Abstract
Gram-positive pili are composed of covalently bound pilin subunits whose assembly is mediated via a pilus-specific sortase(s). Major subunits constitute the pilus backbone and are therefore essential for pilus formation. Minor subunits are also incorporated into the pilus, but they are considered to be dispensable for backbone formation. The srtG cluster is one of the putative pilus gene clusters identified in the major swine pathogen Streptococcus suis. It consists of one sortase gene (srtG) and two putative pilin subunit genes (sgp1 and sgp2). In this study, by constructing mutants for each of the genes in the cluster and by both immunoblotting and immunogold electron microscopic analysis with antibodies against Sgp1 and Sgp2, we found that the srtG cluster mediates the expression of pilus-like structures in S. suis strain 89/1591. In this pilus, Sgp1 forms the backbone, whereas Sgp2 is incorporated as the minor subunit. In accordance with the current model of pilus assembly by Gram-positive organisms, the major subunit Sgp1 was indispensable for backbone formation and the cognate sortase SrtG mediated the polymerization of both subunits. However, unlike other well-characterized Gram-positive bacterial pili, the minor subunit Sgp2 was required for polymerization of the major subunit Sgp1. Because Sgp2 homologues are encoded in several other Gram-positive bacterial pilus gene clusters, in some types of pili, minor pilin subunits may contribute to backbone formation by a novel mechanism.
Pilus-like structures on the surface of Gram-positive bacteria were first observed in Corynebacterium renale by electron microscopy (50), and recently, these surface appendages have been characterized genetically and biochemically in many additional Gram-positive bacterial pathogens (18, 33, 44, 46). Gram-positive bacterial pili are anchored to the cell wall peptidoglycan and consist of covalently cross-linked subunit proteins (18, 33, 44, 46). Polymerized monomers of a single major pilin subunit form the pilus backbone, to which one or more minor (or ancillary) pilin subunits are attached (18, 33, 44, 46). Both the major and minor subunits contain C-terminal cell wall sorting signals (CWSSs), composed of a pentapeptide motif represented by LPXTG (where X is any amino acid), a C-terminal hydrophobic domain, and a charged tail (32). The subunits are assembled via CWSSs by the action of pilus-specific class C sortases (8).
Major pilin subunits have been shown to be indispensable for pilus formation. In contrast, early studies showed that minor subunits are not essential for pilus synthesis and assembly (10, 39, 47). It has only recently been reported that minor pilin subunits of SpaA- and SpaH-type pili of Corynebacterium diphtheriae and Spy0130 (FctB) of a Streptococcus pyogenes serotype M1 strain participate in the termination of pilus polymerization as well as in anchoring the polymer to the cell wall peptidoglycan (17, 35). However, in many Gram-positive bacterial pili, the roles of minor pilin subunits in pilus formation remain to be fully elucidated.
Streptococcus suis is a Gram-positive coccus responsible for a wide range of diseases in pigs, including meningitis, septicemia, endocarditis, and sudden death (14, 37). This bacterium can also affect humans in close contact with diseased pigs or swine products (1, 7, 43, 49, 51). Four putative pilus gene clusters, named the srtBCD, srtE, srtF, and srtG clusters, have so far been identified in S. suis (9, 40). The srtG cluster, identified in strain 89/1591, consists of one putative sortase gene, srtG, and two putative pilin subunit genes, sgp1 and sgp2 (Fig. 1A). The genetic organization of the srtG cluster was similar to that of the fibronectin-binding, collagen-binding, T-antigen 1 (FCT-1) region of S. pyogenes (21). Moreover, the amino acid sequences of SrtG, Sgp1, and Sgp2 showed 35 to 56% identity with those of the proteins encoded by the corresponding genes for the FCT-1 region (40). However, despite the in silico predictions, it is unknown whether this cluster is associated with the expression of pilus fibers in S. suis.
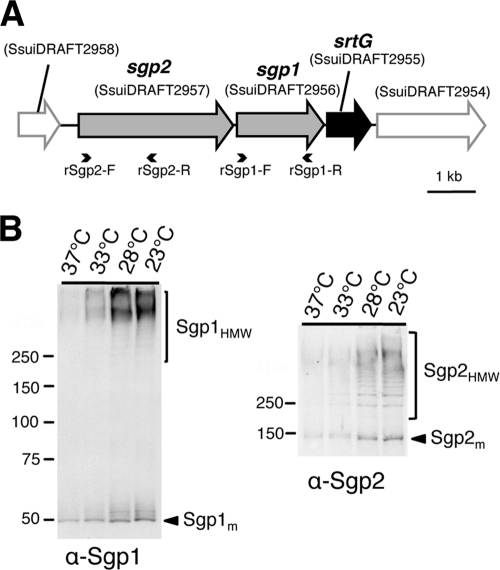
FIG. 1.
Surface expression of Sgp1 and Sgp2 in wild-type strain 89/1591. (A) Genetic organization of the srtG pilus cluster. This schema is constructed on the basis of the draft genome sequence of S. suis 89/1591, updated in February 2009 (GenBank accession no. AAFA03000013). Gray arrows, genes encoding CWSS-containing proteins (sgp1 and sgp2); black arrow, sortase-encoding gene (srtG); open arrows outlined in gray, genes flanking the cluster; small arrowheads, annealing positions of the primers used to generate His-tagged recombinant Sgp1 and Sgp2. (B) Immunoblots with anti-Sgp1 (α-Sgp1; left panel) and anti-Sgp2 (α-Sgp2; right panel) rabbit pAbs using bacterial cells at different growth temperatures (37°C, 33°C, 28°C, or 23°C). Bacteria were grown in THB until the optical density at 600 nm reached 0.35, which corresponds to the mid-log phase. Each lane contains cell wall fractions extracted from cells in 750-μl cultures with an optical density at 600 nm of 0.35. The calculated molecular weights of Sgp1m and Sgp2m are 52,045 and 104,403, respectively. Numbers to the left of the gels are molecular weights (in thousands).
In this study, we generated polyclonal antibodies (pAbs) against the two putative pilin subunits (Sgp1 and Sgp2) and identified reactive pilus-like appendages on the surface of S. suis 89/1591 by both immunoblotting and immunogold electron microscopy (IEM). By constructing a series of isogenic mutant strains, we demonstrated that the srtG cluster mediates pilus formation in S. suis 89/1591 and that the minor subunit Sgp2 is necessary for polymerization of the major subunit Sgp1.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. suis strains were grown in Todd-Hewitt (Becton Dickinson, Sparks, MD) broth (THB) or agar (THA) at 37°C in a 5% CO2 atmosphere, unless otherwise specified. Escherichia coli strains were cultured in Luria-Bertani (Becton Dickinson) broth or agar at 37°C. When required, the following antibiotics were added to the medium at the indicated concentrations: for E. coli, ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml; and spectinomycin, 50 μg/ml; for S. suis, spectinomycin, 100 μg/ml. When necessary, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside was added to the plates at 100 μg/ml.
TABLE 1.
