Abstract
Polyacrylate (PAA) adsorbents selectively bind low density lipoproteins (LDL) from human plasma and blood, whereas very low density lipoproteins (VLDL) are only minimally adsorbed. The adsorption of cholesterol-rich lipoproteins to PAA adsorbents is related to the molecular weight (mw) of the polyanion ligand. Ca++ and Mg++ inhibit the binding of LDL to PAA adsorbents. The chemical composition of the organic hardgels of the adsorbents does not have an influence on adsorption. The selective adsorption of LDL to PAA adsorbents can be explained to result from their low negative surface charge density and the specific colloid-chemical properties of the surface-bound PAA, which do not prevent LDL from binding to charge-like domains of the ligand. By contrast, VLDL and high density lipoproteins (HDL) are repelled from the adsorbents due to their higher negative surface charge density.
Keywords: polyacrylate, adsorbents, surface charge density, cholesterol-rich lipoproteins, lipoprotein apheresis
Abstract
Polyakrylat(PAA)-Adsorbentien binden selektiv Low Density Lipoproteine (LDL) aus menschlichem Plasma oder Blut. Very Low Density Lipoproteine (VLDL) werden hingegen nur geringfügig adsorbiert. Die Adsorption cholesterinreicher Lipoproteine an PAA-Adsorbentien ist von dem Molekulargewicht der PAA-Liganden abhängig. Ca++ und Mg++ hemmen die Bindung von LDL. Die chemische Zusammensetzung der organischen Festphasen hat keinen Einfluss auf die Adsorption. Die selektive Adsorption von LDL an PAA-Adsorbentien beruht auf der geringen negativen Oberflächenladungsdichte dieser Lipoproteine und den spezifischen kolloid-chemischen Eigenschaften Oberflächen-gebundener PAA, welche eine Adsorption von LDL an gleichgeladene Domänen des Liganden nicht beeinträchtigen. Hingegen verhindert die höhere negative Oberflächenladungsdichte von VLDL und High Density Lipoproteinen (HDL) deren Bindung an die PAA-Adsorbentien.
Introduction
Organic and inorganic porous spherical hardgels with polyacrylate (PAA) ligands are used to selectively remove LDL from human plasma or blood of patients. The first ex vivo experiments for LDL-apheresis used a polymethacrylate hardgel with PAA-ligands [1]. The PAA adsorbent most widely used for treatment is prepared from a polyacrylamide hardgel and is marketed since 1996 [2].
The adsorption binding of LDL can not be explained by an immune reaction, since PAA adsorbents do not include antibodies against lipoproteins. Since the cause of the selectivity of adsorption remains unclear, the in vitro experiments described in this report were made to compare and shed light on the typical adsorption properties of PAA adsorbents.
Materials
Polymethacrylate hardgels were from Tosoh Bioscience (Stuttgart, Germany). Various bulk raw materials were used: TOYOPEARL HW 65C®, HW 70EC®, HW 75F®, HW 75C®. For clear understanding of the following, these “untreated” hardgels are distinguished from oxirane-activated hardgels, including FRACTOGEL AF Epoxy® and FRACTOGEL EMD-Epoxy®, a glycidmethacrylate derivative of Toyopearl hardgel from MERCK GmbH (Darmstadt, Germany), both of which contain reactive oxirane residues. Glycidylmethacrylate is also a chemical component of all other Toyopearl type hardgels. Polyacrylamide hardgel EUPERGIT C250L® was from Degussa-Röhm (Darmstadt, Germany).
Unbranched polyacrylic acids with molecular weights between 1,200 dalton and 250,000 dalton were provided from BASF (Ludwigshafen, Germany), Degussa (Krefeld, Germany) and Sigma (München, Germany).
Fluorescamine, N-Ethoxycarbonyl-2-ethoxy-1,2-dihydrochinolin (EEDQ) and epichlorhydrin were from Sigma. Test kits for the measurement of urea, triglyceride, cholesterol, and HDL cholesterol were from Roche Diagnostics (Mannheim, Germany).
Methods
1. Synthesis of oxirane-activated polymethacrylate hardgels
“Untreated” polymethacrylate hardgels were reacted with epichlorhydrin as described elsewhere [3]. The oxirane-activated hardgels were thoroughly washed with distilled water, followed by aceton and dried in the exsiccator.
2. Synthesis of amino-derived hardgels
For the preparation of amino derivatives aliquot 1.5 g of “untreated” polymethacrylate hardgels were suspended in 10 ml of aqueous ammonium hyroxide (10% w/v). The mixture was slowly shaken overnight. Thereafter, the supernatant solution was removed and the solid phase repeatedly washed with distilled water until the wash solution reached pH 6. Water was removed from the particles by several washes with aceton; the aceton was evaporated by drying the amino-derivative of the hardgel at 60°C for 12 hours. The same procedure was used for oxirane-activated polymethacrylate hardgels and for the preparation of the amino derivative of EUPERGIT C250L®, in which the reactive oxirane residues are equally provided from glycidylmethacrylate [4].
3. Synthesis of polyacrylic acid (PAA) adsorbents
3a. “Wet” synthesis
Aqueous PAA solutions (20 g PAA/l H2O) were adjusted to pH 4.2 with NaOH. The mean molecular weight of PAA varied between 1,200 dalton and 250,000 dalton. The amino-derivatives of the polymethacrylate hardgels (1.3 g aliquots) were mixed in 5 ml of the pH-adjusted PAA solutions for 1 hour at room temperature. Thereafter, 0.2 g EEDQ in 5 ml aceton was added dropwise. The mixture was rotated for 1 hour. The supernatant solution was removed and the PAA-polymethacrylate adsorbent washed 5 times with 50 ml aceton, thereafter with 100 ml 1 mol/l NaCl, thereafter with 1 l distilled water, washed again with 100 ml aceton to remove the water, and dried at 60°C.
