Abstract
Insulin sensitivity is impaired in obesity, and insulin resistance is the primary risk factor for type 2 diabetes. Here we show that lipocalin-13 (LCN13), a lipocalin superfamily member, is a novel insulin sensitizer. LCN13 was secreted by multiple cell types. Circulating LCN13 was markedly reduced in mice with obesity and type 2 diabetes. Three distinct approaches were used to increase LCN13 levels: LCN13 transgenic mice, LCN13 adenoviral infection, and recombinant LCN13 administration. Restoration of LCN13 significantly ameliorated hyperglycemia, insulin resistance, and glucose intolerance in mice with obesity. LCN13 enhanced insulin signaling not only in animals but also in cultured adipocytes. Recombinant LCN13 increased the ability of insulin to stimulate glucose uptake in adipocytes and to suppress hepatic glucose production (HGP) in primary hepatocyte cultures. Additionally, LCN13 alone was able to suppress HGP, whereas neutralization of LCN13 increased HGP in primary hepatocyte cultures. These data suggest that LCN13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. LCN13 and LCN13-related molecules may be used to treat insulin resistance and type 2 diabetes.
Insulin controls glucose homeostasis by both suppressing hepatic glucose production and stimulating glucose uptake into skeletal muscle and adipose tissues. Impaired insulin action (insulin resistance) contributes to multiple metabolic disorders, including type 2 diabetes, dyslipidemia, and cardiovascular diseases (16). Insulin resistance is not only a hallmark but also a determinant of type 2 diabetes (16). A major therapeutic goal in treating type 2 diabetes is to improve insulin sensitivity. It is extremely important to identify molecules that sensitize insulin action.
Obesity is the primary risk factor for insulin resistance, and multiple factors contribute to insulin resistance (7). Abnormal lipid accumulation impairs insulin action in skeletal muscle and the liver, thereby contributing to systemic insulin resistance in obesity (4, 11, 14, 17, 21, 22). Obesity is associated with chronic, low-grade inflammation that also contributes to insulin resistance (7, 19). Additionally, adipocytes secrete a variety of polypeptides, collectively called adipokines, which regulate insulin sensitivity (1, 6). Many proinflammatory cytokines and adipokines have been documented to regulate insulin sensitivity (1, 7, 19).
Lipocalin family members are small secretory proteins involved in a variety of biological processes, including chemical communication, cell proliferation and differentiation, and metabolism (2, 8, 23-26). The diverse lipocalin superfamily members share a relatively low level of homology in amino acid sequences; however, their tertiary structures are highly conserved, containing a characteristic β-barrel at the center (18, 26). Lipocalins bind via this central cavity to small lipophilic molecules, including fatty acids, retinol, steroids, odorants, and pheromones (18, 26). Thus, lipocalins are predicted to act as carriers to regulate the transportation, stability, release, and activity of these small bioactive molecules (26). Additionally, lipocalins may also bind to their cognate receptors and directly stimulate cellular responses (3, 12).
Several lipocalin family members appear to be involved in the regulation of insulin action (5, 8, 9, 23-26). For instance, the levels of retinol-binding protein 4 (RBP4) are increased in obesity (5, 9). RBP4 promotes insulin resistance (5, 9, 24). Lipocalin-2 also induces insulin resistance (10, 23, 24). We recently reported that major urinary protein 1 (MUP1), a lipocalin family member primarily expressed in hepatocytes, improves insulin sensitivity and glucose metabolism in mice (25). MUP1 overexpression markedly improves hyperglycemia and glucose intolerance in mice with type 2 diabetes (25). Another group also reported independently that MUP1 improves insulin sensitivity in skeletal muscle (8). In a search for additional lipocalins that regulate insulin sensitivity and glucose metabolism, we profiled gene expression patterns in the livers of db/db mice by using the Affymetrix GeneChip analysis. Lipocalin-13 (LCN13) was identified as a potential candidate. The LCN13 gene was initially identified by its genomic location in the epididymal lipocalin cluster in mouse chromosome 2 (20). Based on its predicted amino acid sequences, LCN13 belongs to the lipocalin superfamily (20). The LCN13 gene contains 7 exons and is predicted to encode 176 amino acids with a putative N-terminal signal peptide (1 to 18 amino acids) (20).
In this study, we show that LCN13 is expressed by multiple cell types and secreted into the bloodstream. Circulating LCN13 is markedly reduced in mice with either genetic or diet-induced type 2 diabetes, and restoration of LCN13 improves insulin resistance, hyperglycemia, and glucose intolerance. LCN13 also directly enhances insulin action in cultured adipocytes and hepatocytes. Therefore, LCN13 acts as an endogenous insulin sensitizer to regulate glucose metabolism.
MATERIALS AND METHODS
Generation of LCN13 transgenic mice.