Plasmids and bacterial strains used in this study
| Plasmid or strain | Relevant properties | Source or reference |
|---|---|---|
| Plasmids | ||
| pCR2.1 | Apr Kmr, pUC ori, f1 ori, lacZΔM15 | Invitrogen |
| pDIA17 | Cmr, p15A ori, Tet promoter, lacI | 22 |
| pIVEX2.4d | Apr, pUC ori, T7 promoter, His tag-coding sequence | Roche |
| pSET4s | Spcr, pUC ori, thermosensitive pG+host3 ori, lacZΔM15 | 42 |
| pMX1 | Spcr, pSSU1 ori, malX promoter of S. suis, derivative of pSET2 | This study |
| pSrtG | pMX1 carrying intact srtG gene | This study |
| pSgp1 | pMX1 carrying intact sgp1 gene | This study |
| pSgp2 | pMX1 carrying intact sgp2 gene | This study |
| S. suis strains | ||
| 89/1591 | Wild-type strain isolated from a pig with septicemia and meningitis | 30 |
| ΔsrtG | 89/1591 derivative strain carrying an in-frame deletion in srtG | This study |
| Δsgp1 | 89/1591 derivative strain carrying an in-frame deletion in sgp1 | This study |
| Δsgp2 | 89/1591 derivative strain carrying an in-frame deletion in sgp2 | This study |
| CPS2B | 89/1591 derivative strain carrying an insertionally inactivated cps2B (cps2B::pSET4s) | This study |
| E. coli strains | ||
| TOP10 | Host for pCR2.1 and pSET4s derivatives | Invitrogen |
| BL21(DE3)/pDIA17 | Host for pIVEX2.4d derivatives | 28 |
| MC1061 | Host for pMX1 derivatives | 6 |
DNA techniques.
Restriction enzymes and DNA-modifying enzymes were purchased from Takara Bio (Otsu, Japan) and used according to the manufacturer's recommendations. Purification of plasmid DNA from E. coli and transformation of E. coli were performed using standard procedures (31). Genomic DNA of S. suis was extracted by the method described previously (25), and S. suis was transformed by electroporation, as described previously (41). Ex Taq polymerase (Takara Bio) and iProof HF master mix (Bio-Rad Laboratories, Hercules, CA) were used for PCR amplification. Primers used in this study are listed in Table S1 in the supplemental material. Sequencing was carried out with a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Foster City, CA) and analyzed on a 3100 genetic analyzer or 3130xl genetic analyzer (Applied Biosystems).
Generation of S. suis mutants.
For the construction of precise in-frame deletions in srtG, sgp1, and sgp2, upstream and downstream regions of these genes were amplified by PCR and fused by overlap-extension PCR (48). For insertional inactivation of cps2B, an internal region of the gene was amplified. The resulting PCR products were cloned into pCR2.1 (Invitrogen, San Diego, CA), excised with EcoRI, and recloned into the EcoRI site of temperature-sensitive S. suis-E. coli shuttle vector pSET4s (42). The resulting plasmids were introduced into S. suis 89/1591 by electroporation. The single-crossover mutant (CPS2B) and double-crossover mutants (ΔsrtG, Δsgp1, and Δsgp2) were generated according to the procedures described previously (42) with slight modifications. Briefly, the single-crossover mutants were obtained by culturing the cells on THA with spectinomycin at 37°C, and the double-crossover mutants were generated by repeated passaging at 28°C on THA without the antibiotic. The deletion or inactivation of target genes was confirmed by PCR and sequence analyses.
Complementation of deletion mutants.
The pMX1 vector was used for the generation of recombinant plasmids for complementation analysis (Table 1). This vector is a derivative of the S. suis-E. coli shuttle cloning vector pSET2 (41) and possesses the S. suis malX promoter for transgene expression in S. suis. The entire srtG, sgp1, and sgp2 genes were amplified from genomic DNA of S. suis 89/1591 and cloned into pMX1 via EcoRI and BamHI sites (srtG and sgp1) or the NcoI site (sgp2), generating complementation vectors pSrtG, pSgp1, and pSgp2, respectively. These plasmids were introduced into E. coli MC1061 for verification of the sequences and then into the respective deletion mutants derived from S. suis 89/1591 to construct srtG-, sgp1-, and sgp2-complemented mutants.
Preparation of His-tagged recombinant proteins and polyclonal antibodies.
Internal fragments of sgp1 and sgp2 were amplified with primer sets rSgp1-F/rSgp1-R and rSgp2-F/rSgp2-R (Fig. 1A), respectively, and cloned into pCR2.1. After excision with NdeI and BamHI, the fragments were ligated into the pIVEX 2.4d vector (Roche Applied Science, Basel, Switzerland). The resulting products were introduced into E. coli TOP10 for verification of the sequences and E. coli BL21(DE3)/pDIA17 (28) for protein expression. Recombinant protein synthesis was induced with isopropyl-β-d-thiogalactopyranoside, as previously described (28). Recombinant proteins were purified by affinity chromatography on nickel-nitrilotriacetic acid columns (Protino protein purification system; Macherey-Nagel, Düren, Germany), according to the manufacturer's instructions. Protein purity was checked by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and protein concentrations were determined accurately using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL).
Purified proteins were used for polyclonal antibody production in rabbits at Iwaki Corporation (Tokyo, Japan) and in mice at Operon Biotechnologies (Tokyo, Japan). As necessary, the resulting rabbit antibodies were purified with a HiTrap Protein G HP column (GE Healthcare, Piscataway, NJ) and a Microcon YM-10 centrifugal filter device (Millipore, Bedford, MA), according to the manufacturers' recommendations. Purification of mouse antiserum was performed by Operon Biotechnologies.
Preparation of bacterial cell wall, protoplast, and culture supernatant fractions.
S. suis cultures were harvested, washed once with TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), suspended in spheroplasting buffer (20 mM Tris-HCl [pH 6.8], 10 mM MgCl2, 26% raffinose, 500 units/ml mutanolysin, 1 tablet/10 ml complete mini-EDTA-free protease inhibitor cocktail [Roche]) (20), and incubated for 1 h at 37°C to digest peptidoglycan with mutanolysin. After centrifugation at 13,000 × g for 5 min at 4°C, the supernatants including digested peptidoglycan and released cell wall-associated proteins were separated from protoplasts and collected as cell wall fractions. The protoplast pellets were washed once with protoplast buffer (20 mM Tris-HCl [pH 6.8], 10 mM MgCl2, 26% raffinose) and suspended in phosphate-buffered saline (PBS; pH 7.4) containing 2% Triton X-100. Culture supernatants were filtered through a 0.22-μm-pore-size filter, precipitated by trichloroacetic acid, washed twice with acetone, and dried.
Coimmunoprecipitation.
The cell wall and protoplast fractions extracted from 15-ml cultures grown overnight at 28°C were incubated with 1 μg purified anti-Sgp1 or anti-Sgp2 rabbit pAb at room temperature (RT) for 1 h with end-over-end rotation. For coimmunoprecipitation, 25 μl protein G Dynabeads (Invitrogen) was added to the mixtures, and the mixtures were incubated at RT for 1 h with end-over-end rotation. Immunoprecipitated complexes bound to protein G beads were then pulled down and washed four times with PBS containing 2% Triton X-100 using a side-pull magnetic isolation apparatus (Invitrogen). Isolated beads were used for immunoblot analysis as described below.
Immunoblot analysis.
