Abstract
The genomic binding sites of Polycomb group (PcG) complexes have been found to cluster, forming Polycomb “bodies” or foci in mammalian or fly nuclei. These associations are thought to be driven by interactions between PcG complexes and result in enhanced repression. Here, we show that a Polycomb response element (PRE) with strong PcG binding and repressive activity cannot mediate trans interactions. In the case of the two best-studied interacting PcG targets in Drosophila, the Mcp and the Fab-7 regulatory elements, we find that these associations are not dependent on or caused by the Polycomb response elements they contain. Using functional assays and physical colocalization by in vivo fluorescence imaging or chromosome conformation capture (3C) methods, we show that the interactions between remote copies of Mcp or Fab-7 elements are dependent on the insulator activities present in these elements and not on their PREs. We conclude that insulator binding proteins rather than PcG complexes are likely to be the major determinants of the long-range higher-order organization of PcG targets in the nucleus.
Transgenes containing Drosophila melanogaster Polycomb response elements (PREs) often show a remarkable degree of pairing-enhanced silencing, the increased repression observed when the transgene is present in two allelic copies (19). A PRE can also silence in trans a reporter gene lacking its own PRE but inserted at the same site on the homologous chromosome (33). Both are consistent with a looping model proposed to explain how the bxd PRE can produce H3K27 trimethylation and silencing of the Ubx promoter many tens of kilobases distant (18). According to this, Polycomb group (PcG) complexes bound to a PRE can contact and interact with chromatin regions in their physical neighborhood, whether on the same chromatin strand or on a separate strand.
More remarkable is the apparent ability of some PRE-containing DNA fragments to interact in trans with copies of the same construct inserted at remote sites, even on different chromosomes, again resulting in enhanced repression. At first sight, this appears to be similar to the homologous pairing effect except that something other than homologous chromosome pairing brings the two remote copies together. This behavior has been observed with constructs containing either of two PcG-binding elements from the bithorax complex, Mcp and Fab-7 (1, 26, 37), and it has been frequently attributed to a general tendency of PcG complexes bound to one genomic site to interact with PcG complexes bound at other sites in the genome.
The idea that PcG-binding sites in the nucleus might tend to cohere is consistent with the observation that, in flies or mammals, staining of diploid nuclei with antibodies against PcG proteins reveals a small number of foci, relative to the many hundreds of binding sites known to be present in the genome. That the PcG complexes might drive this association is supported by the finding that the Drosophila Antennapedia (Antp) gene and the Abdominal-B (Abd-B) gene, several megabases distant from one another, colocalize when both are repressed but not when one of the two is transcriptionally active (12). Furthermore, it has been proposed that this interaction is mediated by RNA interference (RNAi) mechanisms (12), implying their participation in PcG repressive complexes and providing an attractive link to the role of the RNAi machinery in heterochromatin formation (11). In this view, then, PcG complexes are inherently “sticky,” and random or RNAi-mediated encounters in the nucleus would cause PcG binding sites to aggregate and, as in pairing-enhanced silencing, result in stronger or more stable repression.
Against the idea that PcG complexes are intrinsically cohesive is the observation that not all PREs have been found to trans-interact with remotely inserted copies. This has never been observed with constructs containing the powerful bxd PRE silencer although these constructs can exhibit pairing-dependent repression when made homozygous (33; V. Pirrotta, unpublished observations). Both Mcp and Fab-7 elements have been shown to contain two distinct and separable activities: a PRE activity and an enhancer-blocking insulator/boundary activity (13, 15, 38). This raised the possibility that the ability of these elements to enter into long-distance interactions might be mediated by their insulator component. Consistent with this, the bxd PRE 640-bp fragment, although incapable of remote trans interactions by itself, acquired this property when associated with the gypsy Su(Hw) insulator element (33).
Here, we examine the relationship between PRE activity and the ability to mediate long-distance trans interactions, comparing the strong silencer bxd PRE and the weak silencer Mcp. The results show that it is not the PRE that mediates trans interactions but an insulator activity closely associated with the Mcp or Fab-7 PREs. We show in addition that these insulators, but not the PREs, mediate the ability of transgenic insertions to become closely juxtaposed with one another and with the corresponding endogenous element in the nucleus.
MATERIALS AND METHODS
Transgene constructs.
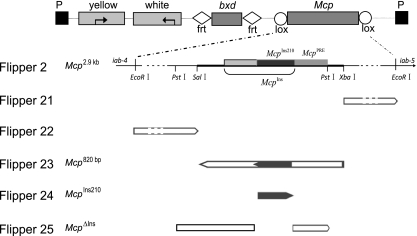
The Flipper constructs were assembled on the pC4YM plasmid backbone. Flipper 2Mcp-bxd was described in Gohl et al. (10).Unique XhoI and NotI sites were used to introduce the various bxd-Mcp cassettes. The bxd PRE is in all cases the 661-bp NdeI-PstI fragment used by Sigrist and Pirrotta (33), flanked by Flp recognition target (FRT) sites. The following Mcp fragments flanked by LoxP sites were tested: Flipper 2 contains a 2.9-kb EcoRI fragment (26); Flipper 21 contains a 0.9-kb fragment extending from XbaI to the distal EcoRI site; Flipper 22 contains a 1.2-kb fragment extending from the SalI site to the proximal EcoRI site; Flipper 23 contains the central 0.8-kb SalI-XbaI fragment; Flipper 24 contains a 210-bp fragment previously described by Kyrchanova et al. (22) and referred to here as McpIns210 (where Ins indicates insulator); Flipper 25 contains McpΔIns, a 755-bp PstI-PstI fragment nearly identical to the 0.8-kb fragment in Flipper 23 but lacking the 210-bp fragment of Flipper 24. The orientation of the Mcp fragments was such that the end normally adjacent to iab4 is closer to the bxd PRE. In Flipper 23Mcp-bxd, Mcp is inserted in the opposite orientation. The McpIns210 insulator fragment was obtained by PCR amplification of the DNA fragments between primers 5′-AAACTTAACTCAGACTTGG-3′ and 5′-CCCAATCGTTGTAAGTGT-3′. As a result, the McpIns210 fragment corresponds to Drosophila genomic chromosome 3R (Chr3R) nucleotides 12694959 to 12695169. The Mcp core carrying a deletion of the 210-bp insulator (McpΔIns) was made by ligation of two fragments obtained by PCR amplification between 5′-GACTTAAATTGATTTAAAG-3′ and 5′-AATCCAAGTCTGAGTTAAG-3′ and between 5′-CTGCAGTCAAACGTCACA-3′ and 5′-CTTACAACGATTGGG-3′. These fragments were cloned between Lox sites and inserted into the FRT-flanked bxd PRE cassette and ligated as XhoI-NotI fragments upstream of the mini-white promoter into the pC4YM plasmid.