The LCN13 transgene constructs were engineered by inserting a full-length mouse LCN13 cDNA into a pCAGGS vector (see Fig. 2A). A “STOP” cassette, which was flanked by two loxP sites, was inserted between the chicken β-actin/rabbit β-globin hybrid promoter and the LCN13 cDNA. The transgenic constructs were linearized with SalI and ApaLI, purified, and microinjected into F2 mouse oocytes (in a C57BL/6 background). The oocytes were surgically transferred to recipients in the University of Michigan Transgenic Animal Model Core. Transgenic animals were identified by PCR-based genotyping assays. The transgenic mice were crossed with EIIA-Cre mice (in a C57BL/6 genetic background) to generate LCN13 and EIIA-Cre double-transgenic mice. Cre was expressed in the germ cells of the LCN13/EIIA-Cre double-transgenic mice and deleted the STOP cassette via the two loxP sites; therefore, the progenies of the LCN13/EIIA-Cre transgenic mice were predicted to express the LCN13 transgene under the control of the chicken β-actin/rabbit β-globin hybrid promoter. This hybrid promoter is constitutively active in multiple tissues of the transgenic mice (13). The EIIA-Cre transgene was removed by a series of backcrosses of the LCN13/EIIA-Cre transgenic mice with wild-type (WT) mice (in a C57BL/6 background). We obtained two independent lines of LCN13 transgenic mice, LCN13-642 and LCN13-783.
ob/ob and db/db mice (in a C57BL/6 background) were from the Jackson Laboratory (Bar Harbor, ME). Mice were housed on a 12-h light and 12-h dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan (ULAM), and fed either normal chow (9% fat; Lab Diet) or a high-fat diet (HFD) (45% fat; Research Diets) ad libitum with free access to water. Animal experiments were conducted following protocols approved by the University Committee on the Use and Care of Animals (UCUCA).
Generation of LCN13 adenoviruses, anti-LCN13 antibody, and recombinant LCN13 protein.
A full-length LCN13 cDNA was inserted 3′ of the cytomegalovirus (CMV) promoter sequences in an adenoviral vector and used to generate recombinant LCN13 adenoviruses using pAdEasy kits (QBiogene, Carlsbad, CA). Recombinant adenoviruses were amplified in QBI-293A cells and purified by CsCl gradient ultracentrifugation. LCN13 or β-galactosidase (β-Gal) (control) adenoviruses were administered to mice via tail vein injection at 2 × 1011 viral particles per mouse. To generate anti-LCN13 antibody (αLCN13), an N-terminally truncated variant of murine LCN13, which lacks amino acids 1 to 18 (a putative signal peptide), was fused to glutathione S-transferase (GST). GST-LCN13 fusion protein was purified from bacteria and used to generate rabbit polyclonal αLCN13. To generate and purify recombinant LCN13 protein, a variant of murine LCN13 cDNA, which lacks the 5′ sequences encoding amino acids 1 to 18, was inserted into a pET28a(+) bacterial expression vector at 3′-His6 tag sequences. The vector was transformed into Escherichia coli BL21 cells, and His6-LCN13 was purified using a Ni2+-nitrilotriacetic acid column (Qiagen, Valencia, CA) following the manufacturer's instructions. Endotoxins were removed using Detoxi-Gel endotoxin removal kits (Thermo Scientific, Rockford, IL).
Animal experiments.
ob/ob mice (10 weeks) were anesthetized with 2 to 4% isoflurane, and osmotic minipumps (model 2002; Alzet, Cupertino, CA) were implanted subcutaneously at the dorsum of the neck. Minipumps were prefilled with either a sterile 0.9% NaCl vehicle or recombinant LCN13. An LCN13-filled pump released LCN13 at 33 pmol/h/mouse. Blood samples were collected from mouse tail veins by using heparin-pretreated capillary tubes. Blood glucose was measured by a glucometer (Bayer Corp., Tarrytown, NY), and plasma insulin was measured using a rat insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Inc., Chicago, IL). The homeostasis model assessment (HOMA) index was calculated as fasting blood glucose (mmol/liter) × fasting plasma insulin (μU/ml)/22.5. For glucose tolerance tests (GTT), mice were fasted overnight (16 h) and intraperitoneally injected with d-glucose. Blood glucose was measured 0, 15, 30, 60, and 120 min after glucose injection. For insulin tolerance tests (ITT), mice were fasted for 6 h (from 10:00 a.m. to 4:00 p.m.) and intraperitoneally injected with human insulin. Blood glucose was monitored 0, 15, 30, and 60 min after insulin injection.
Adipocyte differentiation and glucose uptake assays.
3T3-L1 preadipocytes were cultured in Dulbecco's modified Eagle medium (DMEM) containing 25 mM glucose and 10% calf serum at 37°C and 5% CO2. Two days postconfluence, 3T3-L1 preadipocytes were cultured for 3 days in a differentiation medium (DMEM supplemented with 10% fetal bovine serum [FBS], 0.1 μM insulin, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine) and for 3 additional days in DMEM supplemented with 10% FBS and 0.1 μM insulin. Differentiated adipocytes were maintained in DMEM supplemented with 10% FBS and pretreated with purified LCN13 for the indicated times in DMEM supplemented with 0.6% bovine serum albumin (BSA). In glucose uptake assays, adipocytes were incubated with DMEM containing 0.6% BSA for 3 h, washed with Krebs-Ringer buffer (130 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, 25 mM HEPES, pH 7.4), and incubated with or without insulin for 30 min. 2-[3H]deoxy-d-glucose was added during the last 5 min of insulin stimulation. Cells were immediately washed three times with ice-cold phosphate-buffered saline (PBS) and solubilized in 0.1% SDS buffer. 2-[3H]deoxy-d-glucose uptake was determined by scintillation counting.
Hepatic glucose production (HGP) assays.