Prepared fractions or isolated protein G beads were boiled in SDS-PAGE sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 0.5% bromophenol blue, 10% glycerol, 60 μl/ml β-mercaptoethanol) for 10 min before gel electrophoresis. The samples were separated by 10% precast polyacrylamide gels (Atto Corp., Tokyo, Japan) and transferred to nitrocellulose (Whatman, Kent, United Kingdom) or polyvinylidene fluoride (Bio-Rad) membranes. The membranes were blocked with blocking solution (Western blocking reagent [Roche] diluted 1:10 in maleic acid buffer [Roche]) for 2 h at RT and were subsequently incubated at RT with anti-Sgp1 or anti-Sgp2 pAb diluted 1:1,000 in blocking solution (for anti-Sgp1 pAb) or Can Get signal immunoreaction enhancer solution 1 (for anti-Sgp2 pAb) (Toyobo Corp., Osaka, Japan) for 1 h. The membranes were then washed three times with washing buffer (diluted 1:10 in distilled water; Roche) and incubated at RT with alkaline phosphatase-conjugated anti-rabbit or anti-mouse IgG (MP Biomedicals, Irvine, CA) diluted 1:5,000 in washing buffer (for Sgp1 detection) or Can Get signal immunoreaction enhancer solution 2 (for Sgp2 detection) for 1 h. After three washes with washing buffer, Sgp1 or Sgp2 was detected with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate substrates (Roche) or CDP-Star ready-to-use chemiluminescent substrates (Roche). Densitometry analysis of immunoblots developed with chemiluminescent substrates was performed using a VersaDoc imaging system and Quantity One software (Bio-Rad). For detection of differences, densities were compared by Student's t test.
Partial purification of srtG pili.
S. suis strains were grown for 12 h at 28°C in 200 ml THB, harvested, washed once in PBS, suspended in 1 ml modified spheroplasting buffer (containing 20% sucrose instead of 26% raffinose), and incubated at 37°C for 7 h with gentle shaking to digest peptidoglycan with mutanolysin. After incubation, supernatants containing released pilus material were loaded onto sucrose gradients (25 to 55% in 20 mM Tris-HCl [pH 6.8], 10 mM MgCl2) and centrifuged at 25,000 × g for 20 h by a Beckman L-60 ultracentrifuge (Beckman-Coulter, Fullerton, CA) with an SW41Ti rotor at 4°C. Collected gradient fractions were tested for the presence of pilus subunits by immunoblotting. The fractions containing subunit polymers were pooled, treated with benzonase nuclease (Novagen, Madison, WI) to remove DNA and RNA impurities, and dialyzed against 20 mM Tris-HCl (pH 6.8)-10 mM MgCl2 to remove sucrose.
IEM.
S. suis strains were grown for 12 h at 28°C in 5 ml THB, harvested by centrifugation, washed once with PBS, and suspended in approximately 5 ml PBS containing 2% paraformaldehyde (PFA). After fixation for 5 min, the bacterial suspensions were placed on collodion-coated copper grids (Nisshin EM, Tokyo, Japan) for 10 min and allowed to dry. For mounting partially purified pili, 10-μl samples were placed on grids for 5 min, fixed with PBS containing 2% PFA for 5 min, and allowed to dry. Grids were subsequently blocked with 10% normal horse serum in dilution buffer (PBS containing 1% bovine serum albumin and 1% Tween 20, pH 7.4) for 30 min at RT. Thereafter, the grids were soaked in anti-Sgp1 mouse pAb diluted 1:20 in dilution buffer for 1 h and washed three times with dilution buffer. The grids were then soaked in 10-nm gold-conjugated goat anti-mouse IgG (British BioCell International, Cardiff, United Kingdom) diluted 1:30 in dilution buffer, incubated for 1 h at RT, and washed five times with dilution buffer. For double-labeling experiments, the same procedure was further applied using anti-Sgp2 rabbit pAb and 20-nm gold-conjugated goat anti-rabbit IgG. Finally, samples were fixed in PBS containing 2% PFA for 10 min at RT, washed eight times with distilled water, and allowed to dry. The grids were observed with an H7650 electron microscope (Hitachi Ltd., Tokyo, Japan) at an accelerating voltage of 80 kV.
RESULTS
Sgp1 and Sgp2 are expressed on the bacterial cell surface and polymerized by cognate SrtG.
Polymerized pilin subunits of Gram-positive bacteria cannot be dissociated by boiling in SDS-PAGE sample buffer because of covalent subunit-subunit linkages and thus can be detected as a high-molecular-weight (HMW) ladder by SDS-PAGE immunoblotting (33, 46, 47). To investigate whether HMW polymers of putative pilin subunits Sgp1 and Sgp2 (Sgp1HMW and Sgp2HMW, respectively) are expressed on the cell surface, we extracted the cell wall fractions of S. suis 89/1591 (wild-type strain) from cultures grown at four different temperatures (37°C, 33°C, 28°C, and 23°C) and analyzed them by immunoblotting with anti-Sgp1 and anti-Sgp2 rabbit pAbs. Under all growth conditions, besides the signals corresponding to the predicted monomeric forms of Sgp1 and Sgp2 (Sgp1m and Sgp2m, respectively), HMW ladders were detected by both anti-Sgp1 and anti-Sgp2 pAbs (Fig. 1B and Fig. 2 A and B, lanes 1). The immunoreactive signals in bacterial samples grown at temperatures above 30°C were noticeably weaker than in those grown at temperatures below 30°C (Fig. 1B). Therefore, in subsequent experiments bacteria were grown at 28°C for pilus detection.
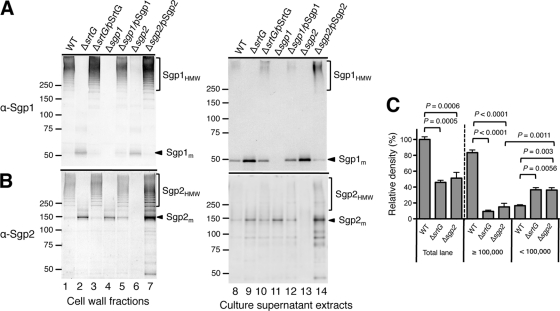
FIG. 2.
Detection of polymerized subunits in cell wall fractions and culture supernatants by immunoblotting with anti-Sgp1 (α-Sgp1; A) or anti-Sgp2 (α-Sgp2; B) rabbit pAb. Immunoblotting was performed using the wild-type (WT) strain and isogenic derivatives (ΔsrtG, Δsgp1, and Δsgp2). The strains were grown in THB at 28°C for 18 h. Each lane contains cell wall fractions extracted from cells in 500-μl cultures adjusted to an optical density at 600 nm of 0.6 or culture supernatants extracted from 500 μl of the adjusted cultures. In panels A and B, numbers to the left of the gels are molecular weights (in thousands). (C) Comparison of the relative density of reactive bands in the mixture of cell wall and culture supernatant fractions on immunoblots with anti-Sgp1 rabbit pAb. Cell wall and culture supernatant fractions of each strain were extracted from an equal volume of the culture adjusted to an optical density at 600 nm of 0.6 and mixed. The mixture corresponding to 500 μl of the adjusted culture was loaded in each lane and analyzed by immunoblotting. The sum of the density values of the bands within the total lane and lanes with molecular weights of ≥100,000 or <100,000 was calculated. Data were collected from five independent experiments, corrected by subtracting the density value of Δsgp1, and are expressed as a percentage of the density values obtained in the total lane of the wild-type strain (mean ± standard deviation). Significant differences between two data are indicated by P values on the basis of Student's t test.
