Abstract
The exported proteins of Mycobacterium tuberculosis that are localized at the bacterial cell surface or secreted into the environment are ideally situated to interact with host factors and to function in virulence. In this study, we constructed a novel β-lactamase reporter transposon and used it directly in M. tuberculosis for genome-wide identification of exported proteins. From 177 β-lactam-resistant transposon mutants, we identified 111 different exported proteins. The majority of these proteins have no known function, and for nearly half of the proteins, our demonstration that they are exported when fused to a β-lactamase reporter is the first experimental proof of their extracytoplasmic localization. The transposon mutants in our banked library were of further value as a collection of mutants lacking individual exported proteins. By individually testing each of 111 mutants for growth in macrophages, six attenuated mutants with insertions in mce1A, mce1B, mce2F, rv0199, ctaC, and lppX were identified. Given that much of the M. tuberculosis genome encodes proteins of unknown function, our library of mapped transposon mutants is a valuable resource for efforts in functional genomics. This work also demonstrates the power of a β-lactamase reporter transposon that could be applied similarly to other bacterial pathogens.
Mycobacterium tuberculosis is the causative agent of tuberculosis, a devastating disease that kills nearly 2 million people each year (57). Following inhalation, M. tuberculosis survives and replicates within phagosomal compartments of macrophages. The phagosome acidification and fusion with lysosomes that normally occur following phagocytosis are blocked by M. tuberculosis (44, 56). Bacterial exported proteins, defined here as both surface-exposed and secreted proteins, are ideally positioned to interact with the host and promote this M. tuberculosis growth in macrophages.
M. tuberculosis has the conserved Sec and twin-arginine translocation (Tat) pathways for exporting proteins with N-terminal signal sequences beyond the cytoplasmic membrane. M. tuberculosis also possesses specialized secretion systems, namely, the SecA2 and ESX systems (35). These specialized systems are not well understood, but at least some of the proteins exported by these pathways lack identifiable export signals. Despite long-standing interest in the exported proteins of M. tuberculosis, many exported proteins have yet to be identified experimentally as such, and few have a demonstrated function.
Mutagenesis with a reporter transposon is a classic approach to first identify exported proteins and then use the resulting transposon mutants to test the importance of these proteins in virulence. TnphoA (30) is a reporter transposon used extensively for this purpose. The toxin-coregulated pilus of Vibrio cholerae (54) and several other virulence factors (5, 16, 39) were discovered by screening TnphoA mutants. However, TnphoA cannot be used directly in M. tuberculosis because of endogenous enzymes that cleave the PhoA substrate (28).
In this study, we used the TEM-1 β-lactamase (BlaTEM-1) as an alternate reporter to identify exported proteins of M. tuberculosis. The BlaTEM-1 reporter exploits the fact that β-lactamases must be exported beyond the cytoplasm in order to cleave β-lactam antibiotics before they target the cell wall (53). A truncated ′BlaTEM-1 enzyme that lacks the endogenous signal for export remains cytoplasmic and fails to protect against β-lactams. If a Sec or Tat signal sequence is fused to ′BlaTEM-1, the reporter is exported and protects against β-lactams (34). Because M. tuberculosis is naturally resistant to β-lactams, a β-lactam-sensitive blaC mutant of M. tuberculosis is used with this reporter (17). A significant advantage of a β-lactamase reporter system is that it employs selection for exported fusions, as opposed to the more labor-intensive screen required with PhoA reporters.
By incorporating the ′BlaTEM-1 reporter coding sequence into a mariner-based Himar1 transposon (43), we created Tn′blaTEM-1, the first reporter transposon that can be used directly in M. tuberculosis for genome-wide identification of exported proteins. This reporter transposon allowed us to select in-frame insertions of the transposon in genes encoding exported proteins by simply plating transposition reaction mixtures onto β-lactam-containing agar. Among 177 β-lactam-resistant mutants, 111 different exported M. tuberculosis proteins were identified. For nearly half of these proteins, the demonstration that they promoted export of a fused ′BlaTEM-1 protein was the first experimental proof of their subcellular location or topology. At the same time the reporter transposon identified an exported protein, it provided an insertion mutant in the respective open reading frame (ORF). Therefore, our library of transposon mutants also represents a valuable resource for assigning function to unknown exported proteins. In this study, we screened each of 111 unique Tn′blaTEM-1 insertion mutants for intracellular growth phenotypes in bone marrow-derived macrophages. Six mutants defective for growth in macrophages were obtained. Thus, our library of mapped Tn′blaTEM-1 mutants not only defined the subcellular location of 111 unique proteins but also provided a useful mutant collection for evaluation in functional assays.
MATERIALS AND METHODS
Bacterial strains and culture.