PAA-polyacrylamide adsorbents were prepared from amino-derived EUPERGIT C250L® by the same procedure.
3b. “Dry” synthesis
Dry amino-derived polymethacylate hardgel (1 g aliquots) was suspended in 10 ml of a 2% PAA solution. In one series of experiments the mean molecular weight of PAA varied between 1,200 dalton and 250,000 dalton. The pH of the PAA solution was adjusted with NaOH to pH 4.5. In a second series of experiments the pH of the PAA (mw 250,000) solution varied between pH 3 and pH 10. The suspensions were rotated at room temperature for 2 hours. Thereafter, the supernatant solution was removed and the hardgel was washed twice with methanol and once with aceton. The hardgel having adsorbed PAA was heated for 40 hours to 70°C, followed by washing with 100 ml 1 mol/L NaCl, subsequently with 1 l distilled water, equilibration to pH 7.4, washing with 60 ml aceton and drying in the exsiccator.
The same procedure was used for the synthesis of PAA adsorbents from amino-derived EUPERGIT C250L®.
Analytical experiments
1. Proof of the presence of oxirane groups in “untreated” hardgels
Untreated polymethacrylate hardgels (0.05 g aliquots) were treated with ammonium hydroxide solution, thoroughly washed with water and dried as described above. The dried hardgels were mixed with of 0.5 ml of 0.01% fluorescamine in acetone. After 5 minutes the solution was removed and the particles thoroughly washed with distilled water. The fluorescence of the particles was examined under the fluorescence microscope. The same procedure was applied to oxirane-derived polymethacrylate hardgels and to polyacrylamide hardgel. Amino-derived hardgels were directly reacted with fluorescamine as described above for examination by fluorecescence microscopy.
2. Proof of PAA covalently bound to amino-derived hardgels
One hundred microlitres of ortho-toluidine blue (o-tb, 10 mg/l) were added to PAA-derived hardgels (0.05 g of each), suspended in 4 ml distilled water at pH 7.8. The decoloration of the solution and concommittant metachromatic staining of the hardgel particles was visually examined after shaking the suspension for 10 minutes. For control, “untreated” hardgels and amino-derived hardgels were correspondingly examined.
3. Proof of amino groups in PAA adsorbents
PAA-polymethacrylate and PAA-polyacrylamide adsorbents were reacted with fluorescamine and examined by fluorescence microscopy as described above.
4. Adsorption of lipoproteins to PAA adsorbents
In one series of experiments aliqout 3 ml of citrated plasma from a human donor having fasted for 16 hrs prior to blood sampling was applied to columns prepared with 0.7 g of Toyopearl HW75F adsorbents with PAA having a mean molecular weight between 1,200 dalton and 250,000 dalton. Corresponding experiments were made using PAA-Eupergit C250L adsorbents.
In another series, adsorbents with PAA covalently bound at pH 3, pH 5, pH 7 and pH 9, respectively, were used. The adsorbents were equilibrated to pH 7.4 prior to perfusion of 3 ml of citrated plasma.
5. Adsorption of lipoproteins to PAA adsorbents in the absence or presence of Ca++ and Mg++ ions
For the assessment of the influence of Ca++ and Mg++ ions on lipoprotein adsorption the PAA adsorbent was perfused with EDTA plasma diluted with 0.15 mol/L NaCl (9:1 by volume); another column was perfused with Ca++ and Mg++ free citrated plasma.
In a second experiment a column was prepared with 1.3 g PAA-Eupergit C250L and perfused with 10 ml of an aqueous solution containing 0.15 mol NaCl, 2.2 mmol CaCl2 and 1 mmol MgCl2 and adjusted to pH 7.4 with NaOH. Thereafter, the column was perfused with 3 ml Ca++ and Mg++ free citrated plasma. A second column with the same adsorbent that was wetted with 0.15 mol NaCl solution (without Ca++ and Mg++) was also perfused with the same plasma.
Triglyceride, cholesterol and HDL cholesterol concentrations were measured using enzymatic methods. LDL cholesterol was calculated according to Friedewald et al. [5]. Urea was also measured to account for dilution.
6. Blood perfusion through PAA-polymethacrylate adsorbents
Three millilitres of citrated blood were perfused through columns of 0.7 g PAA(mw 250,000)-derived TOYOPEARL HW70EC® and PAA(mw 250,000)-derived TOYOPEARL HW75C®, respectively, to examine the retention of blood cells on the adsorbents. Blood cell counts were measured before and after perfusion using an automated blood cell counter.
Observations and results
Reactive oxirane groups in “untreated” polymethacrylate hardgels
Polymethacrylate hardgels are copolymers of ethylene glycol or oligomers of ethylene glycol, glycidmethacrylate and a mixture of erythrol-methacrylate, erythrol-dimethacrylate and erythrol-trimethacrylate. According to the synthesis described in [6] the hardgels contain reactive oxirane groups that may be destroyed by hydrolysis. The polymethacrylate hardgels are not suitable for the preparation of adsorbents with small ligands for affinity chromatography, because of their low contents of reactive oxirane groups. However, for polymeric ligands containing a large number of residues for covalent binding, such as PAA, polymethacrylate hardgels containing only a small number of oxirane residues may still be suitable. Therefore the proof of the presence reactive oxirane groups in “untreated” polymethacrylate hardgels seemed to be pertinent to assess their suitability for the synthesis of PAA adsorbents without the need for additional epoxidation.