No immunoreactive signals of Sgp1 and Sgp2 were detected in the cell wall fractions extracted from Δsgp1 and Δsgp2 mutants, respectively (Fig. 2A, lane 4, and B, lane 6). The signals were restored by introduction of the intact genes into the respective mutants (Fig. 2A, lane 5, and B, lane 7). These results suggest that both putative subunits Sgp1 and Sgp2 are expressed and polymerized on the cell surface of strain 89/1591. Of note, disruption of one pilin subunit gene affected the polymerization of the other subunit (Fig. 2A, lane 6, and B, lane 4). These mutations were complemented by reintroduction of the respective genes (Fig. 2A, lane 7, and B, lane 5).
We also generated a ΔsrtG mutant and investigated whether SrtG, like cognate sortases in the pilus clusters of other Gram-positive bacteria, is necessary for the polymerization of Sgp1 and Sgp2. In the cell wall fraction of the mutant, both Sgp1HMW and Sgp2HMW completely disappeared. Instead, Sgp1m and Sgp2m accumulated (Fig. 2A and B, lanes 2). Polymerization of the subunits was fully restored, and the signal intensities of Sgp1m and Sgp2m were reduced by introducing intact srtG into the mutant (Fig. 2A and B, lanes 3). These results suggest that SrtG is the specific pilin polymerase required for the polymerization of both Sgp1 and Sgp2.
Sgp1 and Sgp2 are components of the same pilus structure.
To investigate whether the HMW polymers on the cell surface of the wild-type strain are composed of both Sgp1 and Sgp2, we performed immunoadsorption on the cell wall fractions using anti-Sgp1 or anti-Sgp2 rabbit pAb. Immunoprecipitated complexes were captured using protein G-coupled magnetic beads and were analyzed by immunoblotting using anti-Sgp1 mouse and anti-Sgp2 rabbit pAbs. Neither Sgp1 nor Sgp2 was detected in the cell wall fraction of the wild-type strain precipitated with preimmune rabbit serum of anti-Sgp1 or anti-Sgp2 pAb (data not shown). As expected, anti-Sgp1 pAb recovered both Sgp1HMW and Sgp1m from the wild-type strain (Fig. 3A, lane 1), but neither form was precipitated from the Δsgp1 mutant (Fig. 3A, lane 5). In addition, the anti-Sgp1 pAb coimmunoprecipitated Sgp2HMW from the wild-type strain (Fig. 3B, lane 1). Similarly, anti-Sgp2 pAb coimmunoprecipitated Sgp1HMW along with Sgp2HMW and Sgp2m from the wild-type strain (Fig. 3, lanes 2) but not from the Δsgp2 mutant (Fig. 3, lanes 6). These results indicate that Sgp1 and Sgp2 are components of the same pilus structure.
FIG. 3.
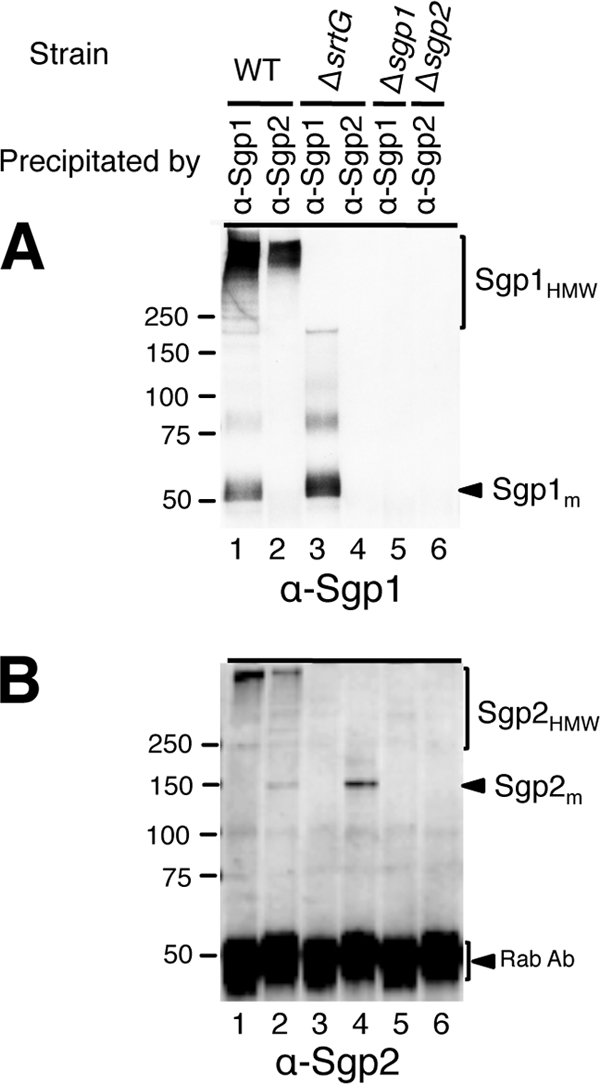
Association of Sgp2 with Sgp1 in cell wall fractions. Cell wall fractions were extracted from cultures grown in THB at 28°C overnight. Each lane contains coimmunoprecipitated complexes of 0.1 μg anti-Sgp1 (α-Sgp1) or anti-Sgp2 (α-Sgp2) rabbit pAb and cell wall fractions extracted from cells in 1.5-ml cultures. The blot was probed with anti-Sgp1 mouse or anti-Sgp2 rabbit pAb. Rab Ab, reactive signals of α-Sgp1 or α-Sgp2 rabbit pAb used for coimmunoprecipitation. Numbers to the left of the gels are molecular weights (in thousands).
Electron microscopic evidence of pilus-like structures and localization of Sgp1 and Sgp2.
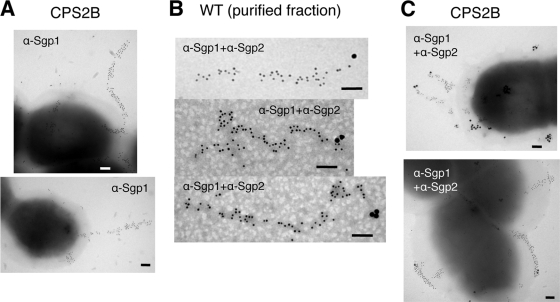
To confirm the formation of fiber structures composed of Sgp1 and Sgp2, we carried out IEM with anti-Sgp1 mouse and anti-Sgp2 rabbit pAbs. IEM using anti-Sgp1 pAb showed the presence of appendages labeled by immunogold particles on the cell surface of the wild-type strain. However, most of the surface appendages did not show the typical extended structure observed in pili of other Gram-positive bacteria (data not shown). We suspected that the thick polysaccharide capsule produced by strain 89/1591 may be cloaking pili, affecting their surface display and/or interfering with antibody binding. We thus constructed and examined the mutant carrying a disruption of the cps2B gene (mutant CPS2B; cps2B::pSET4s), which is necessary for capsule biosynthesis (34). Immunoblot analysis showed that this mutant expressed Sgp1 and Sgp2 (data not shown). On the cell surface of the mutant, typical pilus-like structures were clearly observed and the immunogold-labeled Sgp1 uniformly distributed along the structures (Fig. 4A). We then performed a double-labeling IEM experiment to show the localization of Sgp2 on the pilus structure. In the purified cell wall fraction, Sgp2 appeared to be located at one end of the structure (Fig. 4B). In addition, labeled Sgp2 was positioned both at the tip of Sgp1 pilus structures and on the cell surface of strain CPS2B (Fig. 4C). These results supported the localization of Sgp2 at the end of the pilus structure. In all experiments, preimmune sera of anti-Sgp1 and anti-Sgp2 pAbs did not label pilus fibers. Taken together, IEM and immunoblot analysis results demonstrate that Sgp1 is a major pilin subunit forming the pilus backbone, while Sgp2 is an ancillary subunit which appears to localize at one end of the structure.
