Abstract
Gene regulation in response to environmental stress is critical for the survival of all organisms. From Saccharomyces cerevisiae to humans, it has been observed that splicing of mRNA precursors is repressed upon heat shock. However, a mild heat pretreatment often prevents splicing inhibition in response to a subsequent and more severe heat shock, a phenomenon called splicing thermotolerance. We have shown previously that the splicing regulator SRSF10 (formerly SRp38) is specifically dephosphorylated by the phosphatase PP1 in response to heat shock and that dephosphorylated SRSF10 is responsible for splicing repression caused by heat shock. Here we report that a mild heat shock protects SRSF10 from dephosphorylation during a second and more severe heat shock. Furthermore, this “thermotolerance” of SRSF10 phosphorylation, like that of splicing, requires de novo protein synthesis, specifically the synthesis of heat shock proteins. Indeed, overexpression of one of these proteins, Hsp27, inhibits SRSF10 dephosphorylation in response to heat shock and does so by interaction with SRSF10. Our data thus provide evidence that splicing thermotolerance is acquired through maintenance of SRSF10 phosphorylation and that this is mediated at least in part by Hsp27.
Splicing of mRNA precursors, like other steps in the gene expression pathway, is regulated by environmental stress (25). It was observed over 20 years ago that splicing is interrupted by heat shock in Drosophila melanogaster cells (29). The same phenomenon was subsequently reported in organisms from Saccharomyces cerevisiae to humans (2, 28), suggesting that heat shock-induced repression of splicing is highly conserved. The biological significance of this phenomenon appears to be severalfold (16, 31). First, since most metazoan genes contain introns (21), splicing repression can contribute to the general inhibition of gene expression that occurs upon heat shock. Second, in many species, genes encoding heat shock proteins contain few or no introns while other protein-coding genes are intron rich (12). Therefore, heat shock-induced splicing repression results in inhibition of the expression of most genes, while expression of heat shock genes is generally not affected, thereby ensuring the preferential expression of heat shock proteins. Third, splicing repression following heat shock causes accumulation of unspliced RNAs, some of which are exported into the cytoplasm and translated into abnormal proteins (30). These aberrant products may be partially responsible for the detrimental effects of heat shock.
Thermotolerance refers to the phenomenon that a mild heat treatment induces transient resistance to a second and more severe heat shock (5). Thermotolerance can be observed not only at the cell survival level but also at the molecular level. For example, following a mild heat shock, splicing can occur normally in response to a more severe heat shock that would otherwise inhibit it (2, 29). Similar to heat-induced splicing inhibition, splicing thermotolerance is also conserved from yeast to humans (31). Splicing thermotolerance requires de novo protein synthesis, most likely of heat shock proteins (31). However, it remains unclear which specific heat shock protein(s) is involved and how it might function in establishing thermotolerance. In yeast, Hsp104 and Hsp70 contribute to reactivation of splicing after heat inactivation, but overexpression of these proteins does not lead to development of thermotolerance (27). Hsp27 has recently been reported to play a similar role in human cells (19). Thus, the mechanism of splicing thermotolerance remains unclear.
Earlier work from our lab has identified SRSF10 (previously called SRp38; see reference 17) as a factor that mediates global splicing inhibition, both during M phase of the cell cycle and in response to heat shock (24, 26). SRSF10 belongs to the SR family of splicing regulators, all of which share a domain rich in arginine/serine dipeptide repeats called the RS domain as well as one or two RNP-type RNA binding domains (11, 18). The RS domains of these proteins are extensively phosphorylated. Most SR proteins function as essential but redundant general splicing activators in vitro. In contrast, SRSF10 can activate splicing only in a sequence-dependent manner (7). When dephosphorylated, however, SRSF10 is converted into a potent and general splicing repressor. In keeping with these properties, SRSF10 phosphorylation status is tightly regulated (23). Following heat shock, SRSF10 is specifically dephosphorylated by the phosphatase PP1 and is necessary and sufficient for inhibiting splicing under this condition (24).
Given the central role of SRSF10 in heat shock-induced splicing inhibition, we wondered whether SRSF10 might therefore also be involved in development of splicing thermotolerance. To address this, we investigated whether SRSF10 dephosphorylation in response to heat shock is affected by prior heat treatment. Our data show that, like splicing, SRSF10 dephosphorylation acquires thermotolerance. Also like splicing thermotolerance, it requires de novo protein synthesis of heat shock proteins. Finally we provide evidence that thermotolerance of SRSF10 is mediated at least in part by Hsp27, which interacts with SRSF10 and protects it from dephosphorylation by the heat shock-activated phosphatase PP1.
MATERIALS AND METHODS
Constructs and antibodies.