Mouse hepatocytes were isolated by liver perfusion with type II collagenase (Worthington Biochem, Lakewood, NJ) and grown on collagen-coated plates for 2 h in Williams' medium E (Sigma, St. Louis, MO) containing 2% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were rinsed with PBS and cultured for an additional 4 h in KRB buffer (118 mM NaCl, 2.5 mM CaCl2, 4.8 mM KCl, 25 mM NaHCO3, 1.1 mM KH2PO4, 1.2 mM MgSO4, 10 μM ZnSO4, 0.6% BSA, 10 mM HEPES, pH 7.4) in the presence or absence of recombinant LCN13 protein. For HGP assays, primary hepatocyte cultures were washed twice with PBS and incubated with HGP buffer (KRB buffer supplemented with 10 mM sodium dl-lactate and 5 mM pyruvate) in the presence or absence of 100 nM insulin or Mix (10 μm N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt and 100 nm dexamethasone). Culture medium was collected 4 h later and used to measure glucose using glucose LiquiColor kits (Fisher Scientific Inc., Pittsburgh, PA). In separate experiments, anti-LCN13 antibody (0.3 μl/ml) was added directly to culture medium in HGP assays to neutralize endogenous LCN13.
Immunoprecipitation and immunoblotting.
Tissues were homogenized in lysis buffer (50 mm Tris, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 1 mM benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin; 1 mM phenylmethylsulfonyl fluoride). Tissue extracts were incubated with primary antibodies at 4°C for 2 h and with protein A-agarose beads (RepliGen Corp., Waltham, MA) for an additional hour at 4°C. The immunocomplexes adsorbed on the protein A-agarose beads were washed three times with washing buffer (50 mM Tris, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 2 mM EGTA) and boiled at 95°C for 5 min in loading buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 2% β-mercaptoethanol, 10% glycerol, 0.005% bromphenol blue). Protein was separated by SDS-PAGE, immunoblotted with indicated antibodies, and visualized using the Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) or the ECL kit (Amersham, Piscataway, NJ). Polyclonal anti-LCN13 antibodies were raised against glutathione S-transferase-LCN13 fusion protein. Phospho-Akt (Thr308), albumin, and tubulin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Akt (Ser473) antibody was from Invitrogen (Carlsbad, CA).
qRT-PCR.
Total RNA was extracted from the liver or hepatocytes and used to synthesize the first-strand cDNAs using oligo(dT)12-18 and Moloney murine leukemia virus reverse transcriptase. The mRNA abundance of various molecules was measured using Absolute QPCR SYBR green kits (Thermo Scientific, Waltham, MA) and the Mx3000P real-time PCR system (Stratagene, La Jolla, CA). Primers for quantitative real-time reverse transcription-PCR (qRT-PCR) were as follows: LCN13 forward, 5′-GTCATTCGGGATGGGAAAG-3′; LCN13 reverse, 5′-GCTGTTGCAGACCTGGGTA-3′; β-actin forward, 5′-AAATCGTGCGTGACATCAAA-3′; β-actin reverse, 5′-AAGGAAGGCTGGAAAAGAGC-3′; PEPCK forward, 5′-ATCATCTTTGGTGGCCGTAG-3′, PEPCK reverse, 5′-ATCTTGCCCTTGTGTTCTGC-3′; G6Pase forward, 5′-CCGGTGTTTGAACGTCATCT-3′, G6Pase reverse: 5′-CAATGCCTGACAAGACTCCA-3′; PGC-1α forward, 5′TGGACGGAAGCAATTTTTCA-3′; PGC-1α reverse, 5′-TTACCTGCGCAAGCTTCTCT-3′.
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Differences between groups were determined by two-tailed Student t tests. A P value of <0.05 was considered statistically significant.
RESULTS
Obesity is associated with reduced expression and secretion of LCN13.
To examine tissue distribution of LCN13, total RNAs were prepared from various tissues and used to specifically amplify LCN13 by reverse transcription-PCR using primers unique to LCN13. LCN13 mRNA was detected in multiple tissues, including livers, skeletal muscle, and the pancreas (Fig. 1A). To measure LCN13 protein levels, rabbit polyclonal anti-LCN13 antibody (αLCN13) was raised against GST-LCN13 fusion protein. We also prepared two additional reagents, LCN13 adenoviruses and LCN13 recombinant protein. A full-length mouse LCN13 cDNA was inserted into an adenoviral vector in which LCN13 expression is under the control of the CMV promoter. Generation and purification of recombinant LCN13 have been described in Materials and Methods. To examine the specificity of αLCN13, HEK293 cells were infected with LCN13 or MUP1 (an LCN13-related lipocalin family member [25, 26]) adenoviruses, and cell extracts were immunoblotted with αLCN13. αLCN13 recognized LCN13 but not MUP1 (data not shown). Additionally, preincubation with recombinant LCN13 protein dose-dependently reduced the ability of αLCN13 to detect LCN13 in cell extracts (data not shown), further indicating that αLCN13 specifically recognizes LCN13. To measure endogenous LCN13 protein, tissue extracts were immunoprecipitated with αLCN13 and immunoblotted with αLCN13. Putative LCN13 protein was detected in the liver, skeletal muscle, and pancreas but not in white adipose tissue (WAT); interestingly, two distinct forms were detected by αLCN13 (Fig. 1B).
FIG. 1.
