Abstract
Fructan polymer, synthesized from sucrose by the extracellular fructosyltransferase of Streptococcus mutans, is thought to contribute to the progression of dental caries. It may serve as an extracellular storage polysaccharide facilitating survival and acid production. It may also have a role in adherence or accumulation of bacterial cells on the tooth surface. A number of clinical isolates of S. mutans which produce large, mucoid colonies on sucrose-containing agar as a result of increased production of fructan have been discovered. By using eight independent isolates, we sought to determine if such fructan-hyperproducing strains represented a genetically homogeneous group of organisms. Restriction fragment patterns of total cellular DNA were examined by using pulsed-field and conventional gel electrophoresis. Four genetic types which appeared to correlate with the serotype of the organism and the geographic site of isolation were evident. Southern blot analysis of several genetic loci for extracellular enzymes revealed some minor differences between the strains, but the basic genomic organizations of these loci were similar. To evaluate whether the excess fructan produced by these strains enhanced the virulence of these organisms in the oral cavity, it was of interest to create mutants deficient in fructosidase (FruA), the extracellular enzyme which degrades this polymer. The fruA gene was inactivated by allelic exchange in two fructan-hyperproducing strains as well as in S. mutans GS5, a strain which does not hyperproduce fructan. All of the fruA mutant strains were devoid of fructan hydrolase activity when levan was used as a substrate. However, the fructan-hyperproducing strains retained the ability to hydrolyze inulin, suggesting the presence of a second fructosidase with specificity for inulin in these strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Penders J. E. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-beta-D-fructosidase. Infect Immun. 1992 Nov;60(11):4621–4632. doi: 10.1128/iai.60.11.4621-4632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Schilling K., Bowen W. H., Yasbin R. E. Expression, purification, and characterization of an exo-beta-D-fructosidase of Streptococcus mutans. J Bacteriol. 1987 Oct;169(10):4507–4517. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Caufield P. W., Walker T. M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989 Feb;27(2):274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S., Hsu T. Y., Teng L. J., Chen J. Y., Hahn L. J., Yang C. S. Glucosyltransferase gene polymorphism among Streptococcus mutans strains. Infect Immun. 