The Hsp27 wild type and mutant mammalian expression constructs were kind gifts of Michael Welsh (University of Michigan). Hsp70 and Hsp90 expression constructs were provided by Ulrich Hartl (Max Planck Institute of Biochemistry). The PP1 expression construct was previously described (23). pSuper-based constructs (3) were used for RNA interference (RNAi) of HSF1, and the sequence CCAAGGAGGTGCTGCCCAA within the HSF1 open reading frame (ORF) was targeted. SRSF10 antibody was described previously (26). Hsp/Hsc70 monoclonal antibody was from StressGen (Michigan). Hsp27 and phospho-Hsp27 (Ser82) antibodies were from Cell Signaling (Massachusetts). HSF1 antibody was from Lab Vision (California).
Cell culture and heat shock assays.
HeLa cells were maintained in Dulbecco modified Eagle medium (DMEM) plus 10% fetal bovine serum. Transfections were carried out using either Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions or the calcium phosphate method. Heat shock was carried out by submerging cell culture plates in a circulating water bath as described previously (24).
Cell fractionation.
Nuclear and cytoplasm extracts were prepared as previously described (3a). In brief, nuclear extracts were prepared from cells cultured in a 6-cm plate carefully resuspended in 250 μl of buffer I (10 mM Tris, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 2 mM dithiothreitol) supplemented with protease inhibitor and phosphatase inhibitor (Sigma, St. Louis, MO) and were incubated on ice for 10 min. Then 0.2% NP-40 was added and cell lysates were centrifuged for 4 min at 400 × g. The supernatant and the pellet were kept for cytoplasmic and nuclear fractions. The supernatant was centrifuged twice for 2 min at 10,000 × g. The pellet was washed another time in 1 ml buffer I and centrifuged for 4 min at 400 × g. The pellet was resuspended in buffer II (20 mM Tris, pH 7.4, 40 mM Na4P2O7, 5 mM MgCl2, 50 mM NaF, 100 mM Na3VO4, 10 μM EDTA, 1% Triton, 1% SDS) supplemented with protease inhibitor and phosphatase inhibitor (Sigma), sonicated, and centrifuged for 10 min at 20,000 × g.
Expression of recombinant proteins and in vitro heat shock assays.
6×His-tagged Hsp27, Hsp70, and Hsp90 expression plasmids were transformed into Escherichia coli BL21. Expression of recombinant proteins was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 37°C. Purification was carried out using nickel resin (ProBond; Invitrogen) according to the manufacturer's instructions. For in vitro heat shock assays, 5 μl of HeLa nuclear extract was mixed with various amounts of recombinant Hsp27, Hsp70, and Hsp90 and then incubated at 45°C, as described in the figure legends. Western blotting results in Fig. 3 and 4 were quantified using the Multigauge program (Fuji).
Immunoprecipitations.
For immunoprecipitations, Hsp27-3Flag-pCMV14 was transfected alone and pCDNA3-Hsp27-myc-6His was transfected alone or together with SRSF10-3Flag-pCMV14 into a 10-cm plate of HeLa cells using Lipofectamine 2000 (Invitrogen). Thirty-six hours after transfection, cells were subjected to the heat shock (44°C for 40 min) with or without pretreatment (43°C for 1 h and 37°C for 3 h). Cells were harvested, and nuclear extract was prepared. Cleared nuclear extract was mixed with 20 μl of anti-Flag antibody-conjugated beads (M2 affinity gel; Sigma) for 2 h. Beads were then extensively washed using buffer D containing 250 mM NaCl and then boiled for 5 min at 95°C. Supernatants were analyzed by Western blotting.
RESULTS
A mild heat treatment protects SRSF10 from heat shock-induced dephosphorylation.
Splicing of mRNA precursors in HeLa nuclear extract has long been known to become resistant to heat shock if cells are first subjected to a milder heat treatment (2). Given the central role of dephosphorylated SRSF10 in heat shock-induced splicing inhibition, we set out to examine whether SRSF10 dephosphorylation in response to heat shock is affected by a mild heat pretreatment. To this end, we first subjected HeLa cells to a heat treatment identical to the one previously shown to protect splicing from a second heat shock (43°C for 1 h followed by a 3-h recovery at 37°C [2]). These cells were then subjected to a second heat shock at 44°C. For comparison, HeLa cells without any pretreatment were subjected to the same heat shock. At different time points during the heat shock, cells were harvested and SRSF10 was monitored by Western blotting (Fig. 1). After 40 min at 44°C, in HeLa cells without any pretreatment, significant amounts of SRSF10 were either partially or fully dephosphorylated (Fig. 1, lane 3). In comparison, in heat-pretreated HeLa cells, most SRSF10 remained fully phosphorylated and only trace amounts appeared to be partially dephosphorylated (Fig. 1, lane 6). These data suggest that SRSF10 phosphorylation developed thermotolerance following a mild heat treatment. Therefore, SRSF10 phosphorylation status correlates almost perfectly with the activity of the splicing machinery in the development of thermotolerance.