Obesity is associated with reduced LCN13 expression and secretion. (A) Total RNAs were extracted from male mice (10 weeks) and used to detect the expression of LCN13 and 36B4 by RT-PCR. (B) Tissue extracts were prepared from wild-type males (10 weeks), immunoprecipitated with anti-LCN13 antibody (αLCN13), and immunoblotted with αLCN13. Tissue extracts were also immunoblotted with antibody against the p85 regulatory subunit of phosphatidylinositol 3-kinase. (C) FAO cells were infected with β-Gal or LCN13 adenoviruses. Culture medium and cell extracts were prepared 48 h after infection and immunoblotted with αLCN13. Numbers at left are molecular masses in kilodaltons. (D and E) The plasma (50 μl) was collected from fasted (16 h) or randomly fed males (12 weeks) (D), males (8 weeks) fed normal chow or a high-fat diet (HFD) for 6 weeks (E), or WT and db/db males (8 weeks) (E); immunoprecipitated with αLCN13; and immunoblotted with αLCN13. The plasma was also immunoblotted with antialbumin. (F) C57BL/6 males (8 weeks) were fed normal chow (n = 7) or an HFD (n = 8) for 8 weeks. Mice were fasted overnight. Total RNAs were extracted from the liver and used to measure the abundance of LCN13 mRNA by qRT-PCR. LCN13 expression was normalized to β-actin expression. Error bars represent SEM. *, P < 0.05.
To determine whether LCN13 is a secretary protein, FAO cells (rat hepatoma cells) were infected with LCN13 or β-Gal adenoviruses. Culture medium and cell extracts were prepared 48 h after infection and immunoblotted with αLCN13. Three forms of LCN13 were detected in both culture medium and cell extracts prepared from LCN13- but not β-Gal adenovirus-infected cells; interestingly, the largest form was more abundant than were the smaller forms in the medium (Fig. 1C). Therefore, LCN13 is a secretory protein, and the various forms are likely to result from differential proteolytic cleavages of a common LCN13 precursor.
To determine whether LCN13 is present in the bloodstream, plasma was prepared from fasted (16 h) or randomly fed mice, immunoprecipitated with αLCN13, and immunoblotted with αLCN13. LCN13 was detected in the plasma and greatly decreased in mice under fasting conditions (Fig. 1D). To determine whether obesity is associated with a reduction in circulating LCN13, mice (8 weeks) were fed a high-fat diet (HFD) for 6 weeks to induce obesity. The levels of circulating LCN13 were much lower in HFD-fed mice than in chow-fed mice (Fig. 1E). Consistently, liver LCN13 expression was markedly decreased in mice fed an HFD (Fig. 1F). db/db mice are a genetic model of obesity with leptin receptor deficiency. Similarly, plasma LCN13 levels were also much lower in db/db mice than in wild-type (WT) mice (8 weeks) (Fig. 1E). These results raise a possibility that LCN13 deficiency may contribute to insulin resistance and glucose intolerance in mice with obesity.
Transgenic expression of LCN13 protects against HFD-induced insulin resistance and glucose intolerance.
To determine whether LCN13 regulates insulin sensitivity and glucose metabolism, a full-length mouse LCN13 cDNA was inserted into a transgenic construct under the control of the chicken β-actin/rabbit β-globin hybrid promoter and used to generate LCN13 transgenic mice (Fig. 2A). This hybrid promoter has been reported to be constitutively active in multiple mouse tissues (13). Two independent lines (LCN13-783 and LCN13-642) were generated and used in the following experiments. The expression of the LCN13 transgene was detected in the liver, muscle, and pancreas (Fig. 2B). Circulating LCN13 levels were higher in LCN13 transgenic mice than in WT littermates (Fig. 2C).
FIG. 2.
Generation and characterization of LCN13 transgenic mice. (A) A schematic representation of an LCN13 transgenic construct, Cre-mediated deletion of the STOP cassette, and the LCN13 transgene. (B) Tissue extracts were prepared from WT (lane 1) or LCN13-783 (lanes 2 to 5) mice, immunoprecipitated with αLCN13, and immunoblotted with αLCN13. Lanes 1 and 2, liver; lane 3, muscle; lane 4, pancreas; lane 5, white adipose tissue. (C) Plasma was prepared from LCN13 transgenic mice (LCN13-783) and wild-type (WT) male littermates (8 weeks), immunoprecipitated with αLCN13, and immunoblotted with αLCN13. (D and E) Mice (7 weeks) were fed an HFD. (D) Growth curves of LCN13-783 mice (n = 9) and WT male littermates (n = 10). (E) Growth curves of LCN13-642 mice (n = 9) and WT male littermates (n = 10). Error bars represent SEM.
LCN13-783 and WT male littermates (7 weeks) were fed an HFD. Chronic overexpression of LCN13 did not alter body weight in both LCN13-783 and LCN13-642 mice (Fig. 2D and E); however, LCN13 overexpression protected against HFD-induced hyperinsulinemia and insulin resistance (Fig. 3). Plasma insulin decreased by 47% in LCN13-783 mice (Fig. 3A). The HOMA index, which is commonly used to estimate insulin sensitivity, decreased by 63% in LCN13-783 mice (Fig. 3B). To further analyze insulin sensitivity and glucose metabolism, LCN13-783 and WT littermates were fed an HFD for 12 weeks and subjected to insulin tolerance tests (ITT) and glucose tolerance tests (GTT). Exogenous insulin reduced blood glucose levels to a greater extent in LCN13-783 mice than in WT littermates (Fig. 3C). Glucose injection increased blood glucose to a much lower level in LCN13-783 mice than in WT littermates (Fig. 3D). Chronic overexpression of LCN13 similarly protected against HFD-induced glucose intolerance in the LCN13-642 line (Fig. 3E).