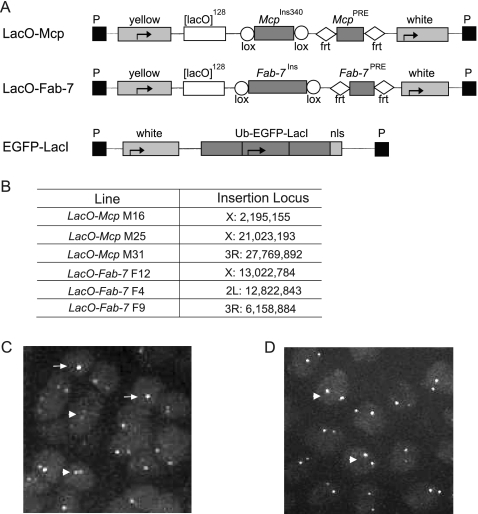
To assemble the LacO-Mcp and LacO-Fab-7 constructs, the insulator and PRE portions of Mcp and Fab-7 were PCR-amplified from BX-C clone BAC R24L18 (obtained from BACPAC Resources Center [http://bacpac.chori.org/]), using the following PCR primers: MI+, GATACTGCAGCTCAGAGTACATAAGCG, and MI−, TGAGGGGCCCAAGCGTTGTAAGTGTG, for the Mcp340 insulator fragment; MP+, CTTGGGATCCTCATGTGTTAGTGCGTGAG, and MP−, ACACAAACGCATCTGCAGTC, for the Mcp PRE; FI+, CAACTGCAGTGAAGACACGAAC, and FI−, CGACGTGAGCGACCGAAACTC, for the Fab-7 minimal insulator; and FP, CGGGGATCCGAGTTTCGGTCGCTCAC, and FP−, GAACTGCAGATGTCGGCAATTCGGATTCC, for the Fab-7 PRE. As a result, the McpIns340 fragment corresponds to genomic Chr3R nucleotides 12694830 to 12695169, the McpIns210 fragment corresponds to Chr3R nucleotides 12694959 to 12695169, and the McpPRE fragment corresponds to Chr3R nucleotides 12695173 to 12695338. All genomic sequences are taken from FlyBase Genome Release 5. The Fab-7Ins fragment corresponds to genomic Chr3R nucleotides 12724265 to 12725516, and the Fab-7PRE fragment corresponds to Chr3R nucleotides 12725499 to 12725765. The amplified fragments were ligated into LoxP and FRT cassette plasmids, and the resulting plasmids were sequenced to verify the inserted sequence. Fragments containing the mini-white gene, the insulator flanked by LoxP, and PRE flanked by FRT were assembled into pBlueScript. The tandem array of 128 copies of LacO was cut from pAFS150 (a gift from J. Vazquez) and inserted into plasmid C4-yellow, and this plasmid was used to accept the LoxP-flanked insulator part, FRT-flanked PRE part, and mini-white gene.
Plasmid pBSKS-Ubq-mRFP-LacI-NLS (where NLS is nuclear localization signal) containing the LacI repressor fused to monomeric red fluorescent proteins (mRFPs) and driven by the ubiquitin (Ubq) promoter was kindly provided by A. Csink (35). The mRFP sequence in this plasmid was replaced by an enhanced green fluorescent protein (EGFP) PCR-amplified fragment cut by ClaI-BamHI. The KpnI-SacI fragment of Ubq-EGFP-LacI-NLS was inserted into pCaSpeR4.
Fly stocks.
Transgenic fly lines were made according to standard procedures (34). Southern blot hybridization was used to verify that the lines contained a single insert, and inverse PCR was used to identify the exact insertion sites. The various deletion derivatives were established with the help of Flipase and Cre recombinase-producing stocks (32), as previously described in Gohl et al. (9), and were verified by PCR analysis. For colocalization studies, two transgene lines on different chromosomes were crossed together through double balancers. In the case of lines with insertions on the same chromosome, the two insertions were recombined to obtain a cis arrangement. PCR was used to verify the presence of both transgenes.
Microscopy.
After crosses of transgenic Mcp or Fab-7 flies with LacI-EGFP flies, the larvae were raised at 18°C and supplemented with active dry yeast. Third-instar larvae were rinsed and dissected in Gibco Schneider's Drosophila medium (Invitrogen Co.). The dissected eye and wing imaginal discs were aligned on a coverslip bottom dish (MatTek Co.) with a drop of Drosophila medium and then covered with a coverslip. Z-stack images were taken with a DeltaVision Image Restoration Microscope system (Applied Precision Instrument LLC, Issaquah, WA), using a 100×/1.35 UplanApo objective, deconvoluted, and processed with the SoftWoRx software (Applied Precision Instruments). The dots in each nucleus were scored, with one dot indicating colocalization and two nonoverlapping dots (center-to-center distance greater than 0.3 μm) indicating no colocalization. Chi-square tests were used for pairwise comparison of any two data sets in each category. All statistical analysis was done using the software JMP (SAS Institute Inc.).
3C analysis.
Chromosome conformation capture (3C) experiments were done as described previously (7, 14) with few modifications. Brain and attached imaginal discs were dissected from 30 third-instar larvae in 1× PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) containing 10% fetal calf serum. The tissue was then fixed in 2% fresh paraformaldehyde-phosphate buffered saline (PBS) for 10 min at room temperature. The cells were lysed in lysis buffer (10 mM Tris-HCl, 10 mM NaCl, 0.2% NP-40, pH 8.0, with Roche protease inhibitor cocktail freshly added) on ice for 10 min, followed by 20 strokes of a Dounce homogenizer. The nuclei were recovered and washed with 1.2× NEB3 buffer (120 mM NaCl, 60 mM Tris-HCl, 12 mM MgCl2, 1.2 mM dithiothreitol, pH 7.9) and then resuspended in 400 μl of 1.2× NEB3 buffer with 0.3% sodium dodecyl sulfate (SDS). After samples were shaken for 2 h at 37°C, Triton X-100 was added to 1.8%, and samples were shaken for another 2 h at 37°C. One-third of the nuclear suspension (160 μl; ∼10 larvae) was used for digestion with EcoRI or HindIII (200 units at 37°C overnight). SDS was added to a concentration of 1.5%, and the solution was incubated at 65°C for 25 min to inactivate the enzyme. Eighty microliters of 10× NEB ligation buffer was added and H2O to 950 μl. Triton X-100 (1%) was used to neutralize the SDS at 37°C for 1 h. The DNA was ligated with 8 μl of ligase (400 U/μl; New England BioLabs) at 16°C for 4.5 h and then for 1 h at room temperature. The 3C template DNA was then de-cross-linked overnight at 65°C and extracted with phenol-chloroform. For PCR analysis, the purified 3C DNA was linearized by digestion with a second restriction enzyme, generally PstI, which cut outside the sequence to be PCR amplified.
3C primers were designed for the regions flanking the religated restriction sites, close to the insertion sites of transgenes. The primers were tested for specificity and efficiency. As a control for the cross-linking and ligation procedure, we used primers 1K and 2K. These lie on adjacent EcoRI fragments in the Brk gene and point in the same direction, close to the EcoRI sites. Since the K2/K3 primer pair yields efficient PCR product with 3C templates, it was chosen as an internal positive control. PCRs were carried out with the following regime: 95°C for 8 min, followed by 36 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final step at 72°C for 10 min. All 3C PCR products were cut out from the gels, purified, and sequenced to confirm that they corresponded to chimeric sequences coming from the insertion sites of the two transgenes.
ChIP analysis.