FIG. 1.
Heat shock-induced SRSF10 dephosphorylation displays thermotolerance. HeLa cells that were untreated (lanes 1 to 3) or treated with a mild heat shock (lanes 4 to 6) were incubated at 44°C for the indicated times. SRSF10 was monitored by Western blotting. SRSF10, dephosphorylated SRSF10 (dSRSF10), and the shorter SRSF10 isoform SRSF10-2 are labeled.
SRSF10 thermotolerance requires synthesis of heat shock proteins.
Next we wished to examine whether development of SRSF10 thermotolerance, like splicing thermotolerance (29), requires de novo protein synthesis. HeLa cells were first treated with a mild heat shock as described above, in either the absence or the presence of the translation inhibitor cycloheximide. After a 3-h recovery, these cells, along with untreated cells, were subjected to a 44°C heat shock as described above. Compared to the untreated cells, cells heat pretreated in the absence of cycloheximide contained more Hsp70 and Hsp27 and showed stronger induction of both these proteins during the second heat shock (Fig. 2A, bottom panels, compare lanes 1 to 3 and 4 to 6; the constitutively expressed Hsc70 serves as a loading control). In these pretreated cells, SRSF10 was again protected from dephosphorylation during the second heat shock (Fig. 2A, top panel, compare lanes 3 and 6), similar to what was observed above (Fig. 1, lanes 3 and 6). However, in the cells pretreated in the presence of cycloheximide, Hsp70 and Hsp27 levels were similar to those in the untreated cells and little, if any, induction was observed during the second heat shock (Fig. 2A, bottom panels, lanes 7 to 9), as expected from the inhibition of protein synthesis. Significantly, SRSF10 was in this case dephosphorylated in response to the second heat shock, similar to what was observed in untreated cells (Fig. 2A, top panel, lanes 7 to 9). These data suggest that, again similar to splicing thermotolerance (29), thermotolerance of SRSF10 phosphorylation requires de novo protein synthesis.
FIG. 2.
Thermotolerance of SRSF10 dephosphorylation requires synthesis of heat shock proteins. (A) Thermotolerance of SRSF10 dephosphorylation requires de novo protein synthesis. HeLa cells that were untreated (lanes 1 to 3) or treated with a mild heat shock (lanes 4 to 9) in the absence (lanes 4 to 6) or presence (lanes 7 to 9) of cycloheximide were incubated at 44°C for the indicated times. SRSF10, Hsp70, Hsc70, and Hsp27 were monitored by Western blotting. (B) HeLa cells transfected with control or HSF1 RNAi constructs were left untreated or treated with a mild heat shock and then incubated at 44°C for 0 or 40 min. SRSF10 and HSF1 were monitored by Western blotting. The arrows indicate the faster- (a) and slower-migrating (b) forms of HSF1.
Since cycloheximide is a general translation inhibitor, it was not clear if synthesis of heat shock proteins or other proteins was required for the thermotolerance of SRSF10 phosphorylation. To address this question, we specifically inhibited heat shock protein synthesis by depleting the transcription factor HSF1 using RNAi. HSF1 binds to the promoters of heat shock protein genes in response to heat shock or other types of cellular stress and is responsible for specifically activating these genes (20, 22). Western blotting showed that HSF1 was efficiently depleted in HeLa cells transfected with an HSF1 short hairpin RNA (shRNA) construct (Fig. 2B, lower panels). Note that the mobility of HSF1 decreases upon heat shock (Fig. 2B, compare arrows a and b), which is consistent with previous studies showing that HSF1 is hyperphosphorylated upon heat shock (6). When HSF1-depleted and control cells were subjected to heat shock as described above, similar levels of SRSF10 dephosphorylation were observed (Fig. 2B, lanes 2 and 4). However, when the same cells were pretreated and then subjected to heat shock, the results obtained were markedly different: in the control cells, very little SRSF10 dephosphorylation was observed (Fig. 2B, upper panel, lane 6), indicating that as shown above SRSF10 phosphorylation became thermotolerant. In HSF1-depleted cells, however, significant SRSF10 dephosphorylation was now observed (Fig. 2B, upper panel, lane 8). Together, these results suggest that heat pretreatment leads to thermotolerance of SRSF10 phosphorylation through inducing heat shock protein synthesis.
Hsp27 functions in thermotolerance of SRSF10 phosphorylation.