The sodium thiosulfate method used for proof of the presence of reactive oxirane groups [7] revealed to be impractical and insensitive for the detection of small amounts of oxirane groups. Amino groups in proteins react with fluorescamine to form fluorescent products [8]. Oxirane groups in hardgels that are converted to amino residues equally react with fluorescamine to form a fluorescent product. Since fluorescamine does not form a stable fluorescent product with ammonia, the method is suitable for sensitive identification of primary amino residues in hardgels.
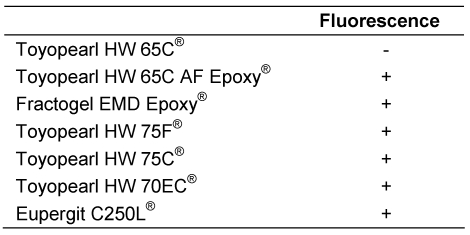
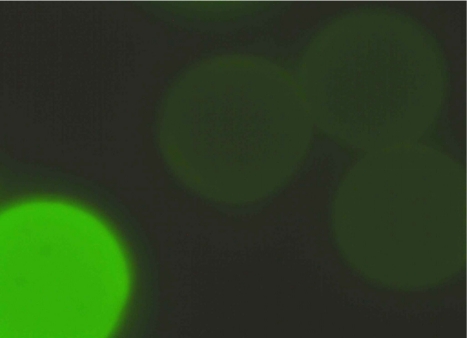
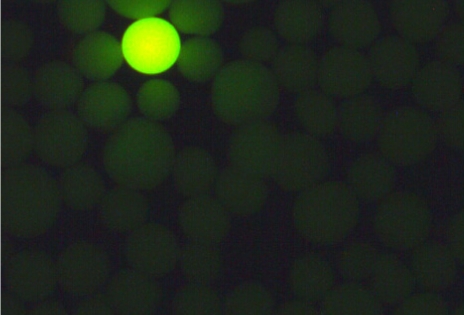
The observations for proof of reactive oxirane groups in “untreated” polymethacrylate hardgels after reaction with ammonia are summarized in Table 1 (Tab. 1). Figures 1 to 4 show fluorescence microscopy images of Toyopearl hardgels. No fluorescence of particles was detectable in TOYOPEARL HW 65C® (Figure 1 (Fig. 1)), whereas fluorescence of TOYOPEARL HW 75C® particles was clearly evident (Figure 2 (Fig. 2)).
Table 1. Oxirane groups in Toyopearl and Eupergit hardgels1) .
1) Hardgels were reacted with ammonia followed by treatment with fluorescamine.
Figure 1. Ammonia treated Toyopearl HW65C particles that were reacted with fluorescamine. A fluorescent particle is shown as impurity to demonstrate the contrast to the bulk of particles that do not exhibit fluorescence. Magnification x200.
Figure 2. “Untreated” Toyopearl HW 75C® particles after reaction with ammonia followed by treatment with fluorescamine. Magnification x200.
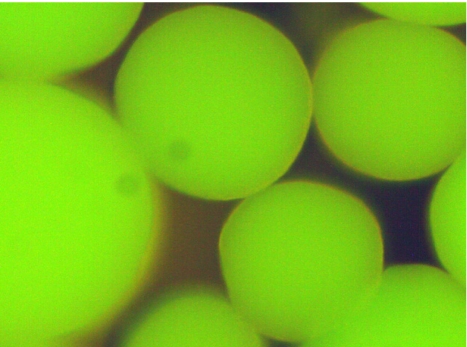
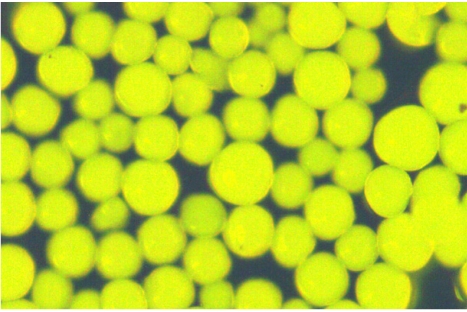
Amino groups in vicinal position of hydroxy groups are selectively destroyed by periodate. For verification, “untreated” TOYOPEARL HW75F® was therefore reacted with ammonia and subsequently treated with fluorescamine for the presence of oxirane groups (Figure 3 (Fig. 3)). The ammonia-treated hardgel was subjected to periodate oxidation followed by reaction with fluorescamine. No fluorescence was detected in these particles (Figure 4 (Fig. 4)). The observation confirms that the position of amino residues is vicinal to hydroxy groups that were formed from reactive oxirane groups in the “untreated” hardgel.
Figure 3. TOYOPEARL HW 75F particles after reaction with ammonia, followed by treatment with fluorescamine. Magnification x100.
Figure 4. Amino-derived TOYOPEARL HW 75F® particles treated with periodate and subsequently reacted with fluorescamine. One particle that was not treated with periodate is shown as impurity to demonstrate the contrast to the bulk of particles that do not exhibit fluorescence. Magnification x100.
PAA covalently bound to amino-derived hardgels
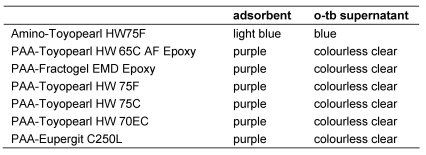
The addition of the dye o-tb to an aqueous PAA solution at pH 7.8 causes a metachromatic shift of light absorption from blue to purple. A suspension of particles with PAA ligands should adsorb the dye, while the solution should rapidly turn colourless clear. The observations of the o-tb test with “untreated”, amino-derived and PAA-derived hardgels are listed in Table 2 (Tab. 2).
Table 2. o-tb binding to PAA ligands in amino-derived adsorbents.
