Abstract
Pseudomonas aeruginosa is an opportunistic pathogen often associated with chronic lung infections in individuals with the genetic disease cystic fibrosis (CF). Previous work from our laboratory revealed that five genes predicted to be important for catabolism of N-acetylglucosamine (GlcNAc) are induced during in vitro growth in CF lung secretions (sputum). Here, we demonstrate that these genes comprise an operon (referred to as the nag operon) and that NagE, a putative component of the GlcNAc phosphotransferase system, is required for growth on and uptake of GlcNAc. Using primer extension analysis, the promoter of the nag operon was mapped and shown to be inducible by GlcNAc and regulated by the transcriptional regulator NagR. Transcriptome analysis revealed that in addition to induction of the nag operon, several P. aeruginosa genes encoding factors critical for extracellular antimicrobial production are also induced by GlcNAc. Finally, we show that the GlcNAc-containing polymer peptidoglycan induces production of the antimicrobial pyocyanin. Based on this data, we propose a model in which P. aeruginosa senses surrounding bacteria by monitoring exogenous peptidoglycan and responds to this cue through enhanced production of an antimicrobial.
Pseudomonas aeruginosa is a ubiquitous Gram-negative bacterium that causes an array of human infections, particularly in patients with compromised systemic immunity or impaired mucosal defenses. In some instances, P. aeruginosa causes persistent infections that are virtually impossible to treat with conventional therapeutics due to the inherent resistance of this bacterium to many antibiotics (48). One of the most prevalent chronic P. aeruginosa infections occurs in the lungs of individuals with the heritable disease cystic fibrosis (CF). CF patients manifest a host defense defect in the conducting airways of the lung that results in the colonization and persistence of numerous bacterial species (12, 15, 22, 37). Within the CF lung, a large proportion of the infecting bacteria proliferate within airway sputum (mucus), growing in highly dense biofilm communities (7, 15, 47). Sputum is a complex mixture of host- and bacterium-derived substances that serves as not only the physical substrate but also the nutritional source for bacterial growth (15, 30, 34, 50).
The nutritional composition of the infection site has a profound impact on the disease pathogenesis of several bacteria, including P. aeruginosa (5). Using sputum harvested from the CF lung as a model system, our laboratory has examined how nutritional cues in sputum, specifically carbon sources, affect the production of factors important for P. aeruginosa host colonization and persistence (31, 32, 34). These studies, along with those of other groups (50), have provided insight into P. aeruginosa carbon catabolism during growth in CF sputum, ultimately implicating amino acids and lipids as primary growth substrates for P. aeruginosa in the CF lung (32, 34, 50). Interestingly, these studies have also provided evidence that some potential carbon sources do not need to be preferentially catabolized to affect processes important for P. aeruginosa pathogenesis (31, 32).
While studies of CF sputum have focused primarily on amino acids and lipids as relevant P. aeruginosa carbon sources, microarray analysis of CF sputum-grown P. aeruginosa revealed that genes annotated to be important for the catabolism of N-acetylglucosamine (GlcNAc) were highly up-regulated during in vitro growth in CF sputum (34). As many catabolic genes are induced in the presence of their substrate, the upregulation of these genes suggests that GlcNAc is present in CF sputum. This was not surprising, as GlcNAc is a component of several polymers in the human body, including mucin (8, 53) and hyaluronic acid (54), and is probably also present in human serum (18). In addition, GlcNAc catabolism has been implicated as an important in vivo carbon source for several bacteria, including Escherichia coli (6) and Salmonella enterica serovar Typhimurium (3, 10, 11).