We next wished to investigate which heat shock protein(s) might be required for thermotolerance of SRSF10 phosphorylation. To address this question, we overexpressed three major heat shock proteins, Hsp27, Hsp70, and Hsp90, by transient transfection and examined their effect on SRSF10 dephosphorylation in response to heat shock. Significantly, we observed that overexpression of Hsp27 (myc-6His-tagged) led to reduced levels of SRSF10 dephosphorylation during heat shock (Fig. 3A, upper panel, compare lanes 3 and 6). In comparison, overexpression of Hsp70 and Hsp90 (hemagglutinin [HA] tagged) had little or no effect (Fig. 3B). These results suggest that Hsp27 is a major contributor to thermotolerance of SRSF10 phosphorylation. (We note that previous studies indicated that the epitope tags [myc-6His and HA] have no significant effect on the functions of heat shock proteins [1, 19].)
FIG. 3.
Overexpression of Hsp27 protects SRSF10 from heat shock-induced dephosphorylation in vivo. (A and B) HeLa cells transfected with empty vector, Hsp27-myc-6His, HA-Hsp70, or HA-Hsp90 expression constructs were incubated at 44°C for 0, 30, or 60 min. SRSF10, Hsp27-myc-6His, and HA-tagged Hsp70 and Hsp90 were monitored by Western blotting. (C) HeLa cells transfected with empty vector or Hsp27-myc-6His or Hsp27-3D-myc-6His expression constructs were incubated at 44°C for 0 or 40 min. SRSF10 and Hsp27 were monitored by Western blotting. (D) HeLa cells transfected with empty vector or Hsp27-myc-6His expression constructs were harvested either after culture at 37°C (lanes 1, 2, 7, and 8) or after a heat shock at 44°C (lanes 3 and 4) or 45°C (lanes 9 and 10) for 1 h and recovery at 37°C for 3 h (lanes 5, 6, 11, and 12).
It has been shown previously that Hsp27 activity is regulated by phosphorylation on three serine residues (8, 15), and phosphorylation at these sites is critical for many of Hsp27's activities, such as nuclear entry and interactions with other proteins (1, 4). To provide additional support for the idea that Hsp27 activity protects SRSF10 from dephosphorylation, we compared the effects of transient expression of Hsp27 and a triple S-to-D mutant derivative (Hsp27-3D [1]) that mimics phosphorylated Hsp27 on SRSF10 dephosphorylation during heat shock. Although the two proteins were expressed at equivalent levels (Fig. 3C, bottom panel), we observed stronger inhibition of SRSF10 dephosphorylation upon heat shock by Hsp27-3D than upon heat shock by wild-type Hsp27 (Fig. 3C, top panel, compare lanes 5 and 6).
It has previously been reported that overexpression of Hsp27 does not prevent dephosphorylation of SRSF10 upon heat shock (19), an apparent contradiction of the results presented here. One possibility is that the slightly harsher conditions (45°C for 1 h instead of 44°C for 1 h) used in the previous study were sufficient to induce a more complete dephosphorylation, which was resistant to Hsp27. We therefore compared the effects of these two heat shock conditions on SRSF10 dephosphorylation (Fig. 3D, upper panel). As previously reported (19), SRSF10 was almost completely dephosphorylated in both vector- and Hsp27-transfected HeLa cells after incubation at 45°C for 1 h (lanes 9 and 10), and dephosphorylation of SRSF10 was sustained during a 3-h recovery period (lanes 11 and 12). However, when cells were incubated at 44°C for 1 h, SRSF10 was only partially dephosphorylated, and this dephosphorylation could be prevented by Hsp27 overexpression (lanes 3 and 4). Furthermore, SRSF10 was mostly rephosphorylated after a 3-h recovery, especially in the presence of exogenous Hsp27 (lanes 5 and 6). Expression of Hsp27 was indistinguishable under the two conditions (Fig. 3D, lower panel, and results not shown). Together, these data suggest that a 1°C increase in temperature during heat shock caused more extensive SRSF10 dephosphorylation and that this overwhelmed the protective effects of Hsp27.
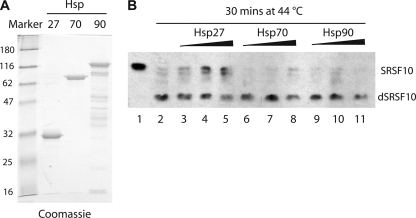
We showed previously that SRSF10 is dephosphorylated when HeLa nuclear extract is incubated at heat shock temperatures, similar to what is observed in vivo (23, 24). To determine whether Hsp27 has the ability to block this in vitro dephosphorylation, we prepared recombinant 6×His-Hsp27, -Hsp70, and -Hsp90 (Fig. 4A) and added them (0.5 to 1.5 μg) to this “in vitro heat shock” assay (Fig. 4B). Although addition of Hsp70 and Hsp90 had at most little effect (compare lanes 6 to 11 to lane 2), addition of Hsp27 consistently, albeit weakly, inhibited SRSF10 dephosphorylation (lanes 3 to 5). Equivalent results were obtained at additional time points (data not shown). These in vitro results provide evidence that Hsp27 plays a direct role in thermotolerance of SRSF10 phosphorylation.