Cultivation of M. tuberculosis strains was performed as described previously (8). Where needed, the growth medium was supplemented with 50 μg/ml hygromycin (Roche), 20 μg/ml kanamycin (Acros Chemicals), and 20 μg/ml carbenicillin (Sigma). Tween was not included in carbenicillin-containing Middlebrook 7H10 agar. E. coli strains were grown in Luria-Bertani medium (LB; Fisher) with 150 μg/ml hygromycin, 40 μg/ml kanamycin, and 100 μg/ml carbenicillin. M. tuberculosis strains used in this study were derivatives of PM638 (17). Strains and plasmids used in this study, including the attenuated mutants identified, are listed in Table 1. The complete list of transposon mutants collected is provided in Table S1 in the supplemental material.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | K-12 DH5α F− φ80dΔlacZM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 glnV44 thi-1 gyrA96 relA1 | Gibco-BRL |
| DH5α λpir | K-12 DH5α F− φ80dΔlacZM15 Δ (lacZYA-argF)U169 deoR recA1 endA1 hsdR17 glnV44 thi-1 gyrA96 relA λpir | W. R. Jacobs, Jr. |
| Mycobacterium | ||
| mc2155 | M. smegmatis ept-1 | 48 |
| H37Rv | Virulent M. tuberculosis | 3 |
| PM638 | H37Rv ΔblaC | 17 |
| MBTB126 | PM638 carrying integrating vector pJES137 | This study |
| MBTB204 | PM638 with a Tn′blaTEM-1 insertion in mce1A (Rv0169) at amino acid 449 of 455 | This study |
| MBTB190 | PM638 with a Tn′blaTEM-1 insertion in mce1B (Rv0170) at amino acid 223 of 337 | This study |
| MBTB183 | PM638 with a Tn′blaTEM-1 insertion in rv0199 at amino acid 74 of 220 | This study |
| MBTB156 | PM638 with a Tn′blaTEM-1 insertion in mce2F (Rv0594) at amino acid 476 of 517 | This study |
| MBTB381 | PM638 with a Tn′blaTEM-1 insertion in ctaC (Rv2200c) at amino acid 351 of 364 | This study |
| MBTB301 | PM638 with a Tn′blaTEM-1 insertion in lppX (Rv2945c) at amino acid 82 of 234 | This study |
| Plasmids | ||
| pCR2.1 | bla aph ColE1; TA cloning vector | Invitrogen |
| pMV261.kan | aph Phsp60oriM ColE1; multicopy mycobacterial shuttle plasmid | 51 |
| pJES102 | aph Phsp60-′blaTEM-1 (E. coli) oriM ColE1; ′blaTEM-1 from pCR2.1 cloned into pMV261.kan | 34 |
| pSH1 | TnHimar1 oriR6Kg Hygr; shuttle vector carrying the Himar1 transposon | 27 |
| pJES123 | hygR oriR6K ′blaTEM-1; intermediate cloning vector | This study |
| pJES124 | hygR oriR6K ColE1 aph Tn′blaTEM-1 Tnpmar λcos; shuttle vector carrying Tn′blaTEM-1 | This study |
| pJES137 | hyg int attP ColE1 Phsp60 ′blaTEM-1; integrating hygromycin-marked vector with constitutively expressed ′BlaTEM-1 | This study |
| pJES153 | aph Phsp60-mce2F oriE oriM; multicopy kanamycin-marked vector with constitutively expressed mce2F | This study |
| pJES178 | aph Phsp60-rv0199 oriE oriM; multicopy kanamycin-marked vector with constitutively expressed Rv0199 | This study |
Construction of Tn′blaTEM-1 and complementation vectors.
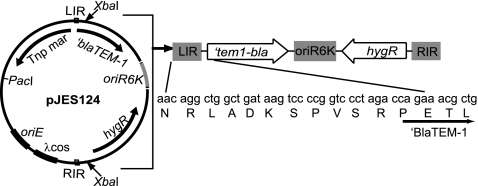
All PCRs were done with an Expand Hi-Fidelity PCR kit (Roche) and 2 to 5% dimethyl sulfoxide (DMSO). The transposon Tn′blaTEM-1 was constructed as follows. The blaTEM-1 gene was PCR amplified without its signal sequence (′blaTEM-1) from pJES102 (34), using primers XbaTem-1F (5′-ATCTAGACCAGAAACGCCTGGTGAA-3′) and SpeITemRev (5′-GACTAGTGCTGGATCCGCAATTGTCTTGG-3′). The resulting product was cloned into pCR2.1 (Invitrogen), creating pJES122. To insert ′blaTEM-1 into the Himar1 transposon, we used plasmid pSH1 (27). pSH1 carries a modified Himar1 transposon that contains within its inverted repeats an oriR6K origin of replication and a hygromycin resistance gene (Fig. 1). Outside the transposon, pSH1 contains the mariner transposase gene (Tnpmar). pSH1 additionally contains a PacI site and a λcos sequence to facilitate cloning of the transposon into mycobacteriophages. pSH1 was digested with XbaI, resulting in a 2.1-kb band and a 3.1-kb band. The 2.1-kb band was ligated to a SpeI and XbaI ′blaTEM-1 fragment of pJES122. The resulting plasmid was pJES123. This ligation abolished the XbaI site at the 3′ end of the ′blaTEM-1 fragment. pJES123 was then digested with XbaI and ligated to the 3.1-kb XbaI fragment of pSH1, containing Tnpmar. In this manner, ′blaTEM-1 was cloned directly downstream of the left inverted repeat of the transposon in the final vector, pJES124 (Fig. 1).
FIG. 1.
Map of Tn′blaTEM-1. Plasmid pJES124 contains the signal sequence-less ′blaTEM-1 gene cloned downstream of the left inverted repeat (LIR) of a Himar1-based mariner transposon derived from pSH1. The codon sequence of the inverted repeat is shown in the same reading frame as the starting codons of ′BlaTEM-1. Tnpmar, mariner transposase; hygR, hygromycin resistance gene.
Construction of Tn′blaTEM-1 delivery phage.
Plasmid pJES124 was cloned into a nonessential region of the conditionally replicating mycobacteriophage phAE159 as described previously (8), with minor modifications. phAE159 DNA was digested with PacI and ligated to PacI-linearized pJES124. The ligated phasmid was then packaged into λ phage heads via the λcos sites present on pJES124, using a Max Plax kit (Epicentre). The λ packaging step allowed recovery of the newly ligated phasmid following transduction into E. coli. Phasmid DNA phAE159::Tn′blaTEM-1 was isolated from hygromycin-resistant E. coli and electroporated into Mycobacterium smegmatis mc2155. Electroporated cells were mixed with late-log-phase M. smegmatis in top agar, poured onto solid agar, and incubated at the permissive temperature of 30°C. A single plaque was isolated, tested for temperature sensitivity, and expanded by permissive growth in M. smegmatis. A high-titer lysate of phAE159::Tn′blaTEM-1 (>1010 PFU per ml) was prepared.
Transposon mutagenesis in M. tuberculosis.
