1999. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3:361-370. [DOI] [PubMed] [Google Scholar]
- 29.Jover, R., R. Bort, M. J. Gomez-Lechon, and J. V. Castell. 2001. Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. Hepatology 33:668-675. [DOI] [PubMed] [Google Scholar]
- 30.Kamiya, A., Y. Inoue, T. Kodama, and F. J. Gonzalez. 2004. Hepatocyte nuclear factors 1alpha and 4alpha control expression of proline oxidase in adult liver. FEBS Lett. 578:63-68. [DOI] [PubMed] [Google Scholar]
- 31.Kamiyama, Y., et al. 2007. Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab. Pharmacokinet. 22:287-298. [DOI] [PubMed] [Google Scholar]
- 32.Lee, H. K., U. H. Park, E. J. Kim, and S. J. Um. 2007. MED25 is distinct from TRAP220/MED1 in cooperating with CBP for retinoid receptor activation. EMBO J. 26:3545-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, Y., et al. 2002. Polyamines modulate the interaction between nuclear receptors and vitamin D receptor-interacting protein 205. Mol. Endocrinol. 16:1502-1510. [DOI] [PubMed] [Google Scholar]
- 34.Maglich, J. M., et al. 2003. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 278:17277-17283. [DOI] [PubMed] [Google Scholar]
- 35.Malik, S., et al. 2004. Structural and functional organization of TRAP220, the TRAP/Mediator subunit that is targeted by nuclear receptors. Mol. Cell. Biol. 24:8244-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik, S., and R. G. Roeder. 2005. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem. Sci. 30:256-263. [DOI] [PubMed] [Google Scholar]
- 37.Malik, S., and R. G. Roeder. 2003. Isolation and functional characterization of the TRAP/Mediator complex. Methods Enzymol. 364:257-284. [DOI] [PubMed] [Google Scholar]
- 38.Malik, S., A. E. Wallberg, Y. K. Kang, and R. G. Roeder. 2002. TRAP/SMCC/Mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4. Mol. Cell. Biol. 22:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittler, G., et al. 2003. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22:6494-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muangmoonchai, R., et al. 2001. Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem. J. 355:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osmundsen, H., B. Brodal, and R. Hovik. 1989. A luminometric assay for peroxisomal beta-oxidation. Effects of fasting and streptozotocin-diabetes on peroxisomal beta-oxidation. Biochem. J. 260:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitarque, M., C. Rodriguez-Antona, M. Oscarson, and M. Ingelman-Sundberg. 2005. Transcriptional regulation of the human CYP2A6 gene. J. Pharmacol. Exp. Ther. 313:814-822. [DOI] [PubMed] [Google Scholar]
- 43.Rachez, C., et al. 1999. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398:824-828. [DOI] [PubMed] [Google Scholar]
- 44.Rana, R., et al. 2010. Hepatocyte nuclear factor 4{alpha} regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes. Drug Metab. Dispos. 38:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy, J. K., and T. Hashimoto. 2001. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr. 21:193-230. [DOI] [PubMed] [Google Scholar]
- 46.Remmer, H. 1970. The role of the liver in drug metabolism. Am. J. Med. 49:617-629. [DOI] [PubMed] [Google Scholar]
- 47.Rhee, J., et al. 2003. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 100:4012-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuel, V. T., et al. 2004. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279:32345-32353. [DOI] [PubMed] [Google Scholar]
- 49.Sanyal, A. J. 2005. Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2:46-53. [DOI] [PubMed] [Google Scholar]
- 50.Sato, S., et al. 2004. A set of consensus mammalian Mediator subunits identified by multidimensional protein identification technology. Mol. Cell 14:685-691. [DOI] [PubMed] [Google Scholar]
- 51.Schrem, H., J. Klempnauer, and J. Borlak. 2002. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol. Rev. 54:129-158. [DOI] [PubMed] [Google Scholar]
- 52.Sladek, F. M., and J. E. Darnell. 1992. Mechanisms of liver-specific gene expression. Curr. Opin. Genet. Dev. 2:256-259. [DOI] [PubMed] [Google Scholar]
- 53.Sladek, F. M., W. M. Zhong, E. Lai, and J. E. Darnell, Jr. 1990. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 4:2353-2365. [DOI] [PubMed] [Google Scholar]
- 54.Surapureddi, S., R. Rana, J. K. Reddy, and J. A. Goldstein. 2008. Nuclear receptor coactivator 6 mediates the synergistic activation of human cytochrome P-450 2C9 by the constitutive androstane receptor and hepatic nuclear factor-4α. Mol. Pharmacol. 74:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taatjes, D. J. 2010. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem. Sci. 35:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tirona, R. G., et al. 2003. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat. Med. 9:220-224. [DOI] [PubMed] [Google Scholar]
- 57.van den Berghe, G. 1991. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J. Inherit. Metab. Dis. 14:407-420. [DOI] [PubMed] [Google Scholar]
- 58.Wang, C., I. M. McCarty, L. Balazs, Y. Li, and M. S. Steiner. 2002. A prostate-derived cDNA that is mapped to human chromosome 19 encodes a novel protein. Biochem. Biophys. Res. Commun. 296:281-287. [DOI] [PubMed] [Google Scholar]
- 59.Wang, J. C., J. M. Stafford, and D. K. Granner. 1998. SRC-1 and GRIP1 coactivate transcription with hepatocyte nuclear factor 4. J. Biol. Chem. 273:30847-30850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Z., et al. 2006. Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab. 3:111-122. [DOI] [PubMed] [Google Scholar]
- 61.Wisely, G. B., et al. 2002. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure 10:1225-1234. [DOI] [PubMed] [Google Scholar]
- 62.Xue, Y., et al. 1998. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell 2:851-861. [DOI] [PubMed] [Google Scholar]
- 63.Yang, F., R. DeBeaumont, S. Zhou, and A. M. Näär. 2004. The activator-recruited cofactor/Mediator coactivation subunit ARC92 is a functionally important target of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 101:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan, X., et al. 2009. Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One 4:e5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmet, P., K. G. Alberti, and J. Shaw. 2001. Global and societal implications of the diabetes epidemic. Nature 414:782-787. [DOI] [PubMed] [Google Scholar]