In addition to the CF lung, P. aeruginosa probably encounters GlcNAc in most of the natural environments it inhabits. GlcNAc is present in polymeric form as chitin, which is the second most widely occurring molecule in nature, after cellulose. Many bacteria degrade chitin through the production of extracellular chitinases that release GlcNAc monomers into the environment, where they are subsequently catabolized by the microbiota (19, 28). GlcNAc is also present in peptidoglycan, a structural polymer produced by eubacteria that is shed in large amounts from the surface of Gram-positive bacteria (9, 26, 27). Thus, while our interest in GlcNAc began from studies of the CF lung, P. aeruginosa probably encounters GlcNAc in several environments outside the host. In this study, we characterize the P. aeruginosa GlcNAc (nag) catabolic operon and define the regulatory cascade controlling the transcription of this operon. We also provide evidence that P. aeruginosa enhances the production of phenazine antimicrobials in the presence of GlcNAc. Interestingly, this response is also observed in the presence of peptidoglycan, suggesting that even when it is present in complex polymers, P. aeruginosa senses and responds to GlcNAc through enhanced production of antimicrobial factors.
MATERIALS AND METHODS
Bacterial strains and media.
Bacterial strains and plasmids are listed in Table 1. P. aeruginosa strain PA14 and the isogenic nagR and nagE transposon insertion mutants were obtained from the PA14 nonredundant transposon mutant library (20) (http://ausubellab.mgh.harvard.edu/cgi-bin/pa14/home.cgi). The transposon insertions in nagR and nagE were confirmed by PCR as described previously (20). P. aeruginosa was routinely cultured in LB Miller broth/agar or morpholinepropanesulfonic acid (MOPS)-buffered defined medium (34) (25 mM MOPS [pH 7.2], 93 mM NH4Cl, 43 mM NaCl, 3.7 mM KH2PO4, 1 mM MgSO4, 3.5 μM FeSO4·7H2O) containing 0.1% Socransky vitamin solution (49) and 20 mM specified carbon source. Escherichia coli DH5α or E. coli XL1-Blue was cultured on LB Miller broth/agar. All cultures were grown at 37°C with shaking at 250 rpm. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; carbenicillin, 300 μg/ml for plasmid selection and 150 μg/ml for plasmid maintenance; and tetracycline, 5 μg/ml for selection and 100 μg/ml for plasmid selection for E. coli and 50 μg/ml for plasmid maintenance for P. aeruginosa. For growth curves, P. aeruginosa grown overnight was starved in MOPS-buffered medium without Socransky vitamins or carbon source (MOPS-B) followed by inoculation into MOPS-buffered base with 0.1% Socransky vitamin solution (MOPS-V) and 20 mM carbon source.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 Δ(lacZYA-argF)U169 deoR [φ80dlac Δ(lacZ)M15] | 42 |
| XL1Blue | endA1 gyrA96(Nalr) thi-1 recA1 relA1 lac glnV44 F′[::Tn10 proAB+lacIq Δ(lacZ)M15] hsdR17(rK− mK+) | Stratagene |
| BL21(DE3) | F−ompT gal dcm lon hsdSB(rB− mB−) λ(DE3 [lacI lacUV5-T7 gene1ind1 sam7 nin5]) | Novagen |
| P. aeruginosa strains | ||
| PA14 | Wild type | 20 |
| nagR mutant | PA14 nagR::Mar2×T7 (Gmr) | 20 |
| nagE mutant | PA14 nagE::Mar2×T7 (Gmr) | 20 |
| ΔnagA-glmS strain | PA14 nagA-glmS deletion mutant | This study |
| Plasmids | ||
| pMP220 | Broad-host-range transcriptional fusion vector, Tcr | 51 |
| pEX1.8 | Broad-host-range expression vector, Apr | 36 |
| pET15b | Expression vector, adds N-terminal His6 tag, Apr | Novagen |
| pGemTEasy | Sequencing vector | Promega |
| pEX18Tc | Gene replacement vector, Tcr | 14 |
| pEX18Tc-P1P2 | nagA-glmS deletion vector, Tcr | This study |
| pMP220-nag | pMP220 containing the nag promoter | This study |
| pET15b-8 | pET15b containing nagR | This study |
| pEX1.8nagE | pEX1.8 carrying nagE | This study |
Growth on and consumption of GlcNAc.