1991 May;59(5):1656–1660. doi: 10.1128/iai.59.5.1656-1660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S., Bahary W. S., Garg S. K., Long L. W., Newbrun E. Levans: I. Fractionation, solution viscosity, and chemical analysis of levan produced by Streptococcus salivarius. J Dent Res. 1975 Mar-Apr;54(2):290–297. [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Formation and significance of bacterial polysaccharides in caries etiology. Caries Res. 1968;2(2):164–171. doi: 10.1159/000259554. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Iwami Y., Yamada T., Araya S. Levan synthesis and accumulation by human dental plaque. Arch Oral Biol. 1970 Jun;15(6):563–567. doi: 10.1016/0003-9969(70)90111-1. [DOI] [PubMed] [Google Scholar]
- Honda O., Kato C., Kuramitsu H. K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990 Oct;136(10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
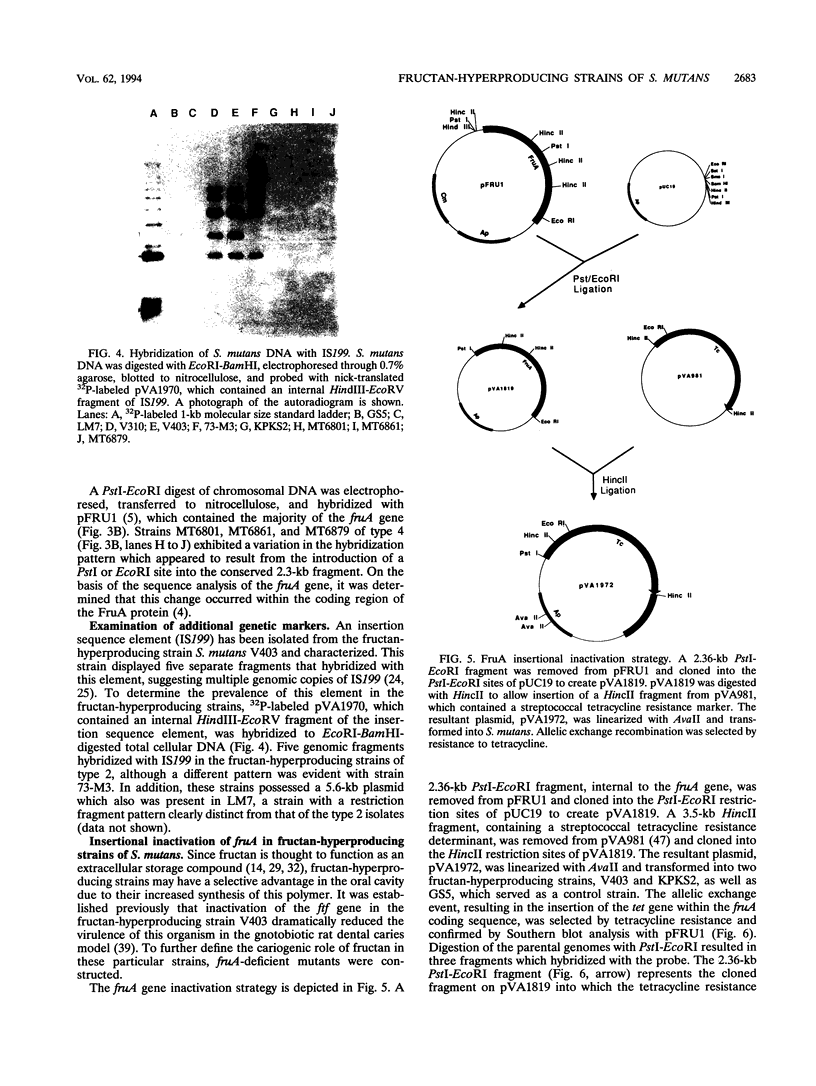
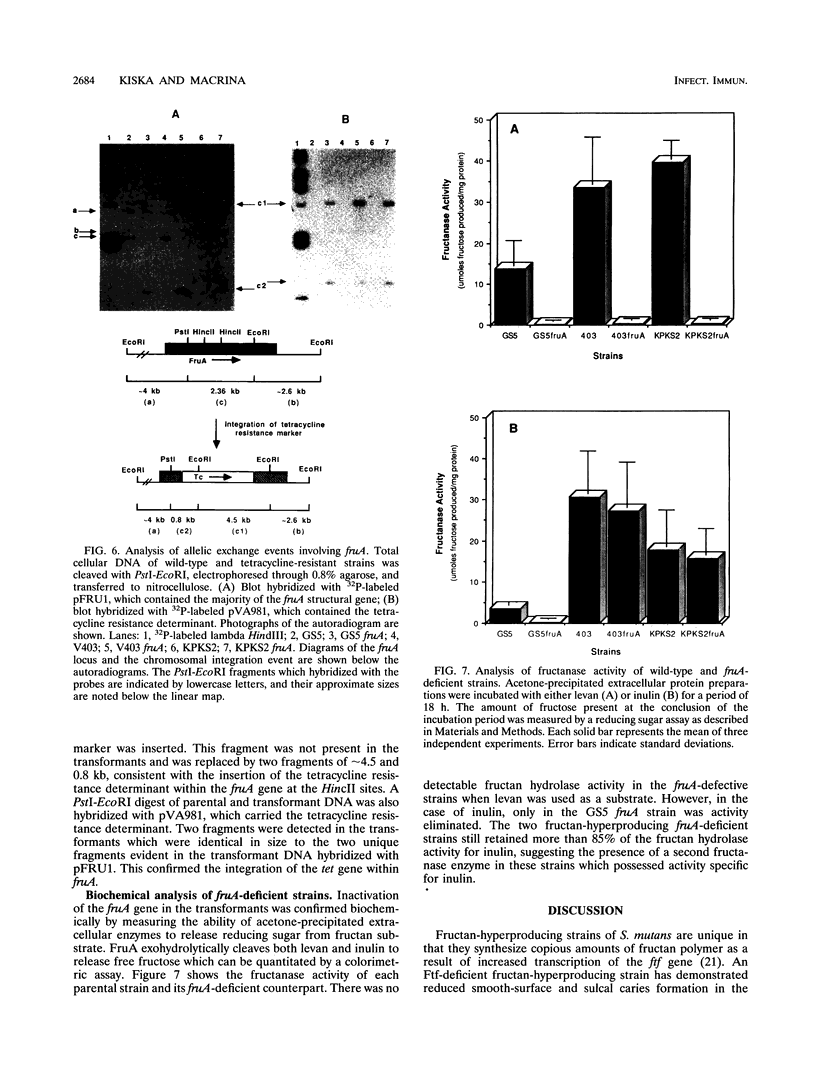
- Kiska D. L., Macrina F. L. Genetic regulation of fructosyltransferase in Streptococcus mutans. Infect Immun. 1994 Apr;62(4):1241–1251. doi: 10.1128/iai.62.4.1241-1251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindler L. E., Macrina F. L. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J Bacteriol. 1986 May;166(2):658–665. doi: 10.1128/jb.166.2.658-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Alpert C. A., Chassy B. M., Michalek S. M. Repeated DNA sequence involved in mutations affecting transport of sucrose into Streptococcus mutans V403 via the phosphoenolpyruvate phosphotransferase system. Infect Immun. 1991 Apr;59(4):1535–1543. doi: 10.1128/iai.59.4.1535-1543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Marshall K., Weigel H. Evidence of multiple branching in the levan elaborated by Streptococcus salivarius strain 51. Carbohydr Res. 1980 Aug 15;83(2):321–326. doi: 10.1016/s0008-6215(00)84544-9. [DOI] [PubMed] [Google Scholar]
- Munro C., Michalek S. M., Macrina F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991 Jul;59(7):2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Asakawa H., Koga T., Masuda N., Hamada S. Clinical isolates of Streptococcus mutans serotype c with altered colony morphology due to fructan synthesis. Infect Immun. 1984 Jun;44(3):617–622. doi: 10.1128/iai.44.3.617-622.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
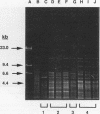
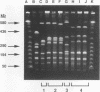
- Okahashi N., Sasakawa C., Okada N., Yamada M., Yoshikawa M., Tokuda M., Takahashi I., Koga T. Construction of NotI restriction map of the Streptococcus mutans genome. J Gen Microbiol. 1990 Nov;136(11):2217–2223. doi: 10.1099/00221287-136-11-2217. [DOI] [PubMed] [Google Scholar]