FIG. 4.
Immunogold staining of pilus structures of S. suis strains. (A) Immunogold-labeled Sgp1 (gold particle size, 10 nm) on the cell surfaces of strain CPS2B. (B and C) Double immunogold labeling with mouse anti-Sgp1 pAbs (α-Sgp1; gold particle size, 10 nm) and rabbit anti-Sgp2 pAbs (α-Sgp2; gold particle size, 20 nm) in the partially purified cell wall fraction of the wild-type (WT) strain (B) and on the cell surface of strain CPS2B (C). Scale bars = 0.1 μm.
Minor subunit Sgp2 is required for polymerization of major subunit Sgp1.
Unlike the minor subunits of most well-characterized pili of Gram-positive bacteria, which were considered to be dispensable for polymerization of the major subunit (4, 10, 15, 29, 39), deletion of the gene encoding the minor subunit Sgp2 affected the polymerization of Sgp1 on the cell surface (Fig. 2A, lane 6). One possible explanation for these results might be that increased release of Sgp1HMW from the cell surface into the culture medium causes the reduction of cell surface Sgp1HMW in the Δsgp2 mutant. To test this hypothesis, we investigated culture supernatant fractions by immunoblotting. In the wild-type strain, both Sgp1HMW and Sgp1m were detected (Fig. 2A, lane 8). In the Δsgp2 mutant, the amount of Sgp1HMW released into the supernatant was noticeably lower than that observed in the wild-type strain (Fig. 2A, lane 13), but the amount of Sgp1m released was as high as that observed in the ΔsrtG mutant (Fig. 2A, lanes 9 and 13). The amount of released Sgp1m diminished when intact sgp2 was reintroduced into the mutant. In addition, in the complemented strain, the amount of released Sgp1HMW increased (Fig. 2A, lane 14). We also compared the density of anti-Sgp1-reactive bands in the mixtures of cell wall and culture supernatant fractions of the Δsgp2 mutant with the densities of the wild-type strain and the ΔsrtG mutant. As shown in Fig. 2C, in the Δsgp2 mutant, the density of bands migrating at molecular weights of <100,000 was significantly higher than that of bands migrating at molecular weights of ≥100,000, accounting for more than 70% of the total density of all reactive bands. Similar results were obtained in the ΔsrtG mutant. In contrast, in the wild-type strain, approximately 83% of the total density of all reactive bands was derived from the bands migrating at molecular weights of ≥100,000. In addition, the density of bands within molecular weights of <100,000 was 2.2-fold higher in the Δsgp2 mutant than in the wild-type strain and was the same as that in the ΔsrtG mutant. Taken together, these results indicate that the weak signal of Sgp1HMW in the cell wall fraction of the Δsgp2 mutant (Fig. 2A, lane 6) was not caused by an increased release of Sgp1 polymers into the culture medium but reflected the abrogation of polymerization of Sgp1 in the absence of Sgp2. It is noteworthy that the total density of all reactive bands observed in the Δsgp2 and ΔsrtG mutants was approximately only 50% of that observed in the wild-type strain. Because no signal was detected in the protoplast fractions of all the strains by immunoblotting (data not shown), it cannot be assumed that Sgp1 is retained in the protoplast of the mutants.
Sgp2 does not associate with Sgp1 in the absence of SrtG.
We investigated whether Sgp2 associates with Sgp1 in the absence of cognate sortase SrtG by coimmunoprecipitation. From the cell wall fraction of the ΔsrtG mutant, only Sgp1m and not Sgp2m was recovered by anti-Sgp1 pAb (Fig. 3, lanes 3). Similarly, Sgp2m but not Sgp1m was precipitated by anti-Sgp2 pAb (Fig. 3, lanes 4). Similar results were observed when the protoplast fraction was used (data not shown), suggesting that Sgp2 does not associate with Sgp1 in the absence of SrtG.
Sgp2 homologues encoded in other Gram-positive bacterial pilus gene clusters.
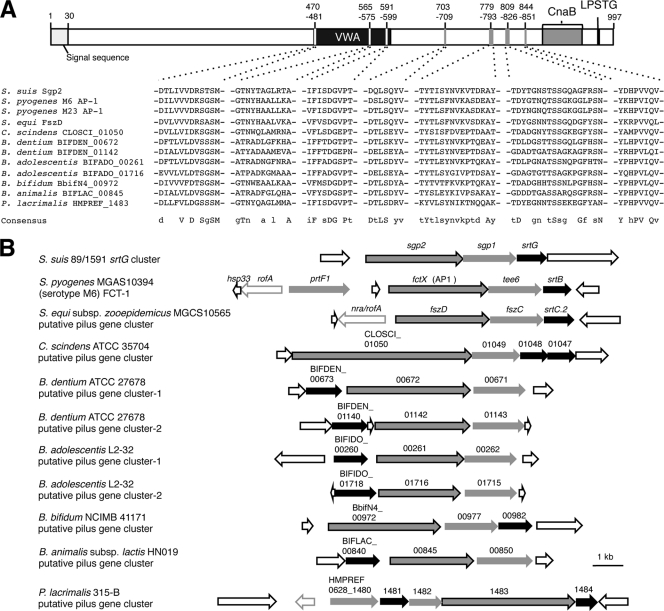
BLAST analysis of Sgp2 showed that it had 32% identity with the fimbrial structural subunit protein FszD of Streptococcus equi subsp. zooepidemicus MGCS10565 and with four minor pilins of FCT-1 regions of S. pyogenes serotype M6 strains (MGAS10394, 2724, and 3650) and a serotype M23 strain (DSM 2071). Several regions of Sgp2 are highly conserved among these proteins (data not shown), and some of the regions also show significant similarities with hypothetical proteins of Clostridium scindens, Peptoniphilus lacrimalis, and four Bifidobacterium species (Fig. 5A). These hypothetical proteins contain CWSSs and are encoded in the close vicinity of putative class C sortase genes (Fig. 5B), suggesting that they are also pilus-associated proteins. Interestingly, these pilus and putative pilus gene clusters share a relatively simple genetic organization with the srtG cluster of S. suis (Fig. 5B). With the exception of the putative pilus gene clusters of C. scindens ATCC 35704 and P. lacrimalis 315-B, all of these clusters consist of a single sortase gene and two putative pilin subunit genes. Although one more CWSS-containing protein, PrtF1, is encoded in the FCT-1 region of S. pyogenes MGAS10394, it has been shown that PrtF1 is not incorporated into the pilus structure (21). In addition, these clusters do not encode signal peptidase homologues, which are considered to be important for pilus synthesis in S. pyogenes and Streptococcus pneumoniae (2, 23, 52).