FIG. 4.
Recombinant Hsp27 protects SRSF10 from heat-induced dephosphorylation in vitro. (A) One microgram of recombinant 6His-Hsp27, -Hsp70, and -Hsp90 was resolved by SDS-PAGE and stained with Coomassie blue. Markers at left show molecular masses in kilodaltons. (B) Five microliters of HeLa nuclear extract was incubated at 44°C for 30 min in the absence (lane 2) or presence (lanes 3 to 11) of 0.5, 1, or 1.5 μg of recombinant 6His-Hsp27, 6His-Hsp70, or 6His-Hsp90. SRSF10 was monitored by Western blotting.
Hsp27 translocates to the nucleus and interacts with SRSF10 in pretreated cells.
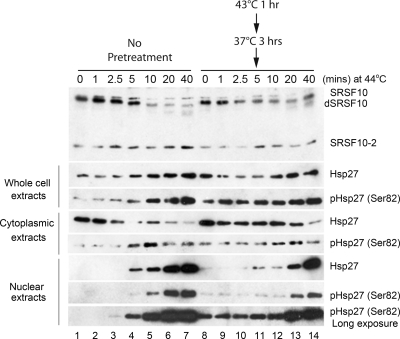
We next wished to gain insight into how Hsp27 blocks SRSF10 dephosphorylation and how this process is activated in vivo during establishment of splicing thermotolerance. As mentioned above, Hsp27 is phosphorylated under stress conditions, and this phosphorylation is necessary for efficient entry into the nucleus (4), as well as for thermotolerance (12a). We first wished to determine how heat pretreatment affects Hsp27 phosphorylation and nuclear localization. To this end, we monitored the subcellular localization and phosphorylation status of Hsp27 during pretreatment and subsequent heat shock, by Western blotting of cell fractions using anti-Hsp27 and anti-phospho-Hsp27 (Ser82) antibodies (Fig. 5). Prior to heat shock, low levels of Hsp27 were detected, located almost exclusively in the cytoplasm (lane 1). Upon heat shock, after a brief lag, total amounts of Hsp27 increased, with nuclear levels increasing dramatically and cytoplasmic levels decreasing (lanes 2 to 7). As previously reported (27a), heat shock induced Hsp27 phosphorylation and accumulation of the phosphorylated Hsp27 in the nucleus (lanes 2 to 7). Strikingly however, after heat pretreatment and a 3-h recovery, significant amounts of phosphorylated Hsp27 were already present in the nucleus prior to heat shock (lane 8, bottom panels, compare with lane 1). Nuclear Hsp27 levels remained constant for some time following heat shock and subsequently increased (lanes 9 to 14). This early accumulation of phosphorylated Hsp27, and nuclear localization, may facilitate interaction with SRSF10, thereby preventing its subsequent dephosphorylation (see Discussion).
FIG. 5.
Heat pretreatment induces accumulation of phosphorylated Hsp27 in the nucleus. HeLa cells that were untreated (lanes 1 to 7) or treated with a mild heat shock as shown (lanes 8 to 14) were incubated at 44°C for indicated times. Whole-cell, cytoplasmic, and nuclear extracts were prepared and subjected to Western blotting using anti-SRSF10, anti-Hsp27, or anti-pHsp27 (Ser82) antibody.
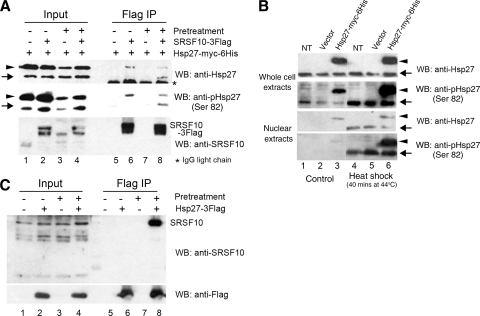
We next set out to examine whether Hsp27 in fact interacts with SRSF10 in vivo and whether heat pretreatment would facilitate such an interaction. As available anti-SRSF10 antibodies functioned poorly in immunoprecipitation, we transiently expressed SRSF10-3Flag together with Hsp27-myc-6His and performed coimmunoprecipitation (co-IP) using anti-Flag antibodies. (Exogenously expressed proteins accumulated to severalfold-higher levels than did their endogenous counterparts [Fig. 6A, left panel].) The transfected cells, with or without heat pretreatment and recovery, were subjected to heat shock (40 min), and co-IPs were analyzed by Western blotting with anti-Hsp27 antibodies. Significantly, our results showed that endogenous Hsp27 was specifically coimmunoprecipitated with SRSF10-3Flag only in the pretreated cells (Fig. 6A, compare lanes 6 and 8), consistent with the idea that this interaction underlies the inhibitory effect of pretreatment on heat shock-induced SRSF10 dephosphorylation. However, in contrast, Hsp27-myc-6His was coimmunoprecipitated with SRSF10-3Flag in both the untreated and pretreated cells (Fig. 6A, lanes 6 and 8). This may reflect the presence of small amounts of Hsp27-myc-6His, but not endogenous Hsp27, in the nucleus even in control untreated cells (Fig. 6B, lane 3). In any case, to examine further whether heat pretreatment facilitates an interaction between Hsp27 and SRSF10, we transiently expressed Hsp27 as an Hsp27-3Flag derivative and, following heat shock with or without pretreatment as described above, performed co-IP using anti-Flag antibodies and Western blotting with anti-SRSF10 antibodies (Fig. 6C). Strikingly, SRSF10 coimmunoprecipitated with Hsp27 in extracts prepared from the pretreated cells (lane 8) but not in extracts from heat-shocked cells that were not subjected to heat pretreatment (lane 6).