Transposon mutagenesis of M. tuberculosis strain PM638 was done via phage infection as described previously (8). A small aliquot of each transposition reaction mix was plated on 7H10 agar containing 50 μg/ml hygromycin to estimate the total number of transposition events for each reaction. The remainder of each transposition reaction mix was plated on 7H10 agar containing 50 μg/ml hygromycin and 20 μg/ml carbenicillin. Individual carbenicillin-resistant colonies were either patched or replated onto 7H10-hygromycin, with or without carbenicillin, to confirm β-lactam resistance.
We calculated the number of transposon mutants needed to evaluate every gene in the genome by using the following equation: N = [ln(1 − P)/ln(1 − f)] × 6 reading frames, where N = the number of transposon insertion mutants required to achieve a probability of P that every 1-kb ORF is represented and f = the fraction of the genome represented by each 1-kb gene (45). We determined the likelihood that additional ORFs could still be identified by using the following Poisson-derived equation: f[0] = e−m, where f[0] represents the still-mutable genes that may potentially be recovered and m is the mean number of hits already recovered per gene (18).
Identification of transposon insertion sites.
Genomic DNAs were isolated from transposon mutants by use of a guanidinium protocol as described previously (38). Genomic DNA was digested with BssHII and self-ligated, producing a plasmid containing the transposon and flanking M. tuberculosis genomic DNA. These plasmids were recovered by transformation into E. coli DH5α λpir and selection on LB agar with hygromycin. Resulting plasmid DNAs were then sequenced to map the insertion sites. The following bioinformatic algorithms were used to predict export signals: TMHMM (49), TopPred (13), TMPRED (19), SignalP (6), Psort (37), LipoP (22), and TatP (7).
Complementation of selected M. tuberculosis Tn′blaTEM-1 mutants.
The gene encoding Rv0199 was PCR amplified from M. tuberculosis H37Rv genomic DNA by use of primers EcoRV199F (5′-AGATATCCAATGCCTGACGGGGAGCAGAGC-3′) and Rv0199Rev (5′-ACGTTCGAAACCCACCACAG-3′). The amplified product was cloned into pCR2.1 (Invitrogen), digested with EcoRV and HindIII, and then ligated into MscI-HindIII-digested pMV261.kan. The resulting plasmid, carrying Rv0199 on a multicopy expression vector, was named pJES178 (Table 1). The gene encoding Mce2F was PCR amplified from M. tuberculosis H37Rv genomic DNA by use of the following primers: mce2FecoRVfor1 (5′-CGATATCACATGCTGACTCGCGCTATCG-3′) and mce2FnheRev1 (5′-CGCTAGCTCAGCCGGTTGGTGCCAGCATC-3′). The amplified product was cloned into pCR2.1 and digested with EcoRV and NheI. The resulting mce2F-containing fragment was ligated into pMV261.kan that had been digested with MscI and NheI, generating pJES153. pJES178 and pJES153 DNAs were electroporated into the M. tuberculosis Rv0199::Tn′blaTEM-1 and mce2F::Tn′blaTEM-1 transposon mutant strains to generate complemented strains (8).
Macrophage infections.
Murine bone marrow-derived macrophage infections were completed as described previously (25). Briefly, bone marrow-derived macrophages were obtained from 6- to 24-week-old female C57BL/6 mice and seeded into 8-well chamber slides at 2 × 105 cells/well 24 h prior to infection. The indicated M. tuberculosis strains were inoculated from freezer stocks grown to mid-exponential phase, washed once in phosphate-buffered saline (PBS)-0.05% Tween 80, resuspended in Dulbecco's modified Eagle's medium (DMEM), and added to macrophages at a multiplicity of infection (MOI) of 1. Following a 4-h uptake period, infected cells were washed with warm DMEM three times. A subset of wells were then lysed, diluted, and plated on 7H10 agar to determine uptake. Remaining infected wells were lysed, diluted, and plated at 5 days postinfection to determine intracellular bacterial growth.
RESULTS AND DISCUSSION
Mutagenesis with the Tn′blaTEM-1 reporter transposon.
Our objective was to develop a reporter transposon that could be used for large-scale identification of exported proteins in M. tuberculosis. We selected the β-lactamase ′blaTEM-1 reporter for this purpose, as we previously established it to work directly in M. tuberculosis to report on proteins exported by either Sec or Tat pathways (34). For the transposon, we chose the mariner-based Himar1 transposon because of its low degree of site specificity—requiring only a TA dinucleotide sequence for insertion—and its prior successful use in M. tuberculosis (46). To construct the reporter transposon, we inserted the truncated ′blaTEM-1 reporter directly downstream of the naturally existing open reading frame running through the left inverted repeat of a modified Himar1 transposon carried on plasmid pSH1 (27) (Fig. 1). Important features of this Himar1 transposon are the presence of a hygromycin resistance gene and an oriR6K origin of replication, which later enabled recovery of the transposon insertion site on a plasmid. A recombinant mycobacteriophage, phAE159::Tn′blaTEM-1, was then constructed and used to deliver the reporter transposon into the β-lactam-sensitive blaC mutant of M. tuberculosis PM638 (17). The average transposition frequency per phage infection was 1.2 × 10−6 transposon mutant/input bacillus, which is similar to the frequency reported for other phage-delivered Himar transposons in M. tuberculosis (4). Transposition events that yielded in-frame insertions of Tn′blaTEM-1 in an ORF for an exported protein produced an exported β-lactamase fusion protein. We selected exported ′BlaTEM-1 fusions by directly plating the transposition reaction mix on agar containing hygromycin plus the β-lactam antibiotic carbenicillin. The average frequency of recovering β-lactam-resistant colonies per phage infection was 1 × 10−8.
Collection of Tn′blaTEM-1 library in M. tuberculosis.