For chromatin immunoprecipitation (ChIP) analysis, chromatin was prepared from approximately 300 third-instar larvae. The larvae were washed in 0.12 M NaCl-0.04% Triton X-100 and then frozen in liquid nitrogen. Frozen larvae were first ground with mortar and pestle and then transferred to a Dounce homogenizer in 5 ml (1.8% formaldehyde, 10 mM HEPES [pH 7.9], 1 mM EGTA, 100 mM NaCl) and given 10 strokes. After neutralization with glycine to 0.125 M the material was washed with 1× phosphate-buffered saline solution, then with ChIP wash buffer A (10 mM HEPES [pH 7.6], 10 mM EGTA [pH 8.0], 0.5 mM EGTA [pH 8.0], 0.25% Triton X-100) for 10 min, and finally with ChIP wash buffer B (10 mM HEPES [pH 7.6], 200 mM NaCl, 1 mM EDTA [pH 8.0], 0.5 mM EGTA [pH 8.0], 0.01% Triton X-100) for 10 min. The pelleted material was stored at −80°C until used. Sonication and ChIP were done essentially as described in Schwartz et al. (30) and analyzed by quantitative real-time PCR. The anti-CP190 antibodies were rabbit polyclonal raised against a peptide containing amino acids 606 to 742 of CP190, fused to glutathione S-transferase (GST). Anti-dCTCF antibody was described by Gerasimova et al. (8) and kindly provided by V. Corces. Anti-PC was described by Horard et al. (17).
PCR primers used for analyses.
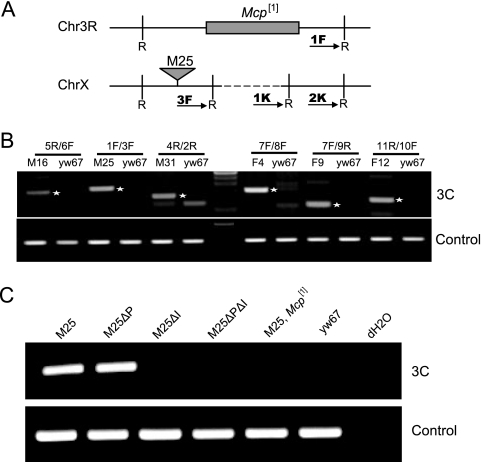
Primers used to analyze the 3C experiments are as follows, written from 5′ to 3′: 1K, CACGGGAAAAACTACTGAAAG; 2K, AAGCCGCAGGAGTTTCTAAC; 1F, GCATGGCGGCATAATTTCTG; 2R, AGCTCATTAGCCGTTAGTTTC; 3F, CTCTCTTGGCCTCGATTAAAC; 4R, CGCCGCACTTTTGGTCCAT; 5R, TAATCCGCTTTACCCAGTAAG; 6F, TGGCGGCAAAGACATTGATG; 7F, TTCCCCCAACCATGCACAC; 8F, CTGCCGAATCGGTTGAAAGG; 9R, ACGTTCTCTAACACTGCAGC; 10F, CAGGCATGCAAGCTAGCTTC; 11R, ACCACCTCAGATACACCTTC.
Primers used for the analysis of the ChIP experiments are as follows: Mcp+, ATAAGGGCTTTTCTGGGGAAG; Mcp−, TGTAAGGAGGAAGACTACATC; Fab7+, AGAGAGCGACTGCTTGAATG; Fab7−, GGGTAAGTAACGGTATTTAGG; W+, ATGCCACGACATCTGACC; W−, TGCCCAAGAAAGCTACCC; BP+, GCCATAACGGCAGAACCAAAG; BP−, ATGAGGCCATCTCAGTCGC; Ubx+54, CCGCTGATAATGTGGATAA; Ubx-177, CACCCCGATAAACTTAAAC; CG+, CGTCTAGTGGTTGATTCCAT; CG−, CAGGACCAAAAGTTTAGTGG.
RESULTS
Comparison of the bxd PRE and the Mcp PRE.
We began by comparing the properties of the bxd PRE and of the Mcp PRE in the same genomic context. For this purpose, we assembled a construct called FlipperMcp-bxd in which the 661-bp bxd PRE and different fragments from the Mcp region are flanked by FRT and LoxP sites, respectively, in a vector containing the two marker genes yellow and mini-white (Fig. 1). Hence, for a given FlipperMcp-bxd insert, bxd and Mcp can be individually deleted in situ with the Flp and Cre recombinases to assess the role of each PRE. The results obtained with the different Mcp fragments (Flipper 21, 22, and 23) show that the essential functions are contained in the 820-bp core fragment. For brevity, we will describe in detail only the experiments done with Flipper 23Mcp-bxd. The results for 13 independent lines with inserts on chromosome 3 are summarized in Table 1. Based on their mini-white phenotypes (Table 1, second and third columns), they could be classified into two groups: (i) 2 lines show pairing-dependent silencing of mini-white, i.e., eye pigmentation is weaker in homozygotes than in heterozygous siblings; (ii) 11 lines have no eye pigmentation at all in hetero- as well as in homozygous conditions; they could be isolated only because of their yellow+ phenotype, which is either uniform or variegated.
FIG. 1.
Maps of the Flipper 2 construct and derivatives. The Flipper 2 transposon construct contains the intronless yellow gene with wing and body enhancers and the mini-white gene as markers and reporters. The bxd PRE 661-bp fragment is flanked by LoxP sites, and the 2.9-kb EcoRI Mcp fragment is flanked by FRT sites to allow independent excision of either element. The Flipper 21 and 22 constructs contain the flanking regions, and Flipper 23 contains the core 800-bp SalI-XbaI Mcp fragment that includes both the PRE and the insulator activities. For Flipper 2, 21, 22, and 23, the orientation of the fragment is indicated by an arrow. In the Flipper 24 and 25 constructs, McpIns210 is the minimal Mcp insulator fragment and McpΔIns is the 755-bp PstI-PstI Mcp core region from which the 210-bp insulator has been deleted. The Mcp segments used in the constructs are indicated in the map of the Mcp genomic region at the bottom.
TABLE 1.
Flipper 23 phenotypes and interactionsa
| Line | Flipper 23Mcp-bxd |
Flipper 23bxd |
Flipper 23Mcp |
Flipper 23bxd/ FlipperMcp | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P/+ eye color | P/P eye color | No. of interactions with Mcp testers/no. of tester lines | P/+ eye color | P/P eye color | No. of interactions with Mcp testers/no. of tester lines | P/+ eye color | P/P eye color | No. of interactions with Mcp testers/no. of tester lines | ||
| 84.7.3 | W | W | 3/12 | pY | W | 0/3 | Or | dOr | 1/3 | W |
| 84.14.1 | W | W | 4/12 | W | W | 1/3 | Or | dOr | 2/3 | W |
| 84.32.3 | W | W | 4/12 | W | W | 0/2 | Or | dOr | 1/2 | W |
| 84.63.1 | W | W | 4/12 | W | W | 0/2 | Or | dOr | 2/2 | W |
| 84.77.1 | W | W | 6/12 | pY | W | 0/4 | NO | NO | ND | ND |
| 84.80.5 | pY | W | 4/12 | ND | ND | ND | ND | ND | ND | ND |
| 84.81.1 | W | L | 6/12 | W | L | 0/2 | Or | L | 0/2 | L |
| 84.95.2 | W | L | 6/10 | W | L | 0/4 | Or | L | 1/4 | L |
| 84.95.5 | pY | W | 4/10 | pY | W | 0/3 | dOr | R | 1/3 | W |
| 84.98.2 | W | W | 6/11 | pY | W | 0/4 | Or | dOr | 0/4 | W |
| 84.110.5 | W | W | 5/12 | W | L | 0/5 | Or | dOr | 1/5 | L |
| 84.131.4 | W | W | 5/12 | pY | W | 0/5 | Or | dOr | 2/5 | pY |
| 84.146.1 | W | W | 6/12 | W | W | 0/3 | pY | dOr | 2/3 | W |
Eye color of PRE heterozygotes (P/+) and PRE homozygotes (P/P) was determined in 13 independent lines with insertions on chromosome 3. Phenotypes of the Flipper 23 line, of Flipper 23 with a deletion of Mcp or bxd, and of the trans combination of Flipper 23bxd and FlipperMcp are shown. W, white; pY, pale yellow; Or, orange; dOr, dark orange; R, red; L, lethal; ND, not done; NO, not obtained.