FIG. 6.
Hsp27 interacts with SRSF10 during heat shock only following heat pretreatment. (A) HeLa cells were transfected with Hsp27-myc-6His-expressing plasmid alone or together with an SRSF10-3Flag construct. After 36 h of transfection, cells were untreated or pretreated as described above and then incubated at 44°C for 40 min. Immunoprecipitation was performed with anti-Flag antibodies. SRSF10 and Hsp27 in input and immunoprecipitated samples were monitored by Western blotting (WB). Arrowheads indicate exogenous Hsp27, and arrows indicate endogenous Hsp27. (B) HeLa cells transfected with empty vector or Hsp27-myc-6His-expressing plasmid were incubated at 44°C for 0 or 40 min. Whole-cell lysate and nuclear extracts were prepared and subjected to Western blotting using anti-Hsp27 or anti-pHsp27 (Ser82) antibodies. Arrowheads indicate exogenous Hsp27, and arrows indicate endogenous Hsp27. NT, nontransfected cells. (C) HeLa cells were transfected with empty vector or a plasmid expressing Hsp27-3Flag. These cells were treated as described for panel A. Extracts were prepared, immunoprecipitated with anti-Flag antibodies, and probed with anti-SRSF10 and anti-Flag antibodies.
Taken together, our data suggest that the heat treatment that facilitates splicing thermotolerance and prevents SRSF10 dephosphorylation also facilitates Hsp27 interaction with SRSF10, at least in part by facilitating nuclear localization of Hsp27 so that it is already accumulating in the nucleus at the beginning of heat shock.
DISCUSSION
In this report, we extended our earlier finding that SRSF10 is a critical regulator of splicing in response to heat shock by showing that a mild heat treatment of cells protects SRSF10 from dephosphorylation during a second heat shock. The thermotolerance of SRSF10 phosphorylation requires heat shock protein synthesis. We show that overexpression of Hsp27 partially protects SRSF10 from dephosphorylation during heat shock, suggesting that Hsp27 plays an important role in the development of the thermotolerance of SRSF10 phosphorylation. Finally our demonstration of an interaction between Hsp27, newly synthesized and translocated to the nucleus, and SRSF10 suggests a potential mechanism by which Hsp27 inhibits SRSF10 dephosphorylation by PP1 during heat shock in thermotolerant cells. Together, our results provide additional evidence that SRSF10 is the central mediator of splicing inhibition in response to heat shock and support the view that control of its phosphorylation status underpins splicing thermotolerance.
Previous studies suggested that overexpression of Hsp27 has no effect on SRSF10 dephosphorylation in response to heat shock but enhances recovery of SRSF10 phosphorylation following heat shock (19). Our study is consistent with the notion that heat shock proteins, including Hsp27, play a role in promoting rephosphorylation of SRSF10 following heat shock. For example, our results showed that in HSF1-depleted cells, SRSF10 rephosphorylation following heat shock was impeded, suggesting that heat shock protein synthesis is required for efficient rephosphorylation of SRSF10 following heat shock. However, in contrast to the aforementioned study, our data indicate that Hsp27 helps to prevent dephosphorylation of SRSF10 in response to heat shock. We showed that the critical difference between our study and the previous one was the heat shock conditions used. The condition that we used, 44°C for times up to 1 h, is relatively mild. It results in only partial dephosphorylation and allows for detection of subtle differences in the rate and efficiency of SRSF10 dephosphorylation (23, 24). In contrast, increasing the temperature by only 1°C (i.e., to 45°C for 1 h, the conditions used by Marin-Vinader et al. [19]) led to essentially complete dephosphorylation of SRSF10, and the protective effects of Hsp27 were insufficient to overcome this more extensive dephosphorylation. Therefore, it is likely that thermotolerance induced by heat pretreatment, or by overexpression of Hsp27, does not necessarily lead to total resistance of SRSF10 to dephosphorylation, and the effects of these treatments may vary and reflect the severity of the heat pretreatment and/or heat shock.