From an estimated 81,000 transposon mutants, we selected 177 mutants and confirmed their carbenicillin resistance (Table 2; see Table S1 in the supplemental material). Because Tn′blaTEM-1 carries the oriR6K origin of replication and the hygromycin resistance gene, the transposon insertion site of each mutant was rescued as a plasmid recovered from genomic DNA by restriction endonuclease digestion, self-ligation, and transformation into E. coli. The transposon insertion site on the recovered plasmids was then identified by DNA sequencing. Of the 177 carbenicillin-resistant mutants, only 5 had insertions that were out of frame or not in an ORF, and these transposon mutants are not discussed further. The remaining 172 mutants had Tn′blaTEM-1 inserted in frame in an ORF. Some ORFs were identified more than once, resulting in a total of 111 different ORFs identified. Each mutant was stocked to create a sequence-defined library of M. tuberculosis transposon mutants. Domain prediction algorithms revealed each of the 111 ORFs to possess some type of predicted export signal: a cleavable standard Sec signal sequence, a cleavable lipoprotein-type Sec signal sequence, a cleavable Tat signal sequence, and/or a transmembrane (TM) domain (Table 2; see Table S1) (see Materials and Methods for the algorithms used). The fact that nearly every β-lactam-resistant mutant possessed an in-frame insertion in an ORF with features consistent with export demonstrated the specificity and selection power of this reporter.
TABLE 2.
Description of M. tuberculosis Tn′blaTEM-1 transposon library
| Parameter | Value |
|---|---|
| Total no. of transposon mutantsa | 81,000 |
| No. of confirmed carbenicillin-resistant insertion mutants | 177 |
| No. of in-frame insertions in ORFs | 172 |
| No. of unique ORFs identified | 111 |
| No. of ORFs with standard Sec signal sequence | 24 |
| No. of ORFs with lipoprotein-type Sec signal sequence | 15 |
| No. of ORFs with Tat signal sequence | 3 |
| No. of ORFs with transmembrane domainsb | 69 |
| No. of ORFs with homologues only in mycobacteria | 41 |
| No. of ORFs with homologues only in pathogenic | |
| mycobacteriac | 16 |
| No. of ORFs with no known or predicted functiond | 66 |
Estimated by plating a fraction of each transposition reaction mix on agar containing hygromycin only.
Includes proteins with at least one predicted transmembrane domain. Proteins with transmembrane domains plus a Sec, Tat, or lipoprotein-type signal sequence are most likely integral membrane proteins, and they are counted in this category.
Pathogenic mycobacteria searched included M. leprae, M. bovis, M. marinum, M. ulcerans, and M. avium.
Functions of individual ORFs are provided in Table S1 in the supplemental material.
For 43% of the ORFs we identified with Tn′blaTEM-1, this was the first experimental evidence that the corresponding protein is exported by M. tuberculosis (see Table S1 in the supplemental material). Of the exported ORFs identified, 37% had homologues only in other mycobacteria and 14% had homologues only in pathogenic Mycobacterium spp. (Table 2; see Table S1). Emphasizing how little is known about M. tuberculosis exported proteins, 59% of the ORFs we identified had no known or predicted function (see Table S1). The demonstration of export, especially for these unknown proteins, is significant. Establishing the location of a protein is an important step in determining its biological function and molecular interactions.
For integral membrane proteins, the site of an active Tn′blaTEM-1 insertion provides additional information about protein topology, since active insertions must be fused to domains localized on the extracytoplasmic side of the membrane. Prediction programs often disagree on the number and location of TM domains. Thus, experimental evidence is critical to establishing the topology of integral membrane proteins. For the 69 membrane proteins identified in this study, the insertion site mapped an extracytosolic domain. While the degree of saturation in our transposon library was insufficient to map the full topology of membrane proteins, there were some integral membrane proteins with multiple independent Tn′blaTEM-1 insertions. One such protein was MmpL4, a protein with an undefined role in M. tuberculosis pathogenesis (15, 26, 46). By combining the sites of active Tn′blaTEM-1 insertions with earlier data obtained using M. smegmatis to identify exported protein domains of MmpL4 (9), a total of seven extracytoplasmic sites could be delineated in this protein (see Fig. S1 in the supplemental material). These experimental data help to build a relatively refined topology map for this virulence factor of unknown function.
At the start, we calculated that evaluation of 80,000 transposon insertion mutants would give a 95% probability that all genes of an average size of 1 kb in the 4.4-Mb M. tuberculosis genome would be represented by at least one transposon insertion in the proper reading frame (see Materials and Methods). After selecting 177 β-lactam-resistant transposon insertions from 81,000 transposon mutants, a Poisson calculation on the number of times we repeatedly identified the same ORF predicted that new ORFs could still be identified among future carbenicillin-resistant mutants. Thus, even more exported M. tuberculosis proteins could be identified in the future. However, as with any experimental approach, there will always be some exported proteins that we cannot identify with this method. Proteins that are essential or not expressed during in vitro growth at a level sufficient for a fusion protein to promote growth on selective media will be missed. While our reporter transposon identifies proteins with N-terminal signal sequences, it misses proteins with C-terminal export signals. This is because the ′blaTEM-1 stop codon on the transposon truncates the C-terminal domains of proteins. Finally, we will miss exported proteins that are incompatible with the ′BlaTEM-1 reporter itself. In fact, the unconventional exported proteins Esat-6 and SodA, which are exported by the M. tuberculosis ESX-1 (1) and SecA2 (41) systems, respectively, appear to fall into this category. We did not identify these proteins with the reporter transposon, and when N-terminal or C-terminal fusions between ′BlaTEM-1 and Esat-6 or SodA were tested directly, they failed to produce β-lactam resistance, despite the fusions being produced at high levels (as determined by immunoblotting [data not shown]). A possible explanation for this incompatibility is that ′BlaTEM-1 is not exported or enzymatically active when fused to these unconventional exported proteins.
Screening the Tn′blaTEM-1 library for mutants with growth defects in macrophages.