No decoloration or metachromatic shift of the o-tb solution occurred after exposure to “untreated” and amino-derived hardgels. By contrast, particles of PAA-derived hardgels stained purple while the blue o-tb solution rapidly decolorised. When stored for several weeks at room temperature no measurable coloration of the supernatant solution occurred, whereas the purple colour of the particles remained stable indicating a firm binding of the dye to the PAA adsorbent and the lack of dissociation of PAA from the particles.
Amino residues in PAA-polymethacrylate and polyacrylamide adsorbents
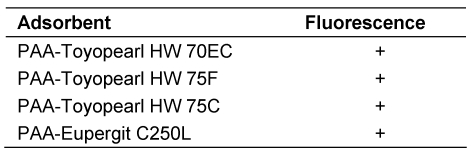
To gain information on the surface properties of PAA-derived polymethacrylate and polyacrylamide hardgels, the adsorbents were investigated for the presence of amino groups. The PAA adsorbents were examined by fluorescence microscopy after treatment with fluorescamine as described above. The observations are listed in Table 3 (Tab. 3). All PAA adsorbents exhibited fluorescence. After treatment with periodate the adsorbent particles no more exhibited fluorescence with fluorescamine. The observations indicate that amino groups were located vicinally to hydroxy groups on the surface of the adsorbents. They also indicate that only parts but not all amino residues on the hardgels reacted with PAA during covalent binding.
Table 3. Fluorescence from amino groups in PAA-Toyopearl and Eupergit adsorbents1) .
1) The PAA adsorbents prepared from amino-derived hardgels after treatment with fluorescamine.
Binding of plasma lipoproteins to PAA adsorbents
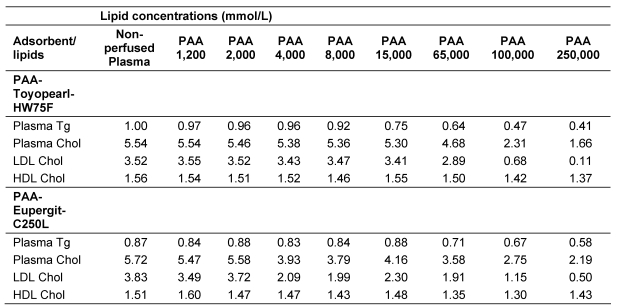
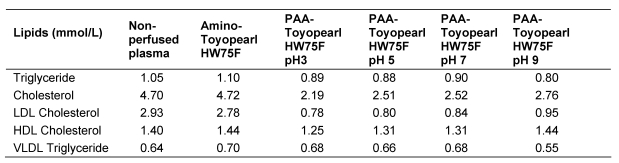
The results of experiments of the adsorption of plasma lipoproteins to TOYOPEARL HW 75F® and EUPERGIT C250L® hardgel with PAA ligands having mean molecular weights between 1,200 dalton and 250,000 dalton are listed in Table 4 (Tab. 4). (A comparison of the chemical, spectroscopical and adsorbtive properties of the widely used commercial polyacrylamide PAA adsorbent and the PAA-Eupergit C250L adsorbent revealed striking identity.) The results of Toyopearl adsorbents were more consistent than those of Eupergit adsorbents. HDL were adsorbed only to a small extent. Interestingly, the adsorption of LDL was related to the molecular weight of the ligand. Hardgels with PAA ligands having a molecular weight <20,000 dalton did not significantly adsorb LDL. Selective and extensive adsorption of LDL occurred to adsorbents having PAA ligands with a molecular weight of 100,000 dalton and above.
Table 4. Lipid concentration in perfused citrated plasma after perfusion through TOYPEARL HW75F® and PAA-derived EUPERGIT C250L® adsorbents with PAA ligands of different molecular weight.
The dissociation of carboxyl groups in PAA changes with pH [9]. At pH 3 all carboxyl groups are hydrogenated, whereas at pH >9 all carboxyl groups are dissociated. As the degree of dissociation of carboxyl groups during covalent binding of PAA to amino-derived hardgel could affect on the adsorption properties of the adsorbent towards lipoproteins, perfusion experiments were made using PAA(mw 250,000)-derived TOYOPEARL HW75F® adsorbent, in which the polyanion had been covalently bound at different pH and equilibrated to physiological pH. The lipid concentrations after perfusion through the adsorbents are listed in Table 5 (Tab. 5). LDL cholesterol in the perfusate was 72% to 81% reduced independent of the adsorbent used, HDL cholesterol concentration was reduced by 5 to 8% .
Table 5. Lipid concentrations (mmol/L) in citrated plasma after perfusion through PAA (mw 250,000)-Toyopearl HW75 F adsorbent. Covalent binding of PAA to amino-derived Toyopearl HW75F was carried out at different pH. The adsorbents were equilibrated to pH 7.4 prior to use.
The adsorption of VLDL can be estimated from VLDL triglyceride concentration using the mean molar triglyceride/cholesterol ratios in HDL and LDL [10] and their respective cholesterol concentrations. VLDL triglyceride concentrations were calculated from the difference of plasma triglyceride and (HDL+LDL) triglyceride. VLDL triglycerides in perfused plasma did not differ significantly from VLDL triglycerides of non-perfused plasma. The results indicate that the reduction of triglyceride concentration in perfused plasma is almost entirely contributed by LDL triglycerides.