In this study, we presented evidence that Hsp27 plays an important role in development of thermotolerance of SRSF10 phosphorylation. Additional support for this might come from analysis of whether depletion of Hsp27 by RNAi results in loss of thermotolerance. However, we were unable to obtain significant depletion of Hsp27 by short interfering RNA (siRNA) (unpublished data), and we are unaware of any studies in which this has been reported, at least during heat treatment. This could be because even if RNAi leads to lower steady-state levels of Hsp27, de novo synthesis of Hsp27 mRNAs and proteins is induced by heat pretreatment. Nonetheless, we showed that overexpression of Hsp27, but not Hsp70 or Hsp90, leads to inhibition of SRSF10 dephosphorylation during heat shock and that recombinant Hsp27, but not Hsp70 or Hsp90, protects SRSF10 from dephosphorylation in vitro. Furthermore, we found that Hsp27 and SRSF10 associate with each other and that this association is induced by the heat pretreatment that induces thermotolerance. The pretreatment not only induces synthesis of Hsp27 but also leads to its phosphorylation and nuclear transport. This may also explain the stronger protective effect on SRSF10 phosphorylation by Hsp27-3D, as this Hsp27 derivative is known to partially localize to the nucleus in the absence of stress (9). It is likely that all of these events contribute to the observed interaction between SRSF10 and Hsp27 upon heat shock. In any event, our results strongly support the conclusion that Hsp27 is a major contributor in the development of thermotolerance of SRSF10 phosphorylation and therefore of splicing. This is consistent with longstanding observations that overexpression of Hsp27 is sufficient to induce thermotolerance at the cell survival level (14).
Our data suggest that the association between Hsp27 and SRSF10 in heat-pretreated cells protects the latter from dephosphorylation. But how does this occur? The protective effect of Hsp27 may be related to the mechanism by which SRSF10 phosphorylation is protected from dephosphorylation in normally growing cells. Here, association with 14-3-3 proteins is important for protection of SRSF10 from dephosphorylation by PP1 (23). Upon heat shock, 14-3-3 dissociates from SRSF10, allowing access to PP1. One possibility is that, in thermotolerance, activated Hsp27 replaces 14-3-3 proteins in protecting SRSF10 from dephosphorylation during heat shock. It may also be that Hsp27 is in some way able to maintain the SRSF10-14-3-3 interaction. For example, it has been reported that during osmotic stress, levels of both phosphorylated Hsp27 and a 14-3-3 protein (14-3-3epsilon) become elevated (21a). In any event, this model explains why rapid accumulation of nuclear phosphorylated Hsp27 induced by heat pretreatment prior to heat shock is essential for thermotolerance: once 14-3-3 is dissociated from SRSF10, dephosphorylation can begin rapidly, and later accumulation of nuclear Hsp27 would be ineffective in blocking dephosphorylation.
Our study has provided further evidence that SRSF10 is the central regulator of splicing in response to heat shock. SRSF10, upon dephosphorylation, mediates splicing repression following heat shock (24). Here we have shown that SRSF10 dephosphorylation displays thermotolerance essentially identical to splicing, and it is thus reasonable to conclude that this plays a major role in splicing thermotolerance: in the absence of SRSF10 dephosphorylation, splicing continues unimpeded (24). Since both heat shock-induced splicing repression and splicing thermotolerance are conserved from yeast to humans, it is possible that phosphorylation control of SRSF10, or a related splicing regulator(s), mediates splicing regulation in response to heat shock in all of these species. Although there is no homologue of SRSF10 in budding yeast, the SR-like protein Npl3 promotes splicing and is regulated by the PP1 homologue Glc7 (10, 13). It will be of interest to determine if Npl3 mediates splicing repression in response to heat shock and splicing thermotolerance in yeast.
Acknowledgments
We thank M. Welsh and U. Hartl for providing reagents and members of the Manley lab for helpful discussions.