In addition to defining the subcellular locations of proteins, our mutant collection is a valuable resource for efforts to assign functions to ORFs. To demonstrate this utility, we took an unbiased approach and screened our library of mutants, each with a transposon insertion in a gene encoding an exported protein, for growth in macrophages. A representative transposon mutant for each of the 111 exported ORFs identified was tested in duplicate for growth in resting murine bone marrow-derived macrophages. For ORFs that received multiple hits, a representative transposon mutant with the most 5′ insertion site was tested. The growth of each transposon mutant was compared to that of M. tuberculosis MBTB126, which is the blaC M. tuberculosis parent engineered to express the nonexported ′BlaTEM-1 reporter. Among 111 transposon mutants tested, 6 mutants had reproducible and statistically significant intracellular growth defects. These mutants had Tn′blaTEM-1 insertions in one of the following genes: mce1A, mce1B, mce2F, rv0199, ctaC, and lppX (see Fig. 2 to 5). All six of these attenuated mutants were tested for growth in broth culture, and all grew at the same rate as the MBTB126 parent strain (data not shown). As discussed below, the macrophage phenotypes of two of these mutants were complemented, proving that the transposon insertions were the cause of the phenotypes. For the remaining four mutants, complementation experiments are pending. Therefore, we cannot yet say with certainty that the disrupted ORF was the cause of the intracellular growth defect.
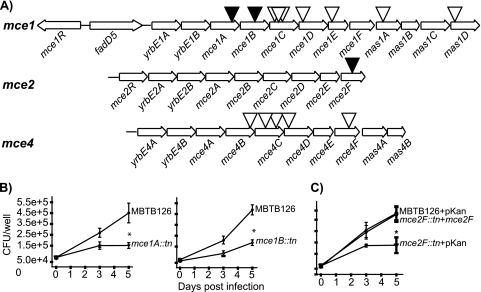
FIG. 2.
Evaluation of M. tuberculosis mce::Tn′blaTEM-1 mutants in macrophages. (A) Representation of mce1, mce2, and mce4 operons in M. tuberculosis H37Rv. Black triangles indicate Tn′blaTEM-1 insertion mutants with macrophage growth defects. White triangles mark Tn′blaTEM-1 insertion mutants that were not attenuated. The mce1 operon has two possible start sites and spans from either fadD5 or yrbE1A to mas1D (12, 20). The mce2 and mce4 operons are transcribed in a single transcript comprised of the genes shown (11, 24). (B) Murine bone marrow-derived macrophages were infected with M. tuberculosis MBTB126, the mce1A::Tn′blaTEM-1 mutant, or the mce1B::Tn′blaTEM-1 mutant. At 4 h (day 0) and 3 and 5 days postinfection, macrophages were lysed and plated to enumerate intracellular bacteria. Error bars indicate standard deviations (SD) of the means. (C) MBTB126 carrying pMV261.kan, the mce2F::Tn′blaTEM-1 mutant carrying pMV261.kan, and the mce2F::Tn′blaTEM-1 mutant carrying a multicopy complementing vector expressing mce2F from a constitutive promoter (pJES153) were used to infect macrophages as described for panel B. *, P ≤ 0.05. Each graph shows representative data from one of at least three independent replicate experiments.
For some of these mutants, our analysis was the first suggestion that the ORF promotes growth in macrophages. For other mutants, the ORF was predicted to have a role in intracellular growth by transposon site hybridization (TraSH) with a saturating pool of M. tuberculosis mutants (40) or a pool of 2,500 Mycobacterium bovis BCG transposon mutants (50). For mutants that fell into the second category, our direct testing provided necessary validation of genes predicted earlier as being important in macrophages.
Mce1A, Mce1B, and Mce2F.
Each of the four mce operons (mce1 to -4) in the M. tuberculosis genome contains six genes encoding Mce protein family members (MceA to MceF) and two YrbE homologues (12). In our Tn′blaTEM-1 library, we obtained 15 unique transposon insertions in 13 genes in mce operons (Fig. 2A). For six of these ORFs, our identification with the reporter transposon is the first demonstration that the protein is exported (see Table S1 in the supplemental material). The function of Mce family members is still unclear, but data suggest that they function as transporters, which is consistent with an extracytoplasmic localization (21, 36).
When tested in macrophages, our Tn′blaTEM-1 insertion mutants in mce1A and mce1B were attenuated for intracellular growth (Fig. 2B). In published studies, there is debate about the phenotype of mce1 mutants. An allelic exchange mutant in which expression of the entire mce1 operon was defective was reported to grow better than wild-type M. tuberculosis in macrophages (47). However, TraSH analysis of pools of M. tuberculosis mutants predicts that transposon mutants with insertions in genes spanning mce1B to mce1F are defective in macrophages (40). Similarly, hybridization analysis of pooled M. bovis BCG transposon mutants predicts that the majority of mce1 genes contribute to intracellular growth (50). Our results agree with the latter predictions. However, unlike the predictions obtained with pooled mutant screens, when we tested Tn′blaTEM-1 insertions in genes downstream of mce1B, we did not observe macrophage growth defects. We speculate that mutations downstream in the mce1 operon have more subtle defects that are not evident when tested individually but are revealed when tested in competition in pools of mutants. Another possibility is that the site of these mce1 downstream insertions failed to disrupt function; however, several insertions in mce1 were positioned early in an ORF, making this an unlikely explanation for all cases (Fig. 2A; see Table S1).
We have yet to complement the mce1A and mce1B transposon mutant phenotypes to rule out the possibility of polar effects on downstream genes in the mce1 operon (spanning genes fadD5 to mas1D) (12) (Fig. 2A). While not giving direct proof, the lack of macrophage phenotypes with transposon insertions in downstream mce1 genes suggests that the impact of polar effects in our experiments is minimal. Published attempts to complement individually disrupted mce1 genes were unsuccessful, suggesting that complementation of our mce1 mutants may be a challenge (47).
A Tn′blaTEM-1 insertion mutation in mce2F also led to a growth defect in macrophages (Fig. 2C). A complementation experiment in which a wild-type copy of mce2F was expressed from the hsp60 promoter in the mce2F::Tn′blaTEM-1 mutant restored intracellular growth, demonstrating that the mutant phenotype was due to the lack of Mce2F. This is the first demonstration of Mce2F being important in macrophage growth. The mce2F gene was not predicted to be important in macrophages among pooled M. bovis BCG strains (50) or by TraSH analysis of M. tuberculosis (40). Furthermore, an mce2 mutant of H37Rv was recently reported to grow normally in the RAW cell line, although the mutant was attenuated in mice (31).