Adsorption of cholesterol-rich lipoproteins in the absence or presence of Ca++ and Mg++
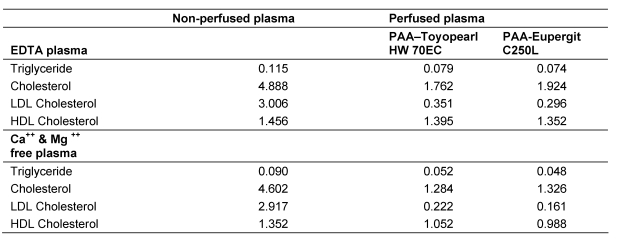
Since PAA adsorbs divalent cations from solution, it seemed pertinent to examine a possible effect of Ca++ and Mg++ in the adsorption of cholesterol-rich lipoproteins from plasma. The results of these experiments are listed in Table 6 (Tab. 6) and Table 7 (Tab. 7). LDL was adsorbed from EDTA plasma (in which the divalent cations are complexed) as well as from Ca++ and Mg++-free citrated plasma. The observations indicate that Ca++ and Mg++ is not required for LDL adsorption.
Table 6. Lipid concentrations (mmol/L) in EDTA plasma and Ca++ and Mg++ free plasma after perfusion through PAA-Toyopearl HW70EC and PAA-Eupergit C250L.
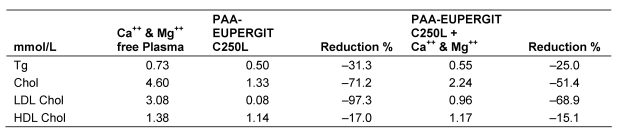
Table 7. Adsorption of lipoproteins from Ca++ and Mg++ free citrated plasma by PAA-Eupergit C250L and by PAA-Eupergit C250 preloaded with Ca++ and Mg++.
In the second experiment citrated Ca++ and Mg++-free plasma was used to exclude a possible effect of these ions, which are normally present in plasma. The adsorbent that was pre-loaded with Ca++ and Mg++ adsorbed less LDL from plasma compared to an absorbent that was not pretreated. No significant difference was observed in HDL adsorption, although the amount of adsorbed HDL was higher than from normal plasma. The observations indicate that the presence of Ca++ and Mg++ impairs rather than enhances LDL adsorption to a PAA adsorbent.
Perfusion of blood through PAA (mw 250,000) polymethacrylate adsorbents
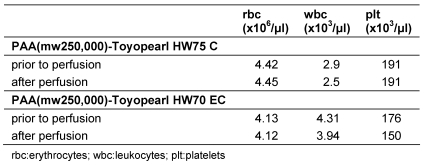
To prove the suitability of PAA-derived polymethacrylate adsorbents for blood perfusion, citrated blood was passed through columns with PAA-derived TOYOPEARL HW 70 EC® and HW 75C® adsorbents. The concentrations of blood cells before and after perfusion are listed in Table 8 (Tab. 8). Neither the concentration of red blood cells nor that of leukocytes or platelets was reduced to an extent that would have clinical significance.
Table 8. Blood cells in citrated blood after perfusion through PAA(mw 250,000)-Toyopearl HW70 EC and PAA(mw 250,000)-Toyopearl HW75 C adsorbent.
Discussion
Perfusion techniques for the elimination of lipoproteins from human blood or plasma ex vivo include adsorbents using specific antibodies for immune reaction [11], [12] or polyanions for physico-chemical adsorption. The ligands of polyanion adsorbents are either polysulfates, such as heparin and dextran sulfate [13], [14] or polyacrylates [1], [2]. The driving force for the binding of lipoproteins to polyanions under pH conditions below the isoelectric point of the lipoproteins is assumed to result from electrostatic attraction [15]. However, the mechanism of adsorption of lipoproteins under physiogical conditions, i.e. above their isoelectric point, is not well understood.
Polyanions, including polysulfates and inorganic polyphosphates, precipitate VLDL and LDL in the presence of unphysiologically high concentrations of divalent cations [16], [17], [18], [19]. Inorganic polyphosphates activate platelets and the plasmatic clotting system. Therefore they are not suitable for lipoprotein elimination ex vivo. Polycarboxylates, such as pectic acid, hyaluronic acid and carrageenin λ, do not precipitate lipoproteins in solution [20], [21]; however, grafted polyacrylic acid does bind lipoproteins [1], [2]. In vitro, polysulfate adsorbents bind VLDL and LDL [22], whereas PAA adsorbents almost exclusively bind LDL.
The PAA adsorbents used in the described experiments have pores with a size exclusion for globular proteins within 40,000 dalton and 50 million dalton. VLDL have a molecular weight up to 10 million; therefore size exclusion effects for VLDL during perfusion can be disregarded; the adsorption properties of PAA adsorbents rather point to specific colloid-chemical features of the polycarboxylic ligands.
The synthesis of the PAA adsorbents includes an intermediate amino derivative of the hardgel. In an aqueous medium above pH 3 and below pH 9 PAA is electrostatically attracted by the amino residues forming a polymeric salt on the surface of the hardgel. Thereafter, free amino groups on the hardgel are covalently linked to carboxyl groups of PAA. The covalent binding of PAA to the particle is multicentric and the PAA ligands are attached in loops rather than as bristles. The presence of free amino groups in the PAA adsorbents further indicates that some but not all amino groups are used for covalent binding so that free carboxylate groups of PAA can electrostatically interact with free amino groups on the hardgel. Consequently, the loops of the PAA ligands on the surface of the adsorbent exhibit a certain flexibility due to the reversable electrostatic association of the amino and carboxyl groups. The flow resistance of such an adsorbent was found to be less than that of a polyanion adsorbent that does not contain additional chargeable amino groups on its surface. Although the physico-chemical properties of such adsorbents have not yet been described, experiments with end-grafted PAA adsorbents revealed observations that should equally apply to the PAA adsorbents described above.