These results show that, together, the two PREs act as a powerful silencer of the mini-white reporter. The yellow reporter is less sensitive to PRE silencing, as has been previously observed (26). To assess the contribution of each of the two PREs to mini-white silencing, we deleted the bxd PRE (yielding Flipper 23Mcp) or the Mcp PRE (yielding Flipper 23bxd). In the Flipper 23bxd flies, more than half of the lines (7/12) remain white-eyed while the others become white when homozygous (Table 1, fifth and sixth columns). None of the Flipper 23Mcp derivatives are white-eyed either when hetero- or homozygous. In all cases, loss of the bxd PRE greatly decreases the pairing-dependent effects of the smaller Mcp element in Flipper 23 (Table 1), though the larger Mcp in Flipper 2 is still strongly pairing dependent (reference 26 and data not shown). These results indicate that, at all insertion sites tested, the bxd PRE is a more potent silencer than the Mcp core fragment.
To ask whether Flipper 23bxd can interact in trans with Flipper 23Mcp on the paired homologue, the ΔMcp and Δbxd derivatives were tested in trans to each other, and the resulting eye color was compared to that of Flipper 23bxd/+ and Flipper 23Mcp/+ flies (Table 1, compare last column with fifth and eighth columns). If the two constructs act independently, we expect that, for a given insertion site, the eye colors of the two inserts would be approximately additive. The results (Table 1, last column), show that, except for cases in which the eye in one of the heterozygotes was already white, the reduction in eye color in flies bearing the trans combination was greater than in heterozygotes bearing one PRE only. This is consistent with the conclusion that, when inserted at a homologous position, Mcp and bxd PREs interact efficiently with each other.
Long-distance interactions.
Two remote insertions of an Mcp-containing transposon are considered to trans-interact if the level of eye pigmentation in the trans-heterozygote is lower than or equal to that seen in either single heterozygote (26). All Flipper 23Mcp-bxd inserts presented in Table 1 can participate in long-distance interactions with a panel of 12 Mcp-containing insertions on chromosome 3 previously reported to be good partners for long-distance interaction (26). Each Flipper 23Mcp-bxd insert shows interactions with at least 3 of the 12 tester lines (Table 1). However, when we compared the long-distance interactions of the Flipper 23Mcp and Flipper 23bxd derivatives, the Flipper 23bxd derivatives had generally lost the ability to interact in trans with distant Mcp testers: only one cross of 40 tested gave an interaction (Table 1). In contrast, only 2 of 11 Flipper 23Mcp lines failed to trans-interact with the Mcp testers used (Table 1, compare results for Flipper 23Mcp and Flipper 23Mcp-bxd). These results support the conclusion that, while the bxd PRE is a powerful silencer, it lacks an activity responsible for mediating long-distance trans interactions. This activity is present in the 800-bp Mcp fragment even though this element contains a much weaker silencing activity. We conclude that the ability of Mcp to interact with other Mcp constructs inserted at remote genomic sites may not be due to the PRE itself but to an associated function present in the Mcp fragment but absent in the bxd PRE.
The insulator/boundary component of Mcp is the trans-interacting element.
Depending on the insertion site, both the bxd PRE and the larger Mcp element can interact with an allelic copy (pairing-dependent interaction) which is juxtaposed by the pairing of homologous chromosomes. However, the functional difference between Mcp and bxd PRE is illustrated by the fact that long-distance trans interactions between remote copies have never been observed among constructs containing the latter even when large fragments of 1.5 to 6 kb were used (V. Pirrotta, unpublished). However, the bxd PRE was notably able to enter into trans interactions when a gypsy Su(Hw) insulator was incorporated into the construct (33). We reasoned therefore that the Mcp fragment might contain a similar insulator activity responsible for the trans interactions. Although Mcp does not bind SU(HW), the core region has been shown to contain an insulator activity that can be separated from the PRE activity (13).
To test the role of the Mcp insulator, we made two new constructs (Fig. 1). In the Flipper 24Mcp Ins210-bxd construct, the bxd PRE, flanked by FRT sites, was inserted next to the 210-bp core insulator from the Mcp element (22), flanked by LoxP sites. We obtained 10 Flipper 24Mcp Ins210-bxd lines on the third chromosome and mapped their insertion sites. In these lines the bxd PRE repressed white expression, as shown by the fact that its deletion darkened the eye color, while deletion of McpIns210 had little effect on eye color (not shown).
To test trans interaction, we again used the panel of 10 Mcp tester lines on the third chromosome (26) and crossed them with 10 Flipper 24Mcp Ins210-bxd candidate lines in all possible pairwise combinations. Eight out of 10 tested Flipper 24Mcp Ins210-bxd lines displayed trans-silencing interactions with two to four of the Mcp tester lines (Table 2, fourth and fifth columns). In general, long-distance interactions were more likely to be observed when the insertions were closer to one another, but in some cases they were found between inserts located on opposite arms of the third chromosome (e.g., line 66D12 and 99B tester line). All trans interactions were lost after excision of the McpIns210 insulator.
TABLE 2.
Flipper 24 and 25 phenotypes and interactions
| Linea | P/+ eye colorb | P/P eye colorb | No. of interactions with Mcp testers/no. of tester linesc |
|---|---|---|---|
| Flipper 24 McpIns210-bxd | |||
| 100E3 | Or | pY | 3/10 |
| 100E3 ΔMcpIns210 | pYv | W | 0/10 |
| 69A | pYv | W | 4/10 |
| 69A ΔMcpIns210 | pYv | W | 0/10 |
| 95F4 | W | W | 0/10 |
| 95F4 ΔMcpIns210 | W | W | 0/10 |
| 82A3 | pY | W | 3/10 |
| 82A3 ΔMcpIns210 | pY | W | 0/10 |
| 66D12 | Yv | W | 3/10 |
| 66D12 ΔMcpIns210 | pYv | W | 0/10 |
| 70A2 | Yv | W | 0/10 |
| 70A2 ΔMcpIns210 | pYv | W | 0/10 |
| 94E8 | W | W | 3/10 |
| 94E8 ΔMcpIns210 | W | W | 0/10 |
| 85B7 | Yv | W | 2/10 |
| 85B7 ΔMcpIns210 | Yv | W | 0/10 |
| 65D5 | pYv | L | 3/10 |
| 65D5 ΔMcpIns210 | pYv | L | 0/10 |
| 88F3 | W | W | 3/10 |
| 88F3 ΔMcpIns210 | W | W | 0/10 |
| Flipper 25 McpΔIns-bxd | |||
| 83B4 | Yv | L | 0/10 |
| 83B4 ΔMcpΔIns | Or | L | 0/10 |
| 70F4 | Y | L | 0/10 |
| 70F4 ΔMcpΔIns | Y | L | 0/10 |
| 100E3 | Yv | W | 0/10 |
| 100E3 ΔMcpΔIns | Yv | W | 0/10 |
| 97E5 | W | W | 0/10 |
| 97E5 ΔMcpΔIns | W | W | 0/10 |
| 72D10 | pY | W | 0/10 |
| 72D10 ΔMcpΔIns | Y | W | 0/10 |
| 100E3-1 | pYv | W | 0/10 |
| 100E3-1 ΔMcpΔIns | Y | W | 0/10 |
| 89B3 | Wv | L | 0/10 |
| 89B3 ΔMcpΔIns | Y | L | 0/10 |
| 92F12 | Y | L | 0/10 |
| 92F12 ΔMcpΔIns | Y | L | 0/10 |
| 86A4 | dY | W | 0/10 |
| 86A4 ΔMcpΔIns | Y | W | 0/10 |
Lines are designated by their cytological insertion sites. Each line is followed by its derivative in which the McpIns210 or McpΔIns fragment has been excised (ΔMIns210 or ΔMcpΔIns, respectively).