P. aeruginosa grown overnight was starved by washing and resuspension in MOPS-B followed by incubation at 37°C for 1.5 h. Starved cells were inoculated into MOPS-V containing 20 mM GlcNAc (Sigma Aldrich), and growth was monitored over time by measurement of optical density at 600 nm (OD600). For GlcNAc consumption, cells were starved as described above and inoculated into MOPS-V with 5 mM GlcNAc. Samples were collected throughout growth by centrifugation at 6,100 × g for 15 min. Supernatants were collected and stored at −20°C. Measurement of GlcNAc in supernatants was performed on a Varian high-performance liquid chromatography (HPLC) instrument fitted with a Varian Metacarb 87H 300- by 6.5-mm column at 35°C. Samples were eluted with 0.025 N H2SO4 isocratic elution buffer with a flow rate of 0.5 ml/min. A Varian refractive index detector at 35°C was used for GlcNAc detection. For quantification, commercially available GlcNAc (Sigma Aldrich) was used to generate a standard curve.
DNA manipulations.
DNA manipulations were performed using standard procedures (1). P. aeruginosa chromosomal DNA was extracted using a DNeasy tissue kit (Qiagen). Plasmid extractions were performed with either a QIAprep spin miniprep kit (Qiagen) or a GeneJET plasmid miniprep kit (Fermentas). Restriction endonucleases were purchased from either New England BioLabs or Fermentas Life Sciences. PCR was performed using an Expand long template PCR system (Roche). DNA sequencing was performed at the University of Texas Institute for Cell and Molecular Biology DNA Core Facility.
Complementation of nagE.
The nagE gene was PCR amplified from P. aeruginosa chromosomal DNA using the primers 61-FcompEcoRI and 61-RcompHindIII (Table 2). The resulting 1,713-bp product was digested with EcoRI and HindIII and ligated into EcoRI/HindIII-digested pEX1.8 (36). The resulting pEX1.8 plasmid containing nagE (pEX1.8nagE) was transformed into the P. aeruginosa nagE mutant by electroporation (1). Transformants were screened for growth on GlcNAc by inoculation into MOPS-V containing 20 mM GlcNAc. The sequence of PCR-amplified nagE was confirmed by standard automated sequencing.
TABLE 2.
Primer sequences
| Use | Primer | Sequence |
|---|---|---|
| DNA contamination | rplU-for | CGCAGTGATTGTTACCGGTG |
| rplu-rev | AGGCCTGAATGCCGGTGATC | |
| RT-PCR | 57RT′+ | CGGCGGTCGGCGATTCCCTC |
| 58RT′− | CAGCGAGCGGCGGCAGTAATC | |
| 58RT+ | CCTGGCCGAAGCCTCGCAACG | |
| 59RT− | GCGAATCCTCGGCATTCACCAG | |
| 59RT+ | GGAGCGCTATCGCTGCTG | |
| 60RT− | GTGTCGAGGCCGACGTGCAGC | |
| 60RT+ | GCTGCGACTGATCGCCCAGAC | |
| 61RT− | GCACCGAGATCATCACCAGG | |
| EMSA probes | 57promF-XbaI | GCTCTAGAGAGAGGTTCCGATGCGGATTG |
| 57promR-EcoRI | GGAATTCCGCAAGACCAGCAACAACTAC | |
| PA3757intF | GCTGCCCTCCGAACGCAACC | |
| PA3757intR | CCGTCCAGGTATTCGTAGAGG | |
| nagA flanking region | 57F-P1 | GGAATTCGAAGACAGCCCACGACCTGC |
| 57R-P1 | CGGGTGACTTTGCTCAGGTGCCGTGTCGGTTCAGCGTCGCAG | |
| glmS flanking region | 60F-P2 | CGTGTCGGTTCAGCGTCGCAGGCACCTGAGCAAAGTCACCCG |
| 60R-P2 | GGGGTACCCGCCGAGGGCCGCCTCGCTGTC | |
| Plasmid constructs | 61-FcompEcoRI | GGAATTCGCAACACTGGCCGCTGGCCTG |
| 61-RcompHindIII | CCCAAGCTTGTAGGGCGGATAACCGCTTGG | |
| 57promF-XbaI | GCTCTAGAGAGAGGTTCCGATGCGGATTG | |
| 57promR-EcoRI | GGAATTCCGCAAGACCAGCAACAACTAC | |
| NdeIPA14_15830F | GGAATTCCATATGAAGACAGCCCACG | |
| 3′N-57NdeI | GGAATTCCATATGTCAGCGTCGCAGT |
Microarray analysis.