PAA is a weak polyanion, in which the dissociation of carboxy groups in solution increases almost linearly with pH [9], [22]. However, in PAA brushes the pH was found to remain almost constant within pH 6 to pH 8 [23], [24]. In such a system charged residues accumulate at the end of the polyelectrolyte chain, whereas there are less charges in the interior of the chain [25]. The hydration of the brushes increases with the dissociation of the carboxyl residues of the ligand. The hydration causes a swelling of the brush thickness. However, the degree of hydration is unevenly distributed. The outer zone is more hydrated than the centre of the polyanionic ligand [26]. Biopolymers or biopolymer composites larger in size will penetrate less into the polyanionic layer than composites of smaller size. Furthermore, composites with a high negative surface charge density will be repelled from the outer zone of the polyanion layer, whereas polymers with a low negative surface charge density, such as LDL, may penetrate through the outer zone and bind in the inner zone of the PAA ligand, which has a lower charge density and is less hydrophilic. Experiments using neutron reflectometry showed that globular bovine serum albumin (BSA) molecules penetrate deeply in the layer of like-charged PAA brushes [27]. The experiments also showed that an increased surface charge density of BSA impairs the penetration of the molecules.
The surface charge density of lipoproteins in a physiological environment has not yet been determined. However, electrophoretic experiments indicate that the negative surface charge density of VLDL and HDL seems to be considerably higher than that of LDL and intermediate density lipoproteins (β-VLDL) [28], [29]. A high negative surface charge density of VLDL and HDL would explain, why at physiological pH conditions these lipoproteins are repelled from the equally charged PAA adsorbent. The larger volume of VLDL additionally contributes to a repulsion of these particles. Therefore the adsorption of VLDL and HDL would be less likely. It is also conceivable that the low negative surface charge density of LDL at physiological pH makes it possible that LDL particles diffuse through the outer, repulsive zone into the inner zone of the PAA ligand, where they are bound to less charged domains. The difference of surface charge densities of lipoproteins under physiological conditions could therefore explain the preferential adsorption of cholesterol-rich lipoproteins by PAA adsorbents.
The influence of the molecular weight of polysulfates on the precipitation of lipoproteins was found to be negligible [21]. Comparative studies on grafted polysulfates have not be published. However, the commercial dextran sulfate adsorbent used for lipoprotein apheresis contains a covalently bound ligand with a molecular weight smaller than that of heparin [30]. PAA adsorbents with ligands of low molecular weight were not found to adsorb LDL; the limit of molecular weight of PAA, above which adsorbents significantly bind LDL, was found near 65,000 dalton. There could be two determining reasons for the limit:
a. The formation of loops of PAA bound to the particle surface decreases with the molecular weight of the ligand. Consequently the functional surface of the adsorbent will be smaller;
b. Since the negative surface charge density should rather impair LDL particles from binding to PAA adsorbents at physiological pH, it is plausible to assume that more (non-dissociated) carboxyl groups of the PAA ligand are involved in the binding of cholesterol-rich lipoproteins. Therefore, PAA ligands should have a minimal chain length to provide at least one binding domain for cholesterol-rich lipoproteins.
Both conditions make it plausible that there is a relation between the molecular weight of the PAA ligand and lipoprotein adsorption. Interestingly, the binding of polyethyleneglycol to PAA was also found to be related to the chain lenght of the polyanion [31].
Ca++ and Mg++ ions precipitate lipoproteins in the presence of polysulfate electrolytes with high surface charge density [16]. The binding to strong polyanions occurs by electrostatic interaction and independent of the pH [24]. Ca++ ions are more strongly bound than Mg++ ions [32] to sulfate and sulfaminogroups of a tetrasacharide unit in heparin [33], [34]. The binding of divalent cations to PAA is pH dependent and involves two neighbouring carboxylate residues in the same chain [35], [36]. In light of these observations a possible effect of divalent cations on the adsorption of lipoproteins to surface-bound polyacrylate had to be considered. The adsorption experiments showed that the presence of divalent cations for the binding of cholesterol-rich lipoproteins from plasma to the PAA adsorbents is not prerequisite. On the contrary, the adsorption of LDL by PAA adsorbent preloaded with Ca++ and Mg++ ions is impaired. Since the intramolecular binding of the divalent cations to PAA reduces the surface charge and causes a dehydration of the grafted polyelectrolyte [37], the effect of Ca++ and Mg++ on LDL adsorption may be the consequence of a shrinking of the PAA layer rather than a competitive effect of the ions blocking specific LDL binding sites on the PAA ligand [38].
The blood perfusion experiments show that adsorbents with particle diameters above 50 μm C- and EC-type Toyopearl hardgels and other types with larger particle dimensions are equally suitable for apheresis of blood and plasma. The physical dimensions of these hardgels provide sufficient room for blood cells to flow through the interparticle space of an adsorbent, whereas the use of adsorbents with smaller particle sizes is limited to plasmapheresis only.