Eye color of PRE heterozygotes (P/+) and PRE homozygotes (P/P) are indicated as follows: W, white; Y, yellow; pY, pale yellow; Or, orange; L, lethal. The superscript “v” indicates variegation.
We also constructed a Flipper 25Mcp ΔIns-bxd transgene containing the 660-bp bxd PRE as before and an Mcp element that included the Mcp PRE but from which the 210-bp insulator fragment was deleted (Fig. 1). We obtained nine transgenic lines on the third chromosome, in which the PREs repressed white expression (Table 2, seventh and eighth columns). In all possible pairwise combinations with the Mcp tester panel, we observed no trans interactions (Table 2, 9th and 10th columns), and this was not changed by deletion of the insulator-less McpΔIns. These results suggest that the Mcp insulator is essential for trans interactions while neither the Mcp PRE nor the bxd PRE can mediate such interactions.
Colocalization of Mcp insulator constructs.
The ability of two remote constructs to affect one another's expression strongly suggests that they are able to make contact in the nucleus. To demonstrate this physical interaction, we used two methods. In one, colocalization is visualized by in vivo imaging of fluorescence-tagged loci (5). For this purpose we made constructs containing a 340-bp Mcp insulator fragment flanked by Lox sites and a 138-bp Mcp PRE fragment flanked by FRTs (Fig. 2A). We used the 340-bp insulator fragment because it has a stronger insulator activity than the 210-bp fragment used in the experiment described in the preceding section (13, 22). To visualize the insertion site, the transposon contained 128 copies of the Lac operator (LacO), and the flies were crossed with a line expressing the LacI repressor fused to EGFP fluorescent protein, driven by the ubiquitin promoter. Three LacO-Mcp lines were obtained (Fig. 2B) and were crossed to test all pairwise combinations.
FIG. 2.
Fluorescent tagging of Mcp and Fab-7 components. (A) Structure of the reporter constructs. The Mcp insulator fragment is flanked by LoxP sites, and the Mcp PRE is flanked by FRT sites. A parallel construct contains a similar arrangement of the Fab-7 insulator and the Fab-7 PRE. The constructs utilize the yellow and mini-white genes as markers. The tandem array of 124 lacO sequences is used to bind the lacI repressor fused to EGFP expressed from a different construct driven by the ubiquitin promoter. (B) Mcp and Fab-7 lines obtained and their insertion sites determined by inverse PCR. (C) Representative image of eye imaginal disc nuclei showing one-dot (arrows) and two-dot (arrowheads) nuclei from the F9 F9ΔP-M31ΔP line (ΔP, deletion of PRE). (D). Image of eye membrane cell nuclei showing the two-dot (arrowhead) nuclei from F9ΔI-M31ΔI line (ΔI, deletion of insulator). The images show a single z-axis slice, while the score was obtained by examining all z-axis slices individually.
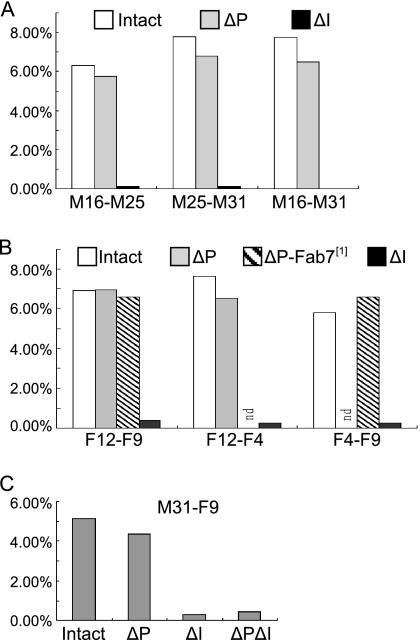
Fluorescence image stacks of eye or wing discs of larvae carrying two transposon insertions were obtained to assemble a three-dimensional representation of the nuclei, and each nucleus was scored as one dot (colocalization) or two dots (no colocalization) when the two signals were nonoverlapping (Fig. 2C). The results show a rather low frequency of colocalization, ranging from 6% to 8% of the nuclei (Fig. 3A). However, when the insulator element was excised, colocalization dropped to 0.1 to 0.2% and chi-square tests indicate a P of <0.0001. Deletion of the insulator from only one of the two transgenes caused the same drop in colocalization as the deletion from both transgenes (data not shown). In contrast, excision of the PRE fragment from one or both transgenes had no appreciable effect on the frequency of colocalization.
FIG. 3.
Frequency of colocalization of Mcp and Fab-7 transgenes. (A) Interactions between Mcp transgenes. For each of the pairwise combinations between three lines bearing the Mcp construct, the histogram shows frequencies of colocalization (one-dot nuclei) for the starting lines (Intact), for the lines after PRE deletion (ΔP), or for the lines after insulator deletion (ΔI). (B) Interactions between Fab-7 transgenes. The histogram shows the frequencies of colocalization for the pairwise combinations between three lines bearing the Fab-7 construct. In both sets of experiments, the frequency of colocalization drops more than 30-fold when the insulator is deleted but is not affected by deletion of the PRE (data not shown). Some combinations were tested in a background with a deletion of the endogenous Fab-7 element (Fab-71), showing that it is not required for the trans interactions. (C) Interaction between Mcp and Fab-7 transgenes. The histograms show the frequencies of colocalization between the F9 and the M31 transgenes before or after deletion of the PRE or insulator segments. Numerical data are available upon request.
A parallel construct was made using fragments containing the insulator and PRE portions of the Fab-7 element (Fig. 2A). Colocalization between different insertions of this construct was observed at a frequency similar to that seen with the Mcp construct (Fig. 3B). In this case also, excision of the insulator caused loss of the colocalization while excision of the PRE fragment had no effect. To ask if the colocalization that we detect between Fab-7 transgenes is dependent on the endogenous element, we tested two pairs of inserts, F4ΔP; F9ΔP and F12ΔP; F9ΔP (where ΔP indicates deletion of the PRE), in a genetic background homozygous for the Fab-71 deletion of the endogenous element. No significant difference was observed in the frequency of colocalization. Thus, although, as shown by the 3C experiments below, the transgenes do interact with the endogenous copy, this is not a prerequisite for interaction between transgenic copies, as was also shown by Bantignies et al. (1).
We also tested whether Mcp inserts could interact with Fab-7 inserts on the same chromosome. The results show that the two inserts do colocalize at a frequency of 5.15%, somewhat lower than that seen between Mcp inserts (Fig. 3C). Deletion of the PRE elements from both constructs gives a slight decrease in the incidence of colocalization to 4.34%, but deletion of the insulator elements reduces it to 0.29%. These results show that a degree of interaction can be observed between Mcp and Fab-7 elements.
Trans interactions are detected by 3C.