FIG. 3.
Transgenic overexpression of LCN13 protects against diet-induced hyperglycemia, glucose intolerance, and insulin resistance. Male mice (7 weeks) were fed an HFD. (A and B) Fasting (16 h) plasma insulin (A) and HOMA index (B) in WT (n = 10) and LCN13-783 (n = 5) mice fed an HFD for 11 weeks. (C) Insulin tolerance tests (ITT) were performed on WT (n = 10) and LCN13-783 (n = 8) mice fed an HFD for 12 weeks (insulin, 1 U/kg body weight). (D) Glucose tolerance tests (GTT) were performed on WT (n = 10) and LCN13-783 (n = 8) mice fed an HFD for 12 weeks (d-glucose, 2 g/kg body weight). (E) GTT (2 g/kg body weight) were performed on LCN13-642 mice (n = 8) and WT male littermates (n = 10) fed an HFD for 12 weeks. Error bars represent SEM. *, P < 0.05.
LCN13 attenuates insulin resistance, hyperglycemia, and glucose intolerance in mice with type 2 diabetes.
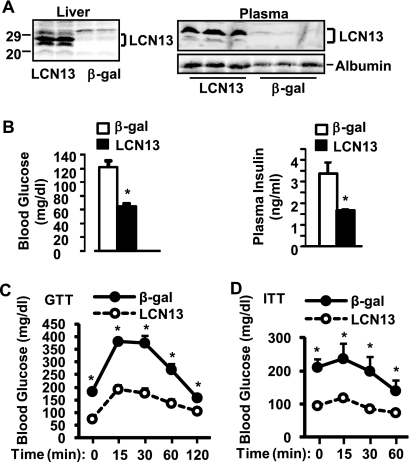
To determine whether restoration of circulating LCN13 improves insulin resistance and glucose intolerance in mice with type 2 diabetes, db/db mice were infected with LCN13 or β-Gal adenoviruses via tail vein injection. Recombinant adenoviruses primarily infect liver cells under these conditions (15, 25). Recombinant LCN13 was detected as two isoforms in the livers of LCN13- but not β-Gal adenovirus-infected mice (Fig. 4A). These results support the observations in Fig. 1B and C that the multiple isoforms are likely to result from proteolytic cleavages of a common LCN13 precursor. Plasma LCN13 levels were significantly elevated in LCN13 adenovirus-infected mice (Fig. 4A).
FIG. 4.
Adenovirus-mediated overexpression of LCN13 ameliorates hyperglycemia and glucose intolerance in db/db mice. db/db males (9 weeks) were infected with β-Gal or LCN13 adenoviruses via tail vein injection. (A) The plasma and liver extracts were prepared 12 and 16 days after infection, respectively, and immunoblotted with anti-LCN13. The plasma was also immunoblotted with antialbumin. Numbers at left are molecular masses in kilodaltons. (B) Fasting (16 h) blood glucose and plasma insulin levels in mice 6 days after β-Gal (n = 8) or LCN13 (n = 8) adenoviral infection. (C) GTT were performed on mice 10 days after β-Gal (n = 8) or LCN13 (n = 8) adenoviral infection (d-glucose, 0.8 g/kg body weight). (D) ITT were performed on mice 14 days after β-Gal (n = 8) or LCN13 (n = 8) adenoviral infection (insulin, 2 U/kg body weight). Error bars represent SEM. *, P < 0.05.
Restoration of LCN13 markedly ameliorated hyperglycemia and hyperinsulinemia in db/db mice (Fig. 4B). In LCN13 adenovirus-infected mice, blood glucose and plasma insulin levels decreased by 46% and 51%, respectively. Restoration of circulating LCN13 also improved glucose intolerance and insulin resistance in db/db mice. In GTT, glucose injection increased blood glucose levels to a lesser extent in LCN13- than in β-Gal adenovirus-infected mice (Fig. 4C). In ITT, blood glucose levels were significantly lower in LCN13- than in β-Gal adenovirus-infected mice at each time point after insulin injection (Fig. 4D).
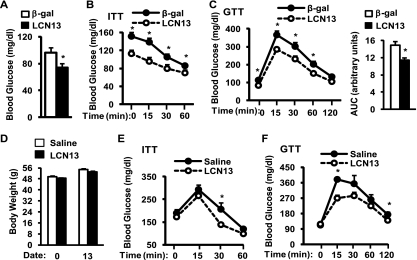
To determine whether LCN13 overexpression reverses insulin resistance and glucose intolerance in dietary fat-induced type 2 diabetes, C57BL/6 mice were fed an HFD for 10 weeks and infected with LCN13 or β-Gal adenoviruses. Fasting blood glucose levels were 29% lower in LCN13- than in β-Gal adenovirus-infected mice (Fig. 5A). In ITT, blood glucose levels were also lower in LCN13- than in β-Gal adenovirus-infected mice at each time point after insulin injection (Fig. 5B). Adenovirus-expressed LCN13 also improved glucose intolerance in HFD-fed mice as revealed by a >23% reduction in the area under the curve (AUC) in GTT (Fig. 5C). Taken together, these data suggest that restoration of circulating LCN13 attenuates insulin resistance, hyperglycemia, and glucose intolerance in both genetic and diet-induced type 2 diabetes.