Conclusion
LDL adsorbents prepared from amino-derived polymethacrylate hardgels and PAA-polyacrylamide hardgel with colavently bound polyacrylate ligands have the same adsorption properties. The properties are defined by the ligand rather than the chemical composition of the hardgel or the spacer between the hardgel and the ligand. The adsorption properties of PAA adsorbents are explained by the specific colloid-chemical properties of surface-bound PAA, which differ from those of polysulfate ligands.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Thies K, Prigent S, Heuck CC. Selective removal of low density lipoproteins from plasma by polyacrylate-coated Fractogel in vitro and in experimental extracorporeal perfusion. Artif Organs. 1988;12(4):320–324. doi: 10.1111/j.1525-1594.1988.tb02780.x. Available from: http://dx.doi.org/10.1111/j.1525-1594.1988.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 2.Bosch T, Schmidt B, Blumenstein M, Gurland HJ. Lipid apheresis by hemoperfusion: in vitro efficacy and ex vivo biocompatibility of a new low-density lipoprotein adsorber compatible with human whole blood. Artif Organs. 1993;17(7):640–652. doi: 10.1111/j.1525-1594.1993.tb00609.x. Available from: http://dx.doi.org/10.1111/j.1525-1594.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto I, Ito Y, Seno N. Preparation of affinity adsorbents with Toyopearl gels. J Chromatogr A. 1982;239:747–754. doi: 10.1016/S0021-9673(00)82034-8. Available from: http://dx.doi.org/10.1016/S0021-9673(00)82034-8. [DOI] [Google Scholar]
- 4.Krämer DM. Jahrbuch der Biotechnologie. München, Wien: Carl Hanser Verlag; 1987. Die Entwicklung von Eupergit C; pp. 398–413. [Google Scholar]
- 5.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 6.Sasaki H, Komiya K, Kato Y. Hydrophilic separating carrier and preparation thereof. EP 0006199. München: European Patent Office; 1982. [Google Scholar]
- 7.Sundberg L, Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifunctional oxiranes. J Chromatogr A. 1974;90(1):87–98. doi: 10.1016/S0021-9673(01)94777-6. Available from: http://dx.doi.org/10.1016/S0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- 8.Böhlen P, Stein S, Dairman W, Udenfriend S. Fluorimetric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973;155(1):213–220. doi: 10.1016/S0003-9861(73)80023-2. Available from: http://dx.doi.org/10.1016/S0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- 9.Manning GS, Holtzer A. Application of polyelectrolyte limiting laws to potentiometric titration. J Phys Chem. 1973;77(18):2206–2212. doi: 10.1021/j100637a010. Available from: http://dx.doi.org/10.1021/j100637a010. [DOI] [Google Scholar]
- 10.Nelson GJ. Blood lipids and lipoproteins, quantitation, composition and metabolism. New York, London, Sydney, Toronto: Wiley-Interscience; 1972. [Google Scholar]
- 11.Stoffel W, Boberg H, Greve V. Application of specific extracorporeal removal of low density lipoprotein in familial hypercholesterolemia. Lancet. 1981;2(8254):1005–1007. doi: 10.1016/S0140-6736(81)91213-7. Available from: http://dx.doi.org/10.1016/S0140-6736(81)91213-7. [DOI] [PubMed] [Google Scholar]
- 12.Saal SD, Parker TS, Gordon BR, Studebaker J, Hudgins L, Ahrens EH, Jr, Rubin AL. Removal of low density lipoproteins in patients with extracorporeal immunoadsorption. Am J Med. 1986;80(4):583–589. doi: 10.1016/0002-9343(86)90811-9. Available from: http://dx.doi.org/10.1016/0002-9343(86)90811-9. [DOI] [PubMed] [Google Scholar]
- 13.Moorjani S, Lupien PJ, Awad J. Extracorporeal removal of plasma lipoproteins by affinity binding to heparin-agarose. Clin Chim Acta. 1977;77(1):21–30. doi: 10.1016/0009-8981(77)90397-7. Available from: http://dx.doi.org/10.1016/0009-8981(77)90397-7. [DOI] [PubMed] [Google Scholar]
- 14.Lupien PJ, Moorjani S, Lou M, Brun D, Gagné C. Removal of cholesterol from blood by affinity binding to heparin-agarose: evaluation on treatment in homozygous familial hypercholesterolemia. Pediatr Res. 1980;14(2):113–117. doi: 10.1203/00006450-198002000-00009. Available from: http://dx.doi.org/10.1203/00006450-198002000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer T, Armstrong VW, Wieland H, Fuchs C, Scheier F, Seidel D. Selective removal of low density lipoproteins (LDL) by precipitation at low pH:first clinical application of the HELP system. Klin Wochenschr. 1987;65(4):161–168. doi: 10.1007/BF01728226. Available from: http://dx.doi.org/10.1007/BF01728226. [DOI] [PubMed] [Google Scholar]
- 16.Burstein M, Scholnik HR. Lipoprotein-polyanion-metal interactions. In: Paoletti R, Kritchevsky D, editors. Advances in Lipid Research. New York: Raven Press; 1973. pp. 67–108. [PubMed] [Google Scholar]
- 17.Srinivasan SR, Lopez A, Radhakrishnamurthy B, Berenson GS. Complexing of serum pre-beta and beta-lipoproteins and acid mucopolysaccharides. Atherosclerosis. 1970;12(3):321–334. doi: 10.1016/0021-9150(70)90036-5. Available from: http://dx.doi.org/10.1016/0021-9150(70)90036-5. [DOI] [PubMed] [Google Scholar]
- 18.Hill P, Dvornik D. Agents affecting lipid metabolism. XXXVII. Separation of rat serum lipoproteins with dextran sulfate. Can J Biochem. 1969;47(11):1043–1047. doi: 10.1139/o69-167. Available from: http://dx.doi.org/10.1139/o69-167. [DOI] [PubMed] [Google Scholar]