To confirm and extend the imaging results, we carried out 3C analysis for physical association between the transgenes (7). We first tested if any one insertion of the Mcp construct was able to interact with the endogenous Mcp element that resides in the bithorax complex. As shown in Fig. 4B, the results were unambiguous: for all three insertion sites tested, insertion of the Mcp construct produced interaction with the endogenous Mcp. Entirely similar results were obtained with the Fab-7 lines. There was no interaction in the absence of the inserted transgene (yw67 host flies). Furthermore, the interaction was dependent on the insulator component and not on the PRE component and was lost when the endogenous Mcp was deleted by the Mcp1 mutation (Fig. 4C).
FIG. 4.
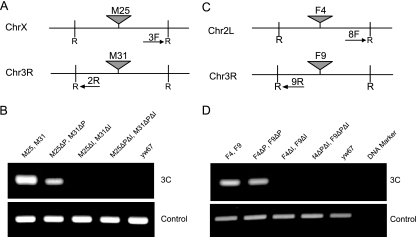
3C assay of trans interactions. (A) Maps of 3C primers used to assay interactions of a typical Mcp transgene with the endogenous Mcp element. Arrows 1F and 3F denote the primer directions. R denotes the EcoRI restriction sites. 1K and 2K are primers for two adjacent restriction fragments used for a control PCR. The map also shows the approximate extent of the Mcp1 deletion of the endogenous Mcp and a transgenic insertion site. A similar scheme was used for the Fab-7 transgene interactions with the endogenous Fab-7. (B) 3C assay between transgene insertion sites and their endogenous partners. The primers and the 3C DNA used for each assay are labeled above each lane, and their sequences are given in the Materials and Methods section. The yw67 host line served as a negative control. The asterisks indicate the bands of the expected molecular weights. The panel shows the reaction with the K1/K2 internal controls for corresponding 3C samples. (C) 3C assay between the M25 line, its derivatives with deletions of PRE (ΔP) and/or the insulator (ΔI), and the endogenous Mcp. For the upper panel, the 3C primer pair 1F/3F was used; for the lower panel, the 3C control primers K1/K2 were used. The 3C DNA from fly lines used for the assay is labeled above each lane.
We then tested the interaction between remote insertions of the Mcp or of the Fab-7 construct. Clear interactions were observed for two of the pairs tested, producing the predicted PCR product (Fig. 5B and D). The identity of the products was confirmed in all cases by excising the bands from the gel and sequencing the DNA fragments. Several other pairs of insertion sites did not give a detectable 3C PCR band. While this may be partly dependent on the choice of primers to detect the 3C interaction, it is likely that the interaction with the endogenous element is stronger than interactions between insertions.
FIG. 5.
3C assay of trans interactions between transgene insertions. Maps of 3C primers used for two Mcp insertions (A) and two Fab-7 insertions (C) are shown. Arrows 3F, 2R, 8F, and 9R denote the primer directions; R denotes the EcoRI restriction sites. (B) 3C assay between the insertion sites of transgenes M25 and M31 and their derivatives using primer pair 2R/3F. The transgene combination used for each assay is labeled above each lane. The lower panel shows the reactions with the control primers K1/K2. (D) 3C assay between the insertion sites of transgenes F4 and F9 and their derivatives using primer pair 8F/9R. The lower panel shows the reactions with 3C control primers K1/K2.
Binding of insulator and PcG proteins.
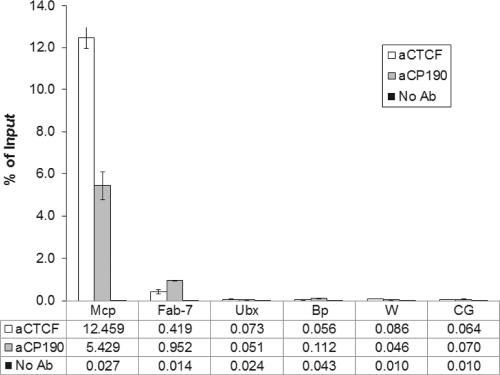
If the insulators of Mcp and Fab-7 are responsible for the trans interactions, the ability of these two elements to interact with one another presupposes that their insulators share some common component. The binding of some insulator proteins to the Mcp and Fab-7 regions has been reported previously (2, 16, 25, 27), and more recently genome-wide ChIP-on-chip analysis of SU(HW), dCTCF, MOD(MDG4), CP190, BEAF, and ZW5 has been carried out by the modENCODE Drosophila Chromatin Consortium (http://www.modencode.org). These results generally agree in detecting dCTCF and CP190 at Mcp but only CP190 at Fab-7. We repeated this analysis using quantitative real-time PCR and concluded that, while Mcp binds both proteins strongly, a low but significant presence of dCTCF is in fact detectable at Fab-7 (Fig. 6), as has also been reported by Holohan et al. (16). Examination of the Mcp and Fab-7 sequences reveals that the Mcp insulator region contains two dCTCF binding consensus sequences, one excellent and one moderately good. No recognizable dCTCF consensus can be found in the Mcp PRE portion. No consensus sequence for any of the known insulator binding proteins is present in the Fab-7 region. GAGAG, the binding sequence of GAF, a BTB/POZ domain protein like CP190, is present in both Mcp (once) and Fab-7 (twice) PREs as well as in the Fab-7 insulator (six times) but not in the Mcp insulator fragment.
FIG. 6.
ChIP binding assay for dCTCF and CP190 to the Mcp and Fab-7 insulators. Quantitative PCR evaluation of ChIP results is expressed as percent input DNA. The primers used are listed in Materials and Methods. For Mcp and Fab-7, they represent the insulator regions. The remaining sites are negative controls. Ubx and w represent the promoters of the corresponding genes. The bxd PRE and gene CG5270 (CG) were also tested, the latter chosen as a particularly active gene in the eye disc. Ab, antibody.
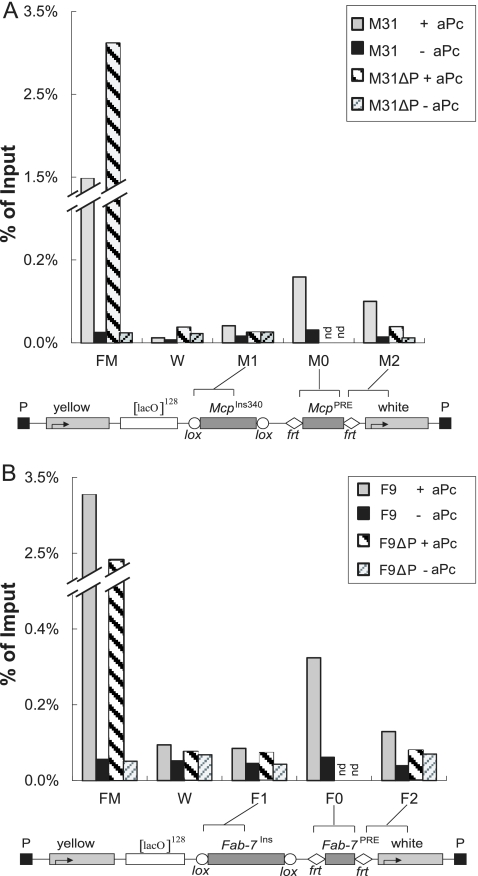
To confirm that the transgenes were in fact able to bind PcG proteins and that this binding was lost when the PRE fragment was excised, we carried out ChIP analysis using chromatin isolated from larvae carrying the LacO-Mcp or LacO-Fab-7 transgene. Figure 7 shows that PC protein binds to the transgenic Mcp or Fab-7 PRE fragments and appears to spread to the region of the white promoter but not to the insulator fragment. The binding is much lower than that observed to the endogenous bxd PRE (Fig. 7, FM), but this is to be expected since the endogenous Mcp or Fab-7 has been shown to bind much less PcG protein than the bxd PRE.