FIG. 5.
(A) Schematic maps of Sgp2 and amino acid sequences conserved in Sgp2 and pilin and putative pilin subunits of several other Gram-positive bacteria. Sgp2 harbors an N-terminal signal sequence (amino acid positions 1 to 30), a von Willebrand factor type A (vWFA) domain (amino acid positions 468 to 601), a Cna protein B-type (CnaB) domain (amino acid positions 870 to 938), and a CWSS (LPSTG; amino acid positions 963 to 967). Alignment was carried out using the ClustalW program (45). Amino acid sequences conserved in all of the aligned sequences (in uppercase letters) or in more than seven sequences (in lowercase letters) were designated consensus sequences. The GenBank accession numbers of the listed proteins are as follows: ancillary protein 1 of S. pyogenes serotype M6 strains, AAL11466 (MGAS10394), ACH87890 (2724), and ACH87891 (3650); ancillary protein 1 of S. pyogenes M23 strain (DSM 2071), ACH87900; FszD of S. equi subsp. zooepidemicus MGCS10565, ACG63149; CLOSCI_01050 of C. scindens ATCC 35704, EDS08034; BIFDEN_00672 and BIFDEN_01142 of B. dentium ATCC 27678, EDT44861 and EDT45313, respectively; BIFADO_00261 and BIFADO_01716 of B. adolescentis L2-32, EDN83355 and EDN82663, respectively; BbifN4_00972 of B. bifidum NCIMB 41171, ZP_03645701; BIFLAC_00845 of B. animalis subsp. lactis HN019, EDT88620; and HMPREF0628_1483 of P. lacrimalis 315-B, EFA89995. (B) Genetic organizations of the srtG cluster, FCT-1 region, and putative pilus gene clusters containing sgp2 homologues or genes partially homologous to sgp2. Black arrows, genes and putative genes encoding class C sortases; gray arrows, genes and putative genes encoding CWSS-containing proteins (sgp2 and genes encoding proteins partially homologous to Sgp2 are outlined in black); open arrows outlined in gray, putative transcriptional regulator genes; open arrows outlined in black, other open reading frames. These genetic maps are constructed on the basis of the whole or draft genome sequences of the respective strains, and the GenBank accession numbers are as follows: S. pyogenes MGAS10394, CP000003; S. equi subsp. zooepidemicus MGCS10565, CP001129; C. scindens ATCC 35704, ABFY02000010; B. dentium ATCC 27678, ABIX02000002; B. adolescentis L2-32, AAXD02000018 and AAXD02000052; B. bifidum NCIMB 41171, ABQP01000004; B. animalis subsp. lactis HN019, ABOT01000008; and P. lacrimalis 315-B, ADDO01000052.
DISCUSSION
The present study demonstrates that the srtG cluster of S. suis strain 89/1591 mediates the expression of pilus structures on the cell surface of this bacterium. Interestingly, our data show that in addition to the cognate sortase SrtG, the minor pilin subunit Sgp2 is required for the formation of the pilus backbone composed of major subunit Sgp1. To our knowledge, this is the first example of a minor pilin-mediated pilus polymerization system studied in detail.
In Gram-positive bacterial pili, minor pilin subunits are incorporated into the pilus, but their role in pilus assembly is poorly understood. In fact, several minor pilin subunits characterized in previous studies, such as SpaF and SpaG of C. diphtheriae, GBS52 and GBS104 of Streptococcus agalactiae, RrgA and RrgC of S. pneumoniae, and BcpB of Bacillus cereus, have been shown to be dispensable for the integrity or synthesis of the pilus backbone (4, 10, 15, 29, 39).
Recently, however, some minor pilin subunits were reported to contribute to pilus assembly. In C. diphtheriae, disruption of spaB, which encodes a minor pilin subunit of SpaA-type pili, resulted in a marked reduction of the amount of surface-associated major subunit polymers, and most of the synthesized polymers were not retained in the cell wall but were secreted into the culture medium (17). Similar results were observed when SpaI, a minor subunit of the SpaH-type pili of C. diphtheriae, and Spy0130, a minor subunit of the FCT-2 type pili of S. pyogenes, were absent. It was thus proposed that incorporation of these minor subunits into the pilus backbone serves as the terminal step in pilus polymerization and triggers concomitant cell wall linkage by the housekeeping sortase (17). In the S. suis Δsgp2 mutant, the amount of surface-associated major subunit polymers (Sgp1HMW) was much lower than that in the wild-type strain. However, in contrast to the spaB mutant of C. diphtheriae, increased secretion of Sgp1HMW into the culture medium did not occur in the Δsgp2 mutant (Fig. 2A), indicating that Sgp2 is not involved in the termination of pilus polymerization. Instead, the Δsgp2 mutant showed increased amounts of Sgp1m both on the cell surface and in the culture medium, in a phenotype similar to that of the ΔsrtG mutant (Fig. 2A and C).
Because reduction of the total amount of the major subunit protein Sgp1 was observed in the Δsgp2 mutant (Fig. 2C), it is conceivable that Sgp2 is involved in the stability of Sgp1 as a chaperone-like protein. In some Gram-negative bacterial pili, such as the type I and pap pili of E. coli (3, 36), the role of chaperone proteins in the stability of pilin proteins has been well studied. In these assembly pathways, named “chaperone-usher” pathways, the chaperone protein accelerates the folding of the pilin subunits and stabilizes them, and thus, the pilin proteins are degraded in the absence of the chaperone protein. In contrast, no chaperone protein had been implicated in the biogenesis of pili in Gram-positive bacteria. Recently, however, Zähner and Scott suggested a possible chaperone-like function of the type I signal peptidase homologue SipA2 in the T3 pilus formation of a S. pyogenes serotype M3 strain. In this strain, when sipA2 was disrupted, the major subunit, T3, was not assembled as the subunits observed in the Δsgp2 mutant were (52). Although SipA2 did not act as a signal peptidase in the polymerization of T3, this protein seemed to interact with T3 independently of the presence of the pilin polymerase SrtC2, and thus, SipA2 was suggested to have a chaperone-like function in pilus formation (52). In our study, coimmunoprecipitation data indicated that Sgp2 does not associate with Sgp1 directly in the absence of SrtG. In addition, Sgp2 did not show any significant amino acid sequence similarities with SipA2 or the chaperone proteins of other species (data not shown). Therefore, it may be difficult to maintain that Sgp2 acts as a chaperone protein for Sgp1 by itself. We think that the reduction of Sgp1 may be due to the degradation of an excessive amount of unassembled monomers by proteinases. Alternatively, excessive amounts of Sgp1m may downregulate sgp1 gene expression. In fact, the reduction of the major subunit protein was also observed in the ΔsrtG mutant that expresses the Sgp2 protein (Fig. 2C), as well as in the cognate sortase gene mutants of other Gram-positive bacterial pili (13, 27). Taken together, our results suggest that Sgp2 participates in the polymerization of the major subunit itself.