We also recovered Tn′blaTEM-1 insertions in five different genes in the mce4 operon (Fig. 2A; see Table S1 in the supplemental material), but none of the resulting mutants was attenuated for growth in macrophages. While Mce4 components are important for bacterial growth in the mouse model of infection (21, 46), our results agree with the TraSH analysis predicting that mce4 is not important for growth in macrophages (40).
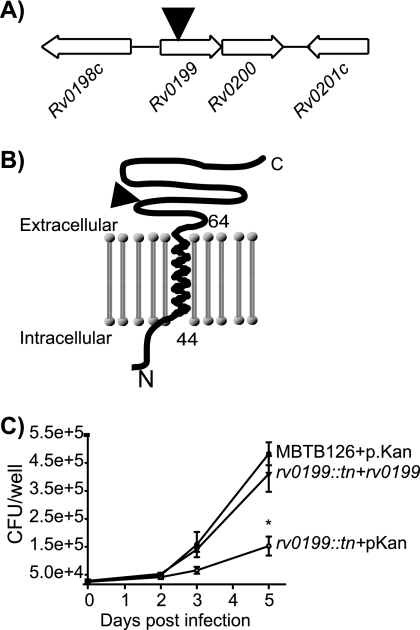
Rv0199.
Rv0199 is a protein with limited homology to some Mce-associated proteins but with no predicted function. However, Rv0199 is conserved in all mycobacteria examined to date (32). The Tn′blaTEM-1 insertion positioned the ′BlaTEM-1 reporter after a single predicted TM domain at the N terminus in Rv0199 (Fig. 3). This indicates that the majority of Rv0199 is located on the extracytoplasmic side of the membrane. The rv0199::Tn′blaTEM-1 mutant was defective for growth in macrophages, and this intracellular growth defect was complemented by addition of a plasmid expressing rv0199 from the hsp60 promoter (Fig. 3C). Rv0199 was not predicted to be important in macrophages by TraSH analysis (40), although it was predicted by another TraSH analysis to be important in a mouse infection model (46). In contrast, Rv0199 was predicted on the basis of pooled infections to be needed for M. bovis BCG to grow in macrophages (50).
FIG. 3.
M. tuberculosis rv0199::Tn′blaTEM-1 has a growth defect in macrophages. (A) Organization of genes surrounding Tn′blaTEM-1 insertion (triangle) in rv0199. (B) Membrane topology of Rv0199 predicted by the TMHMM algorithm (49). The site of ′BlaTEM-1 fusion is indicated with a triangle. (C) Macrophages were infected with MBTB126 carrying the empty vector pMV261.kan, the rv0199::Tn′blaTEM-1 mutant carrying pMV261.kan, or the rv0199::Tn′blaTEM-1 mutant carrying a multicopy complementing vector expressing rv0199 from a constitutive promoter (pJES178). Error bars indicate SD of the means. *, P ≤ 0.05. The graph shows representative data from one of at least three independent replicate experiments.
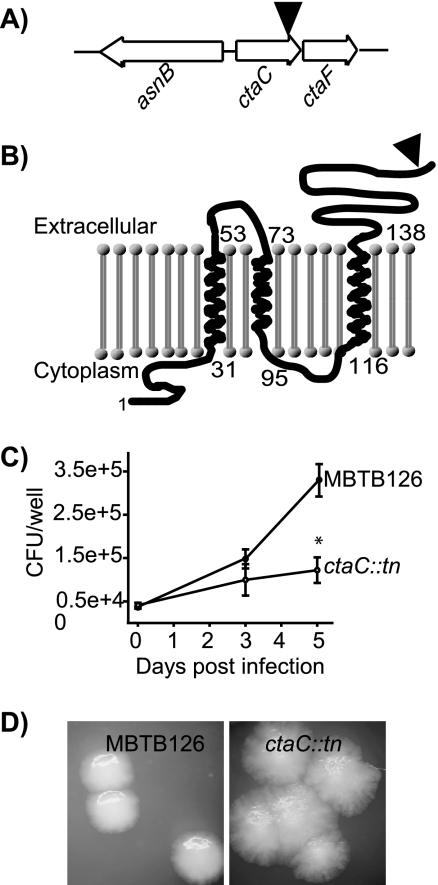
CtaC.
Our ctaC::Tn′blaTEM-1 mutant had a growth defect in macrophages (Fig. 4 C). The ctaC gene encodes subunit II of the cytochrome c oxidase, which is important for growth under aerobic conditions (23). Along with TM and signal sequence predictions (6, 49), the site of our active Tn′blaTEM-1 insertion indicates that the C terminus of CtaC, which contains the cytochrome oxidase domain, is on the extracytoplasmic face of the membrane.
FIG. 4.
M. tuberculosis ctaC::Tn′blaTEM-1 has a growth defect in macrophages and a colony phenotype on agar plates. (A) Relative location of ctaC in the M. tuberculosis genome. The black triangle indicates the site of Tn′blaTEM-1 insertion. (B) Membrane topology of CtaC predicted by the TMHMM algorithm (49). The site of ′BlaTEM-1 fusion is indicated with a triangle. (C) Macrophage infection as described in the legend for Fig. 3. Error bars indicate SD of the means. *, P ≤ 0.05. The graph shows representative data from one of at least three independent replicate experiments. (D) M. tuberculosis MBTB126 and the ctaC::Tn′blaTEM-1 mutant were plated on 7H10 agar plates supplemented with Tween 80. Colonies were examined after 25 days at 37°C.
CtaC is predicted to be essential in M. tuberculosis H37Rv, and an attempt to delete the gene in M. tuberculosis was unsuccessful (33). However, we collected two identical ctaC::Tn′blaTEM-1 mutants, and they did not have growth defects in liquid (data not shown). This is likely due to the ′BlaTEM-1 reporter truncating only the final 13 amino acids of the C terminus. Colonies of the ctaC::Tn′blaTEM-1 mutant were phenotypically rough and spread out in comparison to parental MBTB126 colonies (Fig. 4D).