- Pucci M. J., Jones K. R., Kuramitsu H. K., Macrina F. L. Molecular cloning and characterization of the glucosyltransferase C gene (gtfC) from Streptococcus mutans LM7. Infect Immun. 1987 Sep;55(9):2176–2182. doi: 10.1128/iai.55.9.2176-2182.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell K. G., Birkhed D. An inulin-like fructan produced by Streptococcus mutans, strain JC2. Acta Chem Scand B. 1974;28(5):589–589. doi: 10.3891/acta.chem.scand.28b-0589. [DOI] [PubMed] [Google Scholar]
- Sato S., Kuramitsu H. K. Isolation and characterization of a fructosyltransferase gene from Streptococcus mutans GS-5. Infect Immun. 1986 Apr;52(1):166–170. doi: 10.1128/iai.52.1.166-170.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder V. A., Michalek S. M., Macrina F. L. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun. 1989 Nov;57(11):3560–3569. doi: 10.1128/iai.57.11.3560-3569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A., Tsuboi K., Nagase T., Ito M., Tsumori H., Mukasa H. Structural determination of D-fructans from Streptococcus mutans, serotype b, c, e, and f strains, by 13C-n.m.r. spectroscopy. Carbohydr Res. 1987 Jul 15;165(1):150–154. doi: 10.1016/0008-6215(87)80091-5. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988 Feb;170(2):810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms P. J., Boyko W. J., Edwards J. R. The structural analysis of a levan produced by Streptococcus salivarius SS2. Carbohydr Res. 1990 Dec 15;208:193–198. doi: 10.1016/0008-6215(90)80099-o. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Isolation and properties of levanase from Streptococcus salivarius KTA-19. Infect Immun. 1983 Oct;42(1):231–236. doi: 10.1128/iai.42.1.231-236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Purification and preliminary characterization of exo-beta-D-fructosidase in Streptococcus salivarius KTA-19. Infect Immun. 1985 Jan;47(1):271–276. doi: 10.1128/iai.47.1.271-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Wexler D. L., Penders J. E., Bowen W. H., Burne R. A. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutants strain. Infect Immun. 1992 Sep;60(9):3673–3681. doi: 10.1128/iai.60.9.3673-3681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Bowen W. H., Kuramitsu H. K. Molecular analysis of a Streptococcus mutans strain exhibiting polymorphism in the tandem gtfB and gtfC genes. Infect Immun. 1992 Apr;60(4):1618–1624. doi: 10.1128/iai.60.4.1618-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]