The mechanism(s) by which Sgp2 contributes to the polymerization of the major subunit remains unclear. In the current models of pilus biogenesis, it is envisaged that pilin subunits are added to the base of the growing pilus by repeated transpeptidation reactions, until the terminal subunit is eventually linked covalently to the cell wall peptidoglycan. Pilin polymerization is driven by a pilus-specific class C sortase(s) encoded by genes clustered together with the genes encoding the pilin subunits, while attachment to the cell wall peptidoglycan is mediated by the housekeeping sortase (24, 38). Our IEM analysis showed that Sgp2 was localized at one end of the pilus fibers (Fig. 4B). In addition, labeled Sgp2 was observed both at the tip of the fibers and on the cell surface of the tested strain (Fig. 4C). Although the appearance of Sgp2 on the cell surface might be due to bent pili or pili lying across the bacterial surface, we hypothesize that Sgp2 located at the tip of the pilus represents an assembled minor subunit, while cell surface Sgp2 represents unassembled subunits that have been anchored to the cell wall as monomers by the housekeeping sortase SrtA. If this hypothesis is true, Sgp2 may act as a triggering signal for the cell to initiate the polymerization of Sgp1. Interestingly, as shown in Fig. 2, overexpression of Sgp2 but not that of major subunit Sgp1 resulted in an increase in the signal intensities of both Sgp1HMW and Sgp2HMW. This result may support the possibility that Sgp2 is a trigger molecule to start pilus assembly.
To confirm the tip location of Sgp2 and to test the above possibility, elucidation of the Sgp1-Sgp2 linkage is necessary. The pilin polymerase catalyzes the formation of a peptide bond between the threonine (T) in the CWSS motif of one subunit and an ɛ-amino group of a lysine (K) in the next subunit (5, 12). Therefore, if Sgp2 is the tip pilin, the T in the CWSS motif of Sgp2 should bind to a K in Sgp1, as demonstrated in the linkage between the tip pilin Cpa and the backbone protein T3 of the S. pyogenes serotype M3 strain (27). In C. diphtheriae SpaA, SpaD, and SpaH pilins, the K residue in a conserved pilin motif (WXXXVXVYPK) participates in subunit linkage (10, 39, 47). Although most of the pilin proteins do not have a recognizable pilin motif in S. pyogenes, K161 in the backbone pilin Spy0128 of the serotype M1 strain has been identified as the residue that is linked to T of the CWSS motif of the next subunit (11). Recently, K173 in the T3 pilin of the serotype M3 strain, which corresponds to K161 of Spy0128, was also shown to be required both for T3 polymerization and for the attachment of Cpa to T3 (27). In srtG pili, however, no pilin motif is recognizable in either Sgp1 or Sgp2 (40). In addition, the K residue corresponding to the K residues identified in the S. pyogenes strains was not presented in Sgp1. Furthermore, conserved K residues were not found among Sgp1 and major and putative major subunits encoded in the pilus gene clusters listed in Fig. 5B (data not shown). Thus, we are currently pursuing experiments to identify the K residue required for Sgp1-Sgp2 linkage.
Unlike other well-characterized minor pilin subunits of Gram-positive bacterial pili, our data indicated the essential role of Sgp2 in pilus assembly. These differences may come from differences of the cognate sortases involved in pilus formation. However, SrtG does not contain any conserved domains other than the class C sortase domain and the catalytic active site (histidine, cysteine, and arginine residues) in the domain, which are conserved among pilus-forming sortases of Gram-positive bacteria. In addition, no conserved motif or domain specific to SrtG and cognate sortases in the pilus gene clusters encoding Sgp2 homologues was found by sequence alignment with other well-characterized class C sortases (data not shown). Recently, Manzano et al. analyzed the crystal structures of the pilus-forming sortases SrtC-1 and SrtC-3 of the pneumococcal rlrA islet and revealed the presence of a flexible lid that is absent from sortases not involved in pilus polymerization. Because the lid covered the substrate-recognition cleft in the pilus-forming sortases, it was proposed that this lid sterically blocks the active site and has a substrate-recognition gating function (19). As shown in Fig. S1 in the supplemental material, the sequences corresponding to the lid regions were also present in SrtG and the lid anchor residues (Asp-Pro/Ala-Hyd) were conserved, suggesting that SrtG also has a substrate-recognition gating function. Therefore, although we still do not have any evidence, it is tempting to speculate that only the monomeric form of Sgp2 and not that of Sgp1 can associate with SrtG, open the lid, and initiate pilus assembly. On the other hand, Sgp1 might need some conformational change to associate with SrtG, and the addition of Sgp2 or growing pilus to Sgp1 might drive the major pilin subunit recognizable by SrtG. Of note, Sgp1 and Sgp2 contain identical CWSSs (LPSTG), suggesting that the affinities of the substrate pentapeptides for the SrtG active site are comparable between Sgp1 and Sgp2.
As described above, we have identified the minor pilin subunit Sgp2, which is necessary for the assembly of srtG pili, and discussed the possible role of Sgp2 as a triggering signal to initiate pilus assembly. Interestingly, phenotypes similar to those observed in the Δsgp2 mutant, i.e., reduction of major subunit polymers and increase of the monomeric form of the major subunit, were also observed when the minor pilin subunit-encoding gene in the FCT region of the S. pyogenes serotype M53 strain was disrupted (16). In addition, Sgp2 homologues are encoded in other Gram-positive bacterial pilus clusters, and the clusters share a relatively simple genetic organization with the srtG cluster of S. suis (Fig. 5). Therefore, the participation of the minor subunits in polymerization of the pilus backbone may not be unique to srtG pili but may be common to some types of pili.
In many Gram-positive bacterial pathogens, pili have been reported to contribute to the adherence to and invasion of host cells and to biofilm formation (18, 26, 33, 44). The biological significance of srtG cluster-mediated pili is unknown. However, several S. suis isolates from human patients and diseased pigs possessed all genes in the srtG cluster (40). In addition, most of the isolates expressed at least Sgp1HMW on the cell surface (unpublished results). Our data show that srtG pili are expressed at higher levels when bacteria are grown at temperatures of less than 30°C. Interestingly, the surface temperatures of different external body parts of pigs (snout, ears, vertex, back, and flank) ranged from 20°C to 30°C when the environmental temperature was approximately 20°C (unpublished observations). These findings suggest that this pilus may be important for the interaction of S. suis with the surface structures of host animals. It is noteworthy that, similar to the srtG pilus, the expression of the major subunit FctA of the FCT-3 pili in S. pyogenes serotype M49 also increased with lower temperatures (23). It was suggested that the expression of FctA may be regulated by a bistability mode. In fact, at 37°C, only 20% of all cells express FctA, while at 30°C, approximately 50% of a given population does so (23). Currently, the regulatory mechanism of srtG pilus expression is not known. Further detailed investigations of the mode and molecular background of the temperature-dependent expression of the pili may provide clues to understand the biological advantage of srtG pilus expression by S. suis.
Supplementary Material
Acknowledgments
We thank J. M. Betton (Institut Pasteur, Paris, France) for kindly providing plasmid pDIA17. We also thank T. Fujisawa (National Institute of Animal Health) for preparing the photographs.