FIG. 5.
LCN13 overexpression improves diet-induced insulin sensitivity and glucose intolerance. (A to C) Male mice (7 weeks) were fed an HFD for 10 weeks and infected with β-Gal (n = 8) or LCN13 (n = 8) adenoviruses. (A) Fasting (16 h) blood glucose in mice 6 days after viral infection. (B) ITT were performed on mice 8 days after viral infection (insulin, 1 U/kg body weight). (C) GTT were performed on mice 10 days after infection (d-glucose, 2 g/kg body weight). The area under the curve (AUC) was calculated. (D to F) ob/ob mice (10 weeks) were chronically administered either a sterile 0.9% NaCl vehicle or recombinant LCN13 (33 pmol/h/mouse) via osmotic minipumps. (D) Body weight before (0) and 13 days after LCN13 administration. (E) ITT were performed on mice 10 days after LCN13 treatments (insulin, 4 U/kg body weight). (F) GTT were performed on mice 12 days after LCN13 treatments (d-glucose, 0.5 g/kg body weight). Error bars represent SEM. *, P < 0.05.
To further verify the metabolic function of LCN13, recombinant LCN13 protein was purified from bacteria, and endotoxins were removed. Purified LCN13 was chronically administered to ob/ob mice (10 weeks) via osmotic minipumps (33 pmol/h). Recombinant LCN13 did not alter body weight (Fig. 5D). Insulin sensitivity, measured by ITT, was slightly improved 10 days after LCN13 treatments (Fig. 5E). LCN13 treatments also improved glucose intolerance in ob/ob mice (Fig. 5F). However, the effect of purified LCN13 was modest under these conditions, presumably due to a relatively low concentration of administered LCN13. Additionally, bacterium-produced LCN13 may be less active in vivo than mammalian cell-secreted LCN13.
LCN13 promotes insulin signaling in HFD-fed mice.
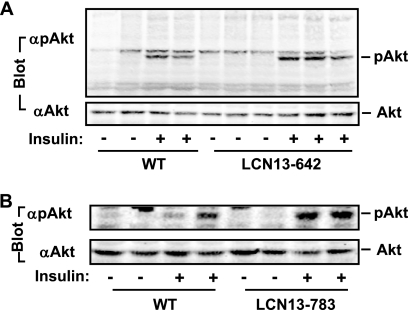
To determine whether chronic overexpression of LCN13 enhances insulin signaling in animals, LCN13-642, LCN13-783, and WT littermates were fed an HFD for 15 weeks. Mice were fasted overnight and treated with PBS or insulin (5 U/kg body weight). Liver extracts were prepared 5 min after insulin stimulation and immunoblotted with anti-phospho-Akt (pThr308). Insulin modestly stimulated Akt phosphorylation in WT mice, indicating that HFD induces insulin resistance (Fig. 6). LCN13 overexpression increased the ability of insulin to stimulate Akt phosphorylation in both LCN13-642 and LCN13-783 mice (Fig. 6). These results suggest that LCN13 improves glucose metabolism in animals at least in part by sensitizing insulin action.
FIG. 6.
LCN13 enhances insulin signaling in LCN13 transgenic mice. LCN13-642 mice (A), LCN13-783 mice (B), and their WT male littermates (7 weeks) were fed an HFD for 15 weeks. Mice were fasted for 16 h and stimulated with insulin (5 U/kg body weight) or PBS. Liver extracts were prepared 5 min after stimulation and immunoblotted with anti-pThr308 or anti-Akt as indicated.
LCN13 directly promotes insulin action in adipocytes.
To determine whether LCN13 directly enhances insulin signaling, 3T3-L1 adipocytes were pretreated with LCN13 or vehicles for 6 h and then stimulated with insulin for 10 min at various concentrations. Cell extracts were immunoblotted with phospho-Akt antibodies. LCN13 alone did not stimulate Akt phosphorylation; however, it increased both insulin sensitivity and the maximal response to insulin (Fig. 7A). Additionally, LCN13 enhanced insulin signaling in a dose-dependent manner (Fig. 7B).
FIG. 7.
LCN13 directly enhances insulin sensitivity in adipocytes. (A and B) 3T3-L1 adipocytes were pretreated with LCN13 or a vehicle for 6 h and then treated with insulin for 10 min. Cell extracts were immunoblotted with anti-phospho-Akt (pThr308 [A] and pSer473 [B]) or anti-Akt, respectively. (C) 3T3-L1 adipocytes were pretreated with 50 nM LCN13 or a vehicle for 3 or 6 h and then treated with insulin or PBS. 2-Deoxyglucose uptake was measured. (D) 3T3-L1 adipocytes were pretreated with LCN13 or a vehicle for 6 h and then treated with 10 nM insulin. 2-Deoxyglucose uptake was measured. Error bars represent SEM. *, P < 0.05.