Until complementation analysis is conducted, we can only speculate on the basis of attenuated mutant phenotypes in macrophages. For the ctaC::Tn′blaTEM-1 mutant, perhaps the mutant has modestly compromised CtaC activity that is problematic to intracellular growth but not to in vitro growth. Another possibility is that the intracellular growth defect of the ctaC::Tn′blaTEM-1 mutant is not caused by the insertion in ctaC but is due to polar effects of the transposon (Fig. 4A). The ctaF gene is directly downstream of ctaC. CtaF shares homology with predicted cytochrome c subunit proteins in other mycobacteria and Rhodococcus (2). It is possible that CtaF works in concert with CtaC in a multisubunit cytochrome c oxidase in mycobacteria, or it may function in another capacity.
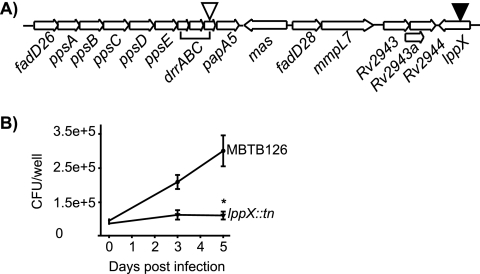
LppX.
We identified active Tn′blaTEM-1 insertions in drrC and lppX. These genes are near each other in the genome and encode proteins required for translocation of the mycobacterium-specific lipid phthiocerol dimycocerosate (PDIM) (10, 14, 55) to the bacterial surface (10, 14, 52) (Fig. 5A).
FIG. 5.
M. tuberculosis lppX::Tn′blaTEM-1 has a growth defect in macrophages. (A) Representation of insertions in the PDIM locus of M. tuberculosis. The black arrowhead represents a Tn′blaTEM-1 insertion that resulted in attenuation in macrophages. The white arrowhead indicates a Tn′blaTEM-1 insertion that did not lead to attenuation. (B) Murine bone marrow-derived macrophages were infected with M. tuberculosis MBTB126 and the lppX::Tn′blaTEM-1 mutant as described in the legend to Fig. 3. Error bars indicate SD of the means. *, P ≤ 0.05. Each graph shows representative data from one of at least three independent replicate experiments.
Our lppX::Tn′blaTEM-1 mutant was defective for growth in macrophages (Fig. 5B). A macrophage phenotype was not, however, observed with our drrC::Tn′blaTEM-1 mutant, even though this mutant had an insertion truncating the protein at amino acid 57 (Fig. 5A). To our knowledge, our results are the first to report a macrophage phenotype for an lppX mutant. While PDIM-deficient mutants are attenuated in virulence in a mouse model of infection, a fadD26 mutant completely devoid of PDIM was not reported to have a defect in resting macrophages (42). Furthermore, TraSH does not predict lppX to be important for growth in macrophages (40).
Conclusions.
In this work, we utilized a new reporter transposon for large-scale identification of exported proteins of M. tuberculosis. We established the subcellular locations of 111 M. tuberculosis proteins and simultaneously collected a library of mapped mutants, each with an insertion in an exported ORF, for subsequent analyses. An important feature of our transposon reporter strategy is that it operates as a selection, not a screen. Screening for an exported fusion phenotype is required with other reporters of protein export, such as PhoA (9), and is a labor-intensive approach that limits the number of colonies that can be surveyed. A positive-selection process made it possible for us to collect in-frame reporter fusions to exported proteins from 81,000 random transposon mutants. Given the remarkable specificity and selection exhibited by our β-lactamase reporter transposon, similar reporters should be considered for comprehensive analyses of exported proteins in other pathogens.
We found that 59% of the exported proteins we identified have no defined or predicted function (see Table S1 in the supplemental material), whereas only 27% of ORFs in the M. tuberculosis genome are annotated as having an unknown function (http://tuberculist.epfl.ch/). This discrepancy emphasizes how little we know about M. tuberculosis exported proteins. The library of mapped transposon mutants reported here is a valuable resource for assigning functions to these unknown exported proteins and for identifying new proteins important to pathogenesis. Furthermore, the mutant collection is of a size where it is feasible to rigorously screen mutants individually for phenotypes. For some of the mutants we identified as attenuated in macrophages (ctaC, mce2F, and lppX mutants), this was the first time that such a phenotype was observed. For other mutants, our results provide necessary validation of predictions from hybridization-based assessment of mutant pools or address conflicting reports (for mce1A, mce1B, and rv0199) (21, 29, 40, 46, 47, 50). Importantly, complementation analysis of the mce2F and rv0199 mutants provides a foundation for unraveling the functions of two new M. tuberculosis exported proteins with roles in macrophage growth.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI070928 and AI076685 to M.B. J.R.M. was supported by training grant NIH 5-T32-GM008581. J.R.M. and J.A.M. were each supported by a Society of Fellows graduate dissertation fellowship, and J.T.S. was the recipient of a Morehead Fellowship from the Graduate School of UNC.
We thank Ellen Perkowski and Jenny Hayden for assistance with data analysis. We also thank Martin Pavelka for strain PM638 and William Jacobs, Sunhee Lee, and Jordan Kriakov for pSH1 and phAE159. Finally, we thank Martin Pavelka, Adrie Steyn, and Braunstein laboratory members for critical readings of the manuscript.