This work was supported by a Grant-in-Aid for Young Scientists (Start-up) (20880039) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 2.Bagnoli, F., et al. 2008. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J. Bacteriol. 190:5480-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnhart, M. M., et al. 2000. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl. Acad. Sci. U. S. A. 97:7709-7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budzik, J. M., L. A. Marraffini, and O. Schneewind. 2007. Assembly of pili on the surface of Bacillus cereus vegetative cells. Mol. Microbiol. 66:495-510. [DOI] [PubMed] [Google Scholar]
- 5.Budzik, J. M., et al. 2008. Amide bonds assemble pili on the surface of bacilli. Proc. Natl. Acad. Sci. U. S. A. 105:10215-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 7.Chang, B., et al. 2006. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn. J. Infect. Dis. 59:397-399. [PubMed] [Google Scholar]
- 8.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 156:289-297. [DOI] [PubMed] [Google Scholar]
- 9.Fittipaldi, N., M. Gottschalk, G. Vanier, F. Daigle, and J. Harel. 2007. Use of selective capture of transcribed sequences to identify genes preferentially expressed by Streptococcus suis upon interaction with porcine brain microvascular endothelial cells. Appl. Environ. Microbiol. 73:4359-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar, A. H., and H. Ton-That. 2006. Assembly of distinct pilus structures on the surface of Corynebacterium diphtheriae. J. Bacteriol. 188:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang, H. J., F. Coulibaly, F. Clow, T. Proft, and E. N. Baker. 2007. Stabilizing isopeptide bonds revealed in gram-positive bacterial pilus structure. Science 318:1625-1628. [DOI] [PubMed] [Google Scholar]
- 12.Kang, H. J., N. G. Paterson, A. H. Gaspar, H. Ton-That, and E. N. Baker. 2009. The Corynebacterium diphtheriae shaft pilin SpaA is built of tandem Ig-like modules with stabilizing isopeptide and disulfide bonds. Proc. Natl. Acad. Sci. U. S. A. 106:16967-16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konto-Ghiorghi, Y., et al. 2009. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 5:e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, G. T., et al. 2008. Streptococcus suis meningitis, United States. Emerg. Infect. Dis. 14:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeMieux, J., S. Woody, and A. Camilli. 2008. Roles of the sortases of Streptococcus pneumoniae in assembly of the RlrA pilus. J. Bacteriol. 190:6002-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lizano, S., F. Luo, and D. E. Bessen. 2007. Role of streptococcal T antigens in superficial skin infection. J. Bacteriol. 189:1426-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandlik, A., A. Das, and H. Ton-That. 2008. The molecular switch that activates the cell wall anchoring step of pilus assembly in gram-positive bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:14147-14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandlik, A., A. Swierczynski, A. Das, and H. Ton-That. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzano, C., et al. 2008. Sortase-mediated pilus fiber biogenesis in Streptococcus pneumoniae. Structure 16:1838-1848. [DOI] [PubMed] [Google Scholar]
- 20.McNab, R., and H. F. Jenkinson. 1998. Lipoproteins and other cell-surface associated proteins in streptococci. Methods Cell Sci. 20:209-216. [Google Scholar]
- 21.Mora, M., et al. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. U. S. A. 102:15641-15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munier, H., et al. 1991. Isolation and characterization of catalytic and calmodulin-binding domains of Bordetella pertussis adenylate cyclase. Eur. J. Biochem. 196:469-474. [DOI] [PubMed] [Google Scholar]
- 23.Nakata, M., et al. 2009. Mode of expression and functional characterization of FCT-3 pilus region-encoded proteins in Streptococcus pyogenes serotype M49. Infect. Immun. 77:32-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobbs, A. H., et al. 2008. Sortase A utilizes an ancillary protein anchor for efficient cell wall anchoring of pili in Streptococcus agalactiae. Infect. Immun. 76:3550-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osaki, M., D. Takamatsu, N. Tsuji, and T. Sekizaki. 2000. Cloning and characterization of the gene encoding O-acetylserine lyase from Streptococcus suis. Curr. Microbiol. 40:67-71. [DOI] [PubMed] [Google Scholar]
- 26.Proft, T., and E. N. Baker. 2009. Pili in Gram-negative and Gram-positive bacteria—structure, assembly and their role in disease. Cell. Mol. Life Sci. 66:613-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quigley, B., D. Zähner, M. Hatkoff, D. Thanassi, and J. Scott. 2009. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Mol. Microbiol. 72:1379-1394. [DOI] [PubMed] [Google Scholar]
- 28.Rogé, J., and J. M. Betton. 2005. Use of pIVEX plasmids for protein overproduction in Escherichia coli. Microb. Cell Fact. 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosini, R., et al. 2006. Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol. Microbiol. 61:126-141. [DOI] [PubMed] [Google Scholar]
- 30.Salasia, S. I., C. Lämmler, and G. Herrmann. 1995. Properties of a Streptococcus suis isolate of serotype 2 and two capsular mutants. Vet. Microbiol. 45:151-156. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 33.Scott, J. R., and D. Zähner. 2006. Pili with strong attachments: Gram-positive bacteria do it differently. Mol. Microbiol. 62:320-330. [DOI] [PubMed] [Google Scholar]
- 34.Smith, H., et al. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, W., et al. 2010. Roles of minor pilin subunits Spy0125 and Spy0130 in the M1 Streptococcus pyogenes strain SF370. J. Bacteriol. 192:4651-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staats, J. J., I. Feder, O. Okwumabua, and M. M. Chengappa. 1997. Streptococcus suis: past and present. Vet. Res. Commun. 21:381-407. [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan, A., et al. 2007. Housekeeping sortase facilitates the cell wall anchoring of pilus polymers in Corynebacterium diphtheriae. Mol. Microbiol. 66:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swierczynski, A., and H. Ton-That. 2006. Type III pilus of corynebacteria: pilus length is determined by the level of its major pilin subunit. J. Bacteriol. 188:6318-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takamatsu, D., et al. 2009. Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet. Microbiol. 138:132-139. [DOI] [PubMed] [Google Scholar]
- 41.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101-113. [DOI] [PubMed] [Google Scholar]
- 42.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140-148. [DOI] [PubMed] [Google Scholar]
- 43.Takamatsu, D., et al. 2008. Streptococcus suis in humans, Thailand. Emerg. Infect. Dis. 14:181-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in Gram-positive pathogens. Nat. Rev. Microbiol. 4:509-519. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ton-That, H., and O. Schneewind. 2004. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 12:228-234. [DOI] [PubMed] [Google Scholar]
- 47.Ton-That, H., and O. Schneewind. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50:1429-1438. [DOI] [PubMed] [Google Scholar]
- 48.Warrens, A. N., M. D. Jones, and R. I. Lechler. 1997. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene 186:29-35. [DOI] [PubMed] [Google Scholar]
- 49.Wertheim, H. F., et al. 2009. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 4:e5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanagawa, R., and K. Otsuki. 1970. Some properties of the pili of Corynebacterium renale. J. Bacteriol. 101:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye, C., et al. 2006. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg. Infect. Dis. 12:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zähner, D., and J. R. Scott. 2008. SipA is required for pilus formation in Streptococcus pyogenes serotype M3. J. Bacteriol. 190:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.