Y.S. was supported by an NIH postdoctoral fellowship (F32GM070113), and this work was supported by NIH grant R01 GM 48259.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Benndorf, R., et al. 2001. HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 ((3D)HSP27). J. Biol. Chem. 276:26753-26761. [DOI] [PubMed] [Google Scholar]
- 2.Bond, U. 1988. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 7:3509-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 3a.Brunet Simioni, M., et al. 2009. Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity. Oncogene 28:3332-3344. [DOI] [PubMed] [Google Scholar]
- 4.Bryantsev, A. L., M. B. Chechenova, and E. A. Shelden. 2007. Recruitment of phosphorylated small heat shock protein Hsp27 to nuclear speckles without stress. Exp. Cell Res. 313:195-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdon, R. H. 1987. Thermotolerance and the heat shock proteins. Symp. Soc. Exp. Biol. 41:269-283. [PubMed] [Google Scholar]
- 6.Cotto, J. J., M. Kline, and R. I. Morimoto. 1996. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J. Biol. Chem. 271:3355-3358. [DOI] [PubMed] [Google Scholar]
- 7.Feng, Y., M. Chen, and J. L. Manley. 2008. Phosphorylation switches the general splicing repressor SRp38 to a sequence-specific activator. Nat. Struct. Mol. Biol. 15:1040-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaestel, M., et al. 1991. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J. Biol. Chem. 266:14721-14724. [PubMed] [Google Scholar]
- 9.Geum, D., G. H. Son, and K. Kim. 2002. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J. Biol. Chem. 277:19913-19921. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert, W., and C. Guthrie. 2004. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell 13:201-212. [DOI] [PubMed] [Google Scholar]
- 11.Graveley, B. R. 2000. Sorting out the complexity of SR protein functions. RNA 6:1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt, C., and R. I. Morimoto. 1985. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl. Acad. Sci. U. S. A. 82:6455-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Kostenko, S., and U. Moens. 2009. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell. Mol. Life Sci. 66:3289-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kress, T. L., N. J. Krogan, and C. Guthrie. 2008. A single SR-like protein, Npl3, promotes pre-mRNA splicing in budding yeast. Mol. Cell 32:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry, J., P. Chretien, H. Lambert, E. Hickey, and L. A. Weber. 1989. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J. Cell Biol. 109:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landry, J., et al. 1992. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 267:794-803. [PubMed] [Google Scholar]
- 16.Lindquist, S. 1986. The heat-shock response. Annu. Rev. Biochem. 55:1151-1191. [DOI] [PubMed] [Google Scholar]
- 17.Manley, J. L., and A. R. Krainer. 2010. A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins). Genes Dev. 24:1073-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manley, J. L., and R. Tacke. 1996. SR proteins and splicing control. Genes Dev. 10:1569-1579. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Vinader, L., C. Shin, C. Onnekink, J. L. Manley, and N. H. Lubsen. 2006. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol. Biol. Cell 17:886-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morano, K. A., and D. J. Thiele. 1999. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 7:271-282. [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsen, T. W., and B. R. Graveley. 2010. Expansion of the eukaryotic proteome by alternative splicing. Nature 463:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Niswander, J. M., and L. A. Dokas. 2006. Phosphorylation of HSP27 and synthesis of 14-3-3epsilon are parallel responses to hyperosmotic stress in the hippocampus. Brain Res. 1116:19-30. [DOI] [PubMed] [Google Scholar]
- 22.Pirkkala, L., P. Nykanen, and L. Sistonen. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15:1118-1131. [DOI] [PubMed] [Google Scholar]
- 23.Shi, Y., and J. L. Manley. 2007. A complex signaling pathway regulates SRp38 phosphorylation and pre-mRNA splicing in response to heat shock. Mol. Cell 28:79-90. [DOI] [PubMed] [Google Scholar]
- 24.Shin, C., Y. Feng, and J. L. Manley. 2004. Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature 427:553-558. [DOI] [PubMed] [Google Scholar]
- 25.Shin, C., and J. L. Manley. 2004. Cell signalling and the control of pre-mRNA splicing. Nat. Rev. Mol. Cell Biol. 5:727-738. [DOI] [PubMed] [Google Scholar]
- 26.Shin, C., and J. L. Manley. 2002. The SR protein SRp38 represses splicing in M phase cells. Cell 111:407-417. [DOI] [PubMed] [Google Scholar]
- 27.Vogel, J. L., D. A. Parsell, and S. Lindquist. 1995. Heat-shock proteins Hsp104 and Hsp70 reactivate mRNA splicing after heat inactivation. Curr. Biol. 5:306-317. [DOI] [PubMed] [Google Scholar]
- 27a.Vos, M. J., B. Kanon, and H. H. Kampingas. 2009. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim. Biophys. Acta 1793:1343-1353. [DOI] [PubMed] [Google Scholar]
- 28.Yost, H. J., and S. Lindquist. 1991. Heat shock proteins affect RNA processing during the heat shock response of Saccharomyces cerevisiae. Mol. Cell. Biol. 11:1062-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yost, H. J., and S. Lindquist. 1986. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45:185-193. [DOI] [PubMed] [Google Scholar]
- 30.Yost, H. J., and S. Lindquist. 1988. Translation of unspliced transcripts after heat shock. Science 242:1544-1548. [DOI] [PubMed] [Google Scholar]
- 31.Yost, H. J., R. B. Petersen, and S. Lindquist. 1990. RNA metabolism: strategies for regulation in the heat shock response. Trends Genet. 6:223-227. [DOI] [PubMed] [Google Scholar]