- 19.Kerscher L, Schiefer S, Draeger B, Maier J, Ziegenhorn J. Precipitation methods for the determination of LDL-Cholesterol. Clin Biochem. 1985;18(2):118–125. doi: 10.1016/S0009-9120(85)80093-X. Available from: http://dx.doi.org/10.1016/S0009-9120(85)80093-X. [DOI] [PubMed] [Google Scholar]
- 20.Bernfeld P, Nisselbaum JS, Berkeley BJ, Hanson RW. The influence of chemical and physicochemical nature of macromolecular polyanions on their interaction with human serum beta-lipoproteins. J Biol Chem. 1960;235(10):2852–2859. [Google Scholar]
- 21.Iverius PH. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972;247(8):2607–2613. [PubMed] [Google Scholar]
- 22.Schiele J, Heuck CC. Modulations of thrombin activity by polyeelctrolytes in the presence of antithrombin III. Semin Thromb Hemost. 1991;17(suppl 1):35–41. [PubMed] [Google Scholar]
- 23.Hollmann O, Czeslik C. Characterisation of a planar poly(acrylic acid) brush as a materials coating for controlled protein immobilization. Langmuir. 2006;22(7):3300–3305. doi: 10.1021/la053110y. Available from: http://dx.doi.org/10.1021/la053110y. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Ballauff M. Spherical polyelectrolyte brushes: comparison between annealed and quenched brushes. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64(5 Pt 1):051406. doi: 10.1103/PhysRevE.64.051406. Available from: http://dx.doi.org/10.1103/PhysRevE.64.051406. [DOI] [PubMed] [Google Scholar]
- 25.Castelnovo M, Sens P, Joanny JF. Charge distribution on anealed polyelectrolytes. Eur Phys J. 2000;E-1:115–125. [Google Scholar]
- 26.Berghold G, van der Schoot P, Seidel Ch. Equilibrium charge distribution on week polyelectrolytes. J Chem Phys. 1997;107:8083–8088. doi: 10.1063/1.475071. Available from: http://dx.doi.org/10.1063/1.475071. [DOI] [Google Scholar]
- 27.Czeslik C, Jansen R, Ballauff M, Wittemann A, Royer CA, Gratton E, Hazlett T. Mechansims of protein binding to spherical polyelectrolyte brushes in-situ using two-photon excitation fluorescence fluctuation spectroscopy. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69(2 Pt 1):021401. doi: 10.1103/PhysRevE.69.021401. Available from: http://dx.doi.org/10.1103/PhysRevE.69.021401. [DOI] [PubMed] [Google Scholar]
- 28.La Belle M, Blanche PJ, Krauss RM. Charge properties of low density lipoproteins. J Lipid Res. 1997;38(4):690–700. [PubMed] [Google Scholar]
- 29.Desrumaux C, Athias A, Masson D, Gambert P, Lallemant C, Lagrost L. Influence of the electrostatic charge of lipoprotein particles on the activity of the human plasma phospholipid transfer protein. J Lipid Res. 1998;39(1):131–142. [PubMed] [Google Scholar]
- 30.Bambauer R, Schiel R, Latza R. Low density lipoprotein apheresis in treatment of hyperlipidemia: Experience with four different technologies. Ther Apher. 2000;4(3):213–217. doi: 10.1046/j.1526-0968.2000.00180.x. Available from: http://dx.doi.org/10.1046/j.1526-0968.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- 31.Osada Y, Sato M. Thermal equilibrium of the intermolecular complexes of polycarboxylic acids realized by cooperative hydrogen bonding. J Polymer Sci Polymer Lett Ed. 1976;14(3):129–134. doi: 10.1002/pol.1976.130140302. Available from: http://dx.doi.org/10.1002/pol.1976.130140302. [DOI] [Google Scholar]
- 32.Mattai J, Kwak JC. Mg and Ca binding to heparinin the presence of univalent salt. Biochim Biophys Acta. 1981;677(2):303–312. doi: 10.1016/0304-4165(81)90100-8. Available from: http://dx.doi.org/10.1016/0304-4165(81)90100-8. [DOI] [Google Scholar]
- 33.Boyd J, Williamson FB, Gettins P. A physico-chemical study on heparin. Evidence for calcium-induced co-operative conformational transition. J Mol Biol. 1980;137(2):175–190. doi: 10.1016/0022-2836(80)90324-1. Available from: http://dx.doi.org/10.1016/0022-2836(80)90324-1. [DOI] [PubMed] [Google Scholar]
- 34.Liang JN, Chakrabarti B. An essential role fort he 2-sulfamino group in the interaction of calcium ions with heparin. Carbohydrate Res. 1982;106(1):101–109. doi: 10.1016/S0008-6215(00)80736-3. Available from: http://dx.doi.org/10.1016/S0008-6215(00)80736-3. [DOI] [Google Scholar]
- 35.Chang DM. The binding of free calcium ions in aqueous solution using chelating agents, phopsphates and poly(acrylic acid) J Am Oil Chem Soc. 1983;60(3):618–622. doi: 10.1007/BF02679800. Available from: http://dx.doi.org/10.1007/BF02679800. [DOI] [Google Scholar]
- 36.Sabbagh I, Delsanti M. Solubiluty of highly charged anionic polyeelctrolytes in the presence of multivalent cations: specific interaction effect. Eur Phys J E Soft Matter Biol Phys. 2000;1(1):75–86. doi: 10.1007/s101890050009. Available from: http://dx.doi.org/10.1007/s101890050009. [DOI] [Google Scholar]
- 37.Sabbagh I, Delsanti M, Lesieur P. Ionic distribution and polymer conformation, near phase separation, in sodium polyacrylate/divalent cation mixtures: small angle X-ray and neutron scattering. Eur Phys J B Condensed Matter Complex Sys. 1999;12:253–260. doi: 10.1007/s100510051002. Available from: http://dx.doi.org/10.1007/s100510051002. [DOI] [Google Scholar]
- 38.Schweins R, Lindner P, Huber K. Calcium induced shrinking of NaPA chains: a SANS Investigation of single chain behaviour. Macromolecules. 2003;36(25):9564–9573. doi: 10.1021/ma0347722. Available from: http://dx.doi.org/10.1021/ma0347722. [DOI] [Google Scholar]