To further verify LCN13 enhancement of insulin action, 3T3-L1 adipocytes were pretreated with LCN13 (50 nM) and then stimulated with insulin, and glucose uptake was measured. LCN13 did not alter basal glucose uptake 3 h after treatments; however, it increased basal glucose uptake 6 h after treatments (Fig. 7C, leftmost three bars). These results suggest that chronic LCN13 treatments are able to promote glucose metabolism independently of insulin. Importantly, both short-term (3-h) and long-term (6-h) LCN13 treatments markedly enhanced the ability of insulin to stimulate glucose uptake (Fig. 7C). LCN13 sensitized insulin action in a dose-dependent manner (Fig. 7D). These data suggest that LCN13 directly promotes glucose uptake in adipocytes by both insulin-dependent and -independent mechanisms.
LCN13 directly suppresses HGP.
To determine whether LCN13 directly regulates liver glucose metabolism, primary hepatocyte cultures were treated for 4 h with or without LCN13 and then subjected to hepatic glucose production (HGP) assays in the presence of PBS (basal), insulin (100 nM), or Mix (a combination of a cyclic AMP [cAMP] analog and dexamethasone). As expected, Mix stimulated HGP (by 91%), whereas insulin suppressed both basal HGP (by 30%) and Mix-stimulated HGP (by 50%) (Fig. 8A). Importantly, LCN13 also dose-dependently suppressed both basal and Mix-stimulated HGP (Fig. 8A). LCN13 (100 nM) inhibited basal HGP by 59% and Mix-stimulated HGP by 68%. A combination of insulin and LCN13 inhibited HGP to a greater extent than did either insulin or LCN13 alone (Fig. 8A).
FIG. 8.
LCN13 directly suppresses hepatic glucose production (HGP). (A) Primary hepatocyte cultures were treated with LCN13 or a vehicle for 4 h and then subjected to HGP assays for an additional 4 h in the presence of PBS (basal), 100 nM insulin, Mix (a combination of 10 μM N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt and 100 nm dexamethasone), or both insulin and Mix. HGP was normalized to total protein levels. (B) Primary hepatocyte cultures were pretreated with 100 nM LCN13 or a vehicle for 4 h and then treated with Mix or both Mix and insulin for an additional 4 h. The expression levels of key gluconeogenic genes were measured by qRT-PCR and normalized to β-actin expression. (C) Primary hepatocyte cultures were pretreated with anti-LCN13 antibody (0.3 μl/ml) or preimmune serum for 4 h and then subjected to HGP assays in the absence (Basal) or presence of Mix. Error bars represent SEM. *, P < 0.05.
To determine whether LCN13 regulates the expression of the genes that control hepatic gluconeogenesis, primary hepatocyte cultures were pretreated with LCN13 and then treated with Mix or Mix plus insulin. The expression levels of key gluconeogenic genes, including phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), and PGC-1α, were measured by quantitative real-time RT-PCR and normalized to β-actin expression. Mix potently stimulated the expression of PEPCK, G6Pase, and PGC-1α; insulin suppressed Mix-stimulated expression of PEPCK (by 88%), G6Pase (by 86%), and PGC-1α (by 81%) (Fig. 8B). LCN13 alone also inhibited the expression of these genes (Fig. 8B). Together, these data suggest that LCN13 is able to directly suppress HGP.
To determine whether depletion of endogenous LCN13 increases HGP, anti-LCN13 antibody was added directly into culture medium to neutralize LCN13 secreted from primary hepatocyte cultures. Neutralization of endogenous LCN13 further increased Mix-stimulated HGP by 37% (Fig. 8C). These data suggest that LCN13 regulates HGP in an autocrine/paracrine fashion.
DISCUSSION
In this study, we showed that LCN13 was expressed by multiple cell types and secreted into the bloodstream. Therefore, LCN13 may regulate glucose metabolism and other cellular activities in an autocrine/paracrine manner as well as in an endocrine fashion. We provided multiple lines of evidence showing that LCN13 functions as a novel endogenous insulin sensitizer. First, obesity-induced insulin resistance was associated with a marked reduction in circulating LCN13 levels. Second, restoration of circulating LCN13 by three distinct approaches (e.g., LCN13 transgenic expression, LCN13 adenoviral infection, and administration of bacterium-produced LCN13 protein) similarly improved hyperglycemia, hyperinsulinemia, insulin resistance, and glucose intolerance in type 2 diabetes. Third, in cultured adipocytes, LCN13 directly enhanced insulin signaling and insulin stimulation of glucose uptake. LCN13 also suppressed hepatic glucose production in primary hepatocyte cultures. These observations suggest that LCN13 sensitizes insulin response. LCN13 deficiency is likely to contribute to insulin resistance, hyperglycemia, and glucose intolerance in type 2 diabetes.
LCN13 appears to target multiple cell types, including adipocytes and hepatocytes. Interestingly, LCN13 not only increased basal glucose uptake in cultured adipocytes but also directly suppressed hepatic glucose production in primary hepatocyte cultures in the absence of insulin. These observations suggest that LCN13 is able to regulate glucose metabolism by an additional insulin-independent mechanism.
The molecular mechanism of LCN13 action remains unclear. LCN13 is predicted to contain a central cavity based on its amino acid sequences. Other lipocalin superfamily members are able to bind to various hydrophobic and bioactive small molecules via their central pockets (18, 26). We speculate that LCN13 may also bind to a distinct subset of bioactive small molecules via its central cavity and may serve as a carrier to regulate the transportation, stability, release, and/or activity of these bioactive molecules. These hydrophobic small molecules in turn regulate insulin sensitivity and glucose metabolism in animals. Interestingly, LCN13 directly enhanced insulin signaling and insulin-stimulated glucose uptake in cultured adipocytes. LCN13 also directly suppressed glucose production in primary hepatocyte cultures independently of insulin. These observations raise a possibility that LCN13 may bind to its own cognate receptors that may have a cross talk with the insulin pathways. These putative LCN13 receptors may also mediate LCN13 suppression of hepatic glucose production. Two isoforms of LCN13 were detected both inside cells and in the bloodstream. These two isoforms are likely to result from a differential proteolytic cleavage of a common LCN13 precursor and may activate the putative LCN13 receptors with different potencies.