FIG. 7.
ChIP binding assay for PC to the LacO-Mcp and LacO-Fab-7 transgenes. Quantitative PCR evaluation of ChIP results is expressed as percent input DNA, using anti-Pc antibody (+aPc) or no antibody (−aPc), in the presence of the PRE or after excision of the PRE (ΔP). FM is the region adjacent to the endogenous bxd PRE, as a positive control, and W is the white gene promoter region as a negative control. The regions M1, M2, and M0 or F1, F2, and F0 used for PCR analysis are shown by brackets in the diagram below each histogram. The results for LacO-Mcp line 31 are given in panel A, while panel B gives the results for LacO-Fab-7 line F9. PC binds to the PRE fragments in the transgenes and appears to spread to the white promoter but not to the insulator region. Note that, as previously reported (reference 30), the bxd PRE binds far more PcG proteins than either endogenous Mcp or endogenous Fab-7 PREs.
DISCUSSION
Polycomb complexes and long-range interactions.
As we along with others have shown, PcG binding sites (PREs) in the nucleus can interact, often even when they are very distant from one another in the genome, resulting in enhanced repression. We have shown here that PcG complexes bound at three different PREs (bxd, Mcp, and Fab-7) are neither necessary nor sufficient to mediate long-distance interaction. The previously described long-range interactions involving the Mcp and Fab-7 elements are not generated by the PcG complexes that bind to their PREs but by the chromatin insulators that flank these PREs. A similar kind of long-range interaction has been proposed for Su(Hw) insulators (4, 5) and, in fact, the gypsy Su(Hw) insulator was shown to mediate an analogous interaction between remote constructs containing the bxd PRE (33) or between the yellow enhancers on one construct and the promoter at a remote site (20). Although we do not exclude the possibility that certain PcG complexes have an intrinsic ability to form clusters, these results suggest that the foci of PcG proteins, the Polycomb “bodies,” that have been visualized in the nucleus are brought together primarily by insulator mechanisms and not by PcG interactions alone. An alternative possibility is that they represent local clusters of PcG-binding sites within a genomic neighborhood that does not require long-range interactions.
Grimaud et al. (12) found that Antp and the Abd-B genes, separated by 10 Mb, colocalized in nuclei in which both were repressed but not when one was active and the other repressed. They concluded, therefore, that the interaction was related to the binding of PcG complexes. Our results indicate that insulators, present in both the antennapedia complex and the bithorax complex, rather than PcG complexes, are the essential requirement for the colocalization of remote elements. PcG complexes may contribute to the stability of the interactions, and other factors might also be involved. One of these is the state of activity of a nearby promoter. Active genes have been reported to become associated with “transcription factories” (6, 28), which are not likely to cohabit with PcG target regions. The different localization of the Antp and Abd-B loci when one of the two is active and one repressed might therefore reflect the transcriptional state rather than the binding of PcG complexes as such. Preliminary evidence suggests that the transcriptional activity of the associated genes has a powerful influence on nuclear colocalization of Mcp elements (H.-B. Li and V. Pirrotta, unpublished observations).
Insulators interactions.
The currently preferred model for the action of chromatin insulators is based largely on the behavior of the gypsy Su(Hw) insulator (5). A DNA-binding protein, Su(Hw), binds to specific DNA sequences found in the insulator; a second layer of proteins capable of extensive protein-protein interactions binds to Su(Hw). These proteins, MOD(MDG4) and CP190, both have POZ/BTB domains that mediate homo- and heterotypic interactions and are thought to be responsible for the association of multiple insulator elements into clusters (2, 9, 29). This clustering and the consequent organization of the chromatin into loops constitute a powerful mechanism that brings together remote chromatin sites.
The binding of some insulator proteins to the Mcp and Fab-7 regions has been reported previously (2, 16, 25, 27), and more recently genome-wide ChIP/chip analysis of SU(HW), dCTCF, MOD(MDG4), CP190, BEAF, and ZW5 binding in cultured cells has been carried out by the modENCODE Drosophila Chromatin Consortium (http://www.modencode.org). Neither Mcp nor Fab-7 binds Su(Hw); however, these analyses and our own results in flies (Fig. 5) agree in finding that Mcp binds dCTCF and CP190 while Fab-7 binds CP190 and a very small amount of dCTCF (16).
The sequence of the Mcp insulator fragment contains at least one CTCF binding consensus, but no obvious match could be found for this consensus in the Fab-7 insulator sequence. In contrast, the Fab-7 insulator contains six GAF-binding GAGAG motifs, and the Fab-7 PRE contains two. GAF binding was found to be important for Fab-7 insulator activity (31) and also for the silencing activity of the Fab-7 PRE (24). GAF binding was also reported to be required for the Mcp silencing activity although the Mcp PRE contains only one GAGAG consensus in the PRE region (3) and none in the insulator region. The GAF protein also contains a POZ/BTB domain that might, in some circumstances, interact with the CP190 POZ/BTB and account for the interaction between Mcp and Fab-7.
Does homology play a role?
Bantignies et al. (1) argued that homology plays a role because they saw no interaction between insertions containing their Fab-7 element and insertions containing the bxd PRE, and although they observed interactions between Fab-7 and Mcp insertions, these interactions were weaker than those between Fab-7 insertions. We now know, however, that the bxd PRE has no associated insulator, and our experiments show that, by itself, a PRE is unable to engage in long-range interactions. In our experiments, all interacting Mcp constructs share at least the mini-white gene and the 200-bp insulator part of Mcp. However, interactions between Mcp insulator constructs and the endogenous Mcp depend on no homology other than the 200-bp insulator. Homology may well contribute to the stability of the interaction, and certainly chromosomal homology is sufficient for the pairing of elements inserted at allelic sites on homologous chromosomes. However, in our constructs, we must conclude that the interaction is largely independent of the extent of homology but depends critically on the presence of the insulator in both interacting partners.
Is the long-distance contact important for gene expression?
In the case of allelic pairing, the proximity of two PREs can have very strong effects on PcG repression, as shown by the fact that the eye color can go from orange in the heterozygous case to entirely white in flies homozygous for the insertion. The effects of long-range interactions on PRE-dependent repression are generally much subtler. How important physiologically and how widespread are such interactions? Several thousand Su(Hw) sites have been mapped in the Drosophila genome, and similar numbers of sites have been mapped for dCTCF and CP190 (2). Some of these, like those in the bithorax complex, may be needed to form higher-order folding to bring together PREs and other regulatory elements, as has been reported to occur at the mammalian Igf2-H19 locus (21) or the globin locus (36).
The low level of colocalization detected by the in vivo GFP-tagged imaging requires explanation. The percent colocalization detected in these experiments is 1 order of magnitude lower than that reported by Vazquez et al. (37) using the same technique to detect trans interactions of the 2.9-kb Mcp fragment. Our analysis of the fragments flanking the 800-bp Mcp core shows that they make no contribution to insulator, PRE, or colocalization. Another possible explanation is that the construct used by Vazquez et al. contained two additional insulator-like elements, the scs and scs′elements, placed at the two ends of the construct. These elements have never been specifically tested for their contribution to trans interactions. Another possible player is the eye enhancer of the white gene, which was included in the construct used by Vazquez et al. but not in the constructs used in the present work. Preliminary results suggest that the eye enhancer may make an important contribution to colocalization (H.-B. Li and V. Pirrotta, unpublished).
The RNAi connection.