For Affymetrix GeneChip analysis, starved P. aeruginosa cells were inoculated into MOPS-V with 20 mM GlcNAc or 20 mM glucose at an OD600 of 0.01 and allowed to grow to an OD600 of 0.45 to 0.50. The cultures were mixed 1:1 with RNAlater (Ambion), and total RNA was isolated using an RNeasy mini kit (Qiagen). Removal of DNA contamination was done by DNase treatment (Promega) and verified by PCR amplification of the rplU gene using the primers rplU-for and rplU-rev (Table 2) (24, 32, 33, 45). The integrity of purified RNA samples was confirmed by agarose gel electrophoresis (10, 12, 13, 16). cDNA synthesis from total RNA and cDNA fragmentation were performed as previously described (24, 32, 33, 45). Processing of the P. aeruginosa Affymetrix GeneChips was performed at the University of Iowa DNA facility. Experiments were performed in duplicate, and data were analyzed using GeneChip operating software (GCOS version 1.4). Differentially regulated genes were identified by pairwise comparisons of all GeneChips.
RNA transcript analyses by RT-PCR.
RNA for reverse transcription (RT)-PCR was prepared as described above for GeneChip experiments. Synthesis of cDNA with the random primer (NS)5 was performed with SuperScript II (Invitrogen) according to the manufacturer's instructions, with 100 ng RNA template. PCR was performed with 50 ng chromosomal DNA, 50 ng cDNA, or 100 ng RNA with primer sets overlapping putative cotranscribed coding regions (Table 2) (33).
Primer extension analysis.
Primer extension was performed using fluorescently labeled 6-carboxyfluorescein (FAM) primers as previously described (4, 21, 31). Briefly, 1 μl of a 0.2 μM 5′-FAM-labeled primer was added to 20 μg total RNA in a final volume of 20 μl and incubated at 70°C for 10 min. Reaction mixtures were quickly chilled in an ice-water bath and then incubated at 65°C for 20 min. The temperature was shifted to 42°C, and reagents for cDNA synthesis (8 μl 5× buffer, 4 μl 0.1 M dithiothreitol [DTT], 4 μl 10 mM deoxyribonucleotides, 4 μl SuperScript II enzyme [Invitrogen]) were added. Reaction mixtures were incubated at 42°C for 2 h, ethanol precipitated, and submitted for DNA sizing analysis at the University of Oklahoma Health Sciences Center Laboratory for Genomics and Bioinformatics. Primers used for primer extension are shown in Fig. 3B below. When more than one fluorescent peak was present, the highest peak, which corresponds to the major primer extension product, was reported. Primer extension analysis was performed with two different primers to confirm the transcriptional start site.
Construction and purification of N-terminally His6-tagged NagR.
The nagR gene was PCR amplified from P. aeruginosa chromosomal DNA using the primers NdeIPA14_15830F and 3′N-57NdeI (Table 2). The resulting 744-bp fragment was digested with NdeI and ligated into NdeI-digested pET15b (Novagen) to generate pET15b-8. This construct adds a 6-histidine tag (His6) to the N terminus of NagR. The sequence of nagR in pET15b-8 was confirmed by DNA sequencing.
For overexpression of His6-NagR, the E. coli strain BL21(DE3) (Novagen) carrying pET15b-8 was grown in LB Miller broth supplemented with ampicillin. During exponential