LCN13 shares a high similarity in amino acids with several other lipocalin family members, including LCN1 (58%), LCN3 (58%), LCN4 (54%), LCN14 (71%), odorant binding protein 2B (OBP2B) (67%), and OBP2A (60%). Like LCN13, these LCN13-related lipocalins may also regulate insulin sensitivity and/or glucose metabolism. It is appealing to hypothesize that LCN13 and LCN13-related lipocalins act coordinately to control insulin sensitivity and glucose metabolism in vivo.
In summary, LCN13 is secreted into the bloodstream by multiple cell types. Circulating LCN13 is markedly reduced in obese animals, and restoration of circulating LCN13 improves hyperglycemia, hyperinsulinemia, and glucose intolerance in type 2 diabetes. LCN13 appears to regulate glucose metabolism by both insulin-dependent and insulin-independent mechanisms. LCN13 or LCN13-related lipocalins may be used to treat insulin resistance and type 2 diabetes.
Acknowledgments
This study was supported by grants DK065122 and DK073601 from the National Institutes of Health and by research award 1-09-RA-156 from the American Diabetes Association. This work utilized the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH 5P60 DK20572), the University of Michigan's Cancer Center (funded by NIH 5P30 CA46592), the University of Michigan Nathan Shock Center (funded by NIH P30AG013283), and the University of Michigan Gut Peptide Research Center (funded by NIH DK34933).
We thank Hong Shen and Lin Jiang for their technical support and for helpful discussions.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Ahima, R. S., and S. Y. Osei. 2008. Adipokines in obesity. Front. Horm. Res. 36:182-197. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, P. A., and K. M. Kendrick. 2006. Mammalian social odours: attraction and individual recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361:2061-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamero, P., et al. 2007. Identification of protein pheromones that promote aggressive behaviour. Nature 450:899-902. [DOI] [PubMed] [Google Scholar]
- 4.Erion, D. M., and G. I. Shulman. 2010. Diacylglycerol-mediated insulin resistance. Nat. Med. 16:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham, T. E., et al. 2006. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354:2552-2563. [DOI] [PubMed] [Google Scholar]
- 6.Halberg, N., I. Wernstedt-Asterholm, and P. E. Scherer. 2008. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 37:753-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil, G. S. 2006. Inflammation and metabolic disorders. Nature 444:860-867. [DOI] [PubMed] [Google Scholar]
- 8.Hui, X., et al. 2009. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J. Biol. Chem. 284:14050-14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloting, N., et al. 2007. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 6:79-87. [DOI] [PubMed] [Google Scholar]
- 10.Law, I. K., et al. 2010. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59:872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, L., et al. 2007. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117:1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.More, L. 2006. Mouse major urinary proteins trigger ovulation via the vomeronasal organ. Chem. Senses 31:393-401. [DOI] [PubMed] [Google Scholar]
- 13.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 14.Postic, C., R. Dentin, and J. Girard. 2004. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 30:398-408. [DOI] [PubMed] [Google Scholar]
- 15.Rui, L., M. Yuan, D. Frantz, S. Shoelson, and M. F. White. 2002. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277:42394-42398. [DOI] [PubMed] [Google Scholar]
- 16.Saltiel, A. R., and C. R. Kahn. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799-806. [DOI] [PubMed] [Google Scholar]
- 17.Schenk, S., and J. F. Horowitz. 2007. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J. Clin. Invest. 117:1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlehuber, S., and A. Skerra. 2005. Lipocalins in drug discovery: from natural ligand-binding proteins to “anticalins.” Drug Discov. Today 10:23-33. [DOI] [PubMed] [Google Scholar]
- 19.Shoelson, S. E., and A. B. Goldfine. 2009. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat. Med. 15:373-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki, K., et al. 2004. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene 339:49-59. [DOI] [PubMed] [Google Scholar]
- 21.Tarantino, G., G. Saldalamacchia, P. Conca, and A. Arena. 2007. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J. Gastroenterol. Hepatol. 22:293-303. [DOI] [PubMed] [Google Scholar]
- 22.Taube, A., K. Eckardt, and J. Eckel. 2009. Role of lipid-derived mediators in skeletal muscle insulin resistance. Am. J. Physiol. Endocrinol. Metab. 297:1004-1012. [DOI] [PubMed] [Google Scholar]
- 23.Yan, Q. W., et al. 2007. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56:2533-2540. [DOI] [PubMed] [Google Scholar]
- 24.Yang, Q., et al. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356-362. [DOI] [PubMed] [Google Scholar]
- 25.Zhou, Y., L. Jiang, and L. Rui. 2009. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J. Biol. Chem. 284:11152-11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, Y., and L. Rui. 2010. Major urinary protein regulation of chemical communication and nutrient metabolism. Vitam. Horm. 83:151-163. [DOI] [PMC free article] [PubMed] [Google Scholar]