If insulators are frequently responsible for the association of remote PREs in the nucleus, this might also account for another puzzling observation. Grimaud et al. (12) reported that the long-range interactions and degree of silencing of reporter genes produced by constructs containing the Fab-7 element were affected by mutations in the RNAi machinery. They attributed this to an involvement of the RNAi machinery in regulating PcG silencing activity. An alternative explanation is suggested if trans interactions are due to insulator elements. Work on the Su(Hw) insulator has revealed that Argonaute genes are needed for efficient insulator activity and that Argonaute mutations were associated with loss of higher-order interactions between insulator elements in the nucleus (23). An attractive synthesis of these results with our observations would explain the effect of RNAi mutations on PcG silencing as due to the loss of long-range interactions brought about by insulator elements such as those found in the Mcp and Fab-7 elements. Since colocalization results in enhancement of repression, loss of colocalization would account for the modest but significant reduction in PcG function at target genes seen in the presence of RNAi mutations.
Acknowledgments
We thank Christian Sigrist, Amy Csink, and Julio Vazquez for plasmids. Jackie Guiard helped produce the Flipper transgenic lines.
This work was supported in part by funds to V.P. from the Swiss National Science Foundation and from Rutgers University, to P.G. from the Russian Foundation for Basic Research (project 10-04-00474-a) and the Ministry of Science and Education of the Russian Federation (project 02.740.11.0289), and to O.K. from the Molecular and Cell Biology Program of the Russian Academy of Sciences.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Bantignies, F., C. Grimaud, S. Lavrov, M. Gabut, and G. Cavalli. 2003. Inheritance of Polycomb-dependent chromosomal interactions in Drosophila. Genes Dev. 17:2406-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bushey, A. M., E. Ramos, and V. G. Corces. 2009. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev. 23:1338-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busturia, A., et al. 2001. The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development 128:2163-2173. [DOI] [PubMed] [Google Scholar]
- 4.Byrd, K., and V. G. Corces. 2003. Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell. Biol. 162:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capelson, M., and V. G. Corces. 2004. Boundary elements and nuclear organization. Biol. Cell 96:617-629. [DOI] [PubMed] [Google Scholar]
- 6.Cook, P. R. 1994. RNA polymerase: structural determinant of the chromatin loop and the chromosome. BioEssays 16:425-430. [DOI] [PubMed] [Google Scholar]
- 7.Dekker, J., K. Rippe, M. Dekker, and N. Kleckner. 2002. Capturing chromosome conformation. Science 295:1306-1311. [DOI] [PubMed] [Google Scholar]
- 8.Gerasimova, T. I., E. P. Lei, A. M. Bushey, and V. G. Corces. 2007. Coordinated control of dCTCF and gypsy chromatin insulators in Drosophila. Mol. Cell 28:761-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh, D., T. I. Gerasimova, and V. G. Corces. 2001. Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 20:2518-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gohl, D., M. Muller, V. Pirrotta, M. Affolter, and P. Schedl. 2008. Enhancer blocking and transvection at the Drosophila apterous locus. Genetics 178:127-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewal, S. I. S., and S. C. R. Elgin. 2007. Transcription and RNA interference in the formation of heterochromatin. Nature 447:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimaud, C., et al. 2006. RNAi components are required for nuclear clustering of Polycomb group response elements. Cell 124:957-971. [DOI] [PubMed] [Google Scholar]
- 13.Gruzdeva, N., O. Kyrchanova, A. Parshikov, A. Kullyev, and P. Georgiev. 2005. The Mcp element from the bithorax complex contains an insulator that is capable of pairwise interactions and can facilitate enhancer-promoter communication. Mol. Cell. Biol. 25:3682-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagege, H., et al. 2007. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2:1722-1733. [DOI] [PubMed] [Google Scholar]
- 15.Hagstrom, K., M. Müller, and P. Schedl. 1996. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 10:3202-3215. [DOI] [PubMed] [Google Scholar]
- 16.Holohan, E. E., et al. 2007. CTCF genomic binding sites in Drosophila and the organisation of the bithorax complex. PLoS Genet. 3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horard, B., C. Tatout, S. Poux, and V. Pirrotta. 2000. Structure of a Polycomb response element and in vitro binding of Polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20:3187-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn, T. G., Y. B. Schwartz, G. I. Dellino, and V. Pirrotta. 2006. Polycomb complexes and the propagation of the methylation mark at the Drosophila Ubx gene. J. Biol. Chem. 281:29064-29075. [DOI] [PubMed] [Google Scholar]
- 19.Kassis, J. A. 2002. Pairing-sensitive silencing, Polycomb group response elements, and transposon homing in Drosophila. Adv. Genet. 46:421-438. [DOI] [PubMed] [Google Scholar]
- 20.Kravchenko, E., et al. 2005. Pairing between gypsy insulators facilitates the Enhancer action in trans throughout the Drosophila genome. Mol. Cell. Biol. 25:9283-9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurukuti, S., et al. 2006. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl. Acad. Sci. U. S. A. 103:10684-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyrchanova, O., S. Toshchakov, A. Parshikov, and P. Georgiev. 2007. Study of the functional interaction between Mcp insulators from the Drosophila bithorax complex: effects of insulator pairing on enhancer-promoter communication. Mol. Cell. Biol. 27:3035-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei, E. P., and V. G. Corces. 2006. RNA interference machinery influences the nuclear organization of a chromatin insulator. Nat. Genet. 38:936-941. [DOI] [PubMed] [Google Scholar]
- 24.Mishra, R. K., et al. 2001. The iab-7 Polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol. Cell. Biol. 21:1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan, M., et al. 2007. The Drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. EMBO J. 26:4203-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, M., K. Hagstrom, H. Gyurkovics, V. Pirrotta, and P. Schedl. 1999. The Mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics 153:1333-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nègre, N. et al. 2010. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 6:e1000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne, C. S., et al. 2004. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat. Genet. 36:1065-1071. [DOI] [PubMed] [Google Scholar]
- 29.Pai, C.-Y., E. P. Lei, D. Ghosh, and V. G. Corces. 2004. The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol. Cell 16:737-748. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz, Y. B., et al. 2006. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 38:700-705. [DOI] [PubMed] [Google Scholar]
- 31.Schweinsberg, S., et al. 2004. The enhancer-blocking activity of the Fab-7 boundary from the Drosophila bithorax complex requires GAGA-factor-binding sites. Genetics 168:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegal, M. L., and D. L. Hartl. 1996. Transgene coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics 144:715-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigrist, C. J. A., and V. Pirrotta. 1997. Chromatin insulator elements block the silencing of a target gene by the Drosophila Polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics 147:209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spradling, A. C., and G. M. Rubin. 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218:341-347. [DOI] [PubMed] [Google Scholar]
- 35.Thakar, R., and A. K. Csink. 2005. Changing chromatin dynamics and nuclear organization during differentiation in Drosophila larval tissue. J. Cell Sci. 118:951-960. [DOI] [PubMed] [Google Scholar]
- 36.Tolhuis, B., R.-J. Palstra, E. Splinter, F. Grosveld, and W. de Laat. 2002. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell 10:1453-1465. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez, J., M. Müller, V. Pirrotta, and J. W. Sedat. 2006. The Mcp element mediates stable long-range chromosome-chromosome interactions in Drosophila. Mol. Biol. Cell 17:2158-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou, J., S. Barolo, P. Szymanski, and M. Levine. 1996. The Fab-7 element of the bithorax complex attenuates enhancer-promoter interactions in the Drosophila embryo. Genes Dev. 10:3195-3201. [DOI] [PubMed] [Google Scholar]