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abdallah, A. M., et al. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883-891. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bange, F. C., F. M. Collins, and W. R. Jacobs, Jr. 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber. Lung Dis. 79:171-180. [DOI] [PubMed] [Google Scholar]
- 4.Bardarov, S., et al. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batisson, I., et al. 2003. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect. Immun. 71:4516-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bendtsen, J. D., H. Nielsen, D. Widdick, T. Palmer, and S. Brunak. 2005. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunstein, M., S. S. Bardarov, and W. R. Jacobs, Jr. 2002. Genetic methods for deciphering virulence determinants of Mycobacterium tuberculosis. Methods Enzymol. 358:67-99. [DOI] [PubMed] [Google Scholar]
- 9.Braunstein, M., et al. 2000. Identification of genes encoding exported Mycobacterium tuberculosis proteins using a Tn552′phoA in vitro transposition system. J. Bacteriol. 182:2732-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camacho, L. R., et al. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276:19845-19854. [DOI] [PubMed] [Google Scholar]
- 11.Casali, N., and L. W. Riley. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casali, N., A. M. White, and L. W. Riley. 2006. Regulation of the Mycobacterium tuberculosis mce1 operon. J. Bacteriol. 188:441-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 14.Cox, J., B. Chen, M. McNeil, and W. J. Jacobs. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 15.Domenech, P., M. B. Reed, and C. E. Barry III. 2005. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect. Immun. 73:3492-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay, B. B., et al. 1988. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol. Microbiol. 2:757-766. [DOI] [PubMed] [Google Scholar]
- 17.Flores, A. R., L. M. Parsons, and M. S. Pavelka, Jr. 2005. Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology 151:521-532. [DOI] [PubMed] [Google Scholar]
- 18.Hawley, R. S. W., and Y. Michelle. 2003. Advanced genetic analysis: finding meaning in a genome. Blackwell Science, Oxford, United Kingdom.
- 19.Hofmann, K., and W. Stoffel. 1993. TMBASE—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 20.Joon, M., S. Bhatia, R. Pasricha, M. Bose, and V. Brahmachari. 2010. Functional analysis of an intergenic non-coding sequence within mce1 operon of M. tuberculosis. BMC Microbiol. 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi, S., et al. 2006. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl. Acad. Sci. U. S. A. 103:11760-11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juncker, A. S., et al. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kana, B. D., et al. 2001. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 183:7076-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, A., M. Bose, and V. Brahmachari. 2003. Analysis of expression profile of mammalian cell entry (mce) operons of Mycobacterium tuberculosis. Infect. Immun. 71:6083-6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz, S., K. P. McKinnon, M. S. Runge, J. P. Ting, and M. Braunstein. 2006. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect. Immun. 74:6855-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamichhane, G., S. Tyagi, and W. R. Bishai. 2005. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect. Immun. 73:2533-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S., et al. 2004. Bxz1, a new generalized transducing phage for mycobacteria. FEMS Microbiol. Lett. 241:271-276. [DOI] [PubMed] [Google Scholar]
- 28.Lim, E. M., et al. 1995. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J. Bacteriol. 177:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima, P., et al. 2007. Enhanced mortality despite control of lung infection in mice aerogenically infected with a Mycobacterium tuberculosis mce1 operon mutant. Microbes Infect. 9:1285-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. U. S. A. 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marjanovic, O., T. Miyata, A. Goodridge, L. V. Kendall, and L. W. Riley. 2010. mce2 operon mutant strain of Mycobacterium tuberculosis is attenuated in C57BL/6 mice. Tuberculosis (Edinburgh) 90:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marmiesse, M., et al. 2004. Macro-array and bioinformatic analyses reveal mycobacterial ‘core’ genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiology 150:483-496. [DOI] [PubMed] [Google Scholar]
- 33.Matsoso, L. G., et al. 2005. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J. Bacteriol. 187:6300-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann, J. R., J. A. McDonough, M. S. Pavelka, and M. Braunstein. 2007. Beta-lactamase can function as a reporter of bacterial protein export during Mycobacterium tuberculosis infection of host cells. Microbiology 153:3350-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonough, J. A., and M. Braunstein. 2008. Protein transport, p. 111-130. In S. H. E. Kaufmann and E. J. Rubin (ed.), Handbook of tuberculosis: molecular biology and biochemistry. Wiley-VCH, Weinheim, Germany.
- 36.Mohn, W. W., et al. 2008. The actinobacterial mce4 locus encodes a steroid transporter. J. Biol. Chem. 283:35368-35374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakai, K., and P. Horton. 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24:34-36. [DOI] [PubMed] [Google Scholar]
- 38.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahme, L. G., et al. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. U. S. A. 94:13245-13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengarajan, J., B. R. Bloom, and E. J. Rubin. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102:8327-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rigel, N. W., and M. Braunstein. 2008. A new twist on an old pathway—accessory Sec [corrected] systems. Mol. Microbiol. 69:291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rousseau, C., et al. 2004. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell. Microbiol. 6:277-287. [DOI] [PubMed] [Google Scholar]
- 43.Rubin, E. J., et al. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. U. S. A. 96:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell, D. G. 2008. Mycobacterium tuberculosis: life and death in the phagosome, p. 307-322. In S. H. E. Kaufmann and E. J. Rubin (ed.), Handbook of tuberculosis: molecular biology and biochemistry. Wiley-VCH, Weinheim, Germany.
- 45.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Sassetti, C. M., and E. J. Rubin. 2003. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. U. S. A. 100:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimono, N., et al. 2003. Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc. Natl. Acad. Sci. U. S. A. 100:15918-15923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 49.Sonnhammer, E. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6:175-182. [PubMed] [Google Scholar]
- 50.Stewart, G. R., J. Patel, B. D. Robertson, A. Rae, and D. B. Young. 2005. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 1:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stover, C. K., et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 52.Sulzenbacher, G., et al. 2006. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J. 25:1436-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tadayyon, M., and J. K. Broome-Smith. 1992. TnblaM: a transposon for directly tagging bacterial genes encoding cell envelope and secreted proteins. Gene 111:21-26. [DOI] [PubMed] [Google Scholar]
- 54.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trivedi, O. A., et al. 2005. Dissecting the mechanism and assembly of a complex virulence mycobacterial lipid. Mol. Cell 17:631-643. [DOI] [PubMed] [Google Scholar]
- 56.Vergne, I., J. Chua, S. B. Singh, and V. Deretic. 2004. Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20:367-394. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. March 2010, revision date. TB fact sheet. http://www.who.int/mediacentre/factsheets/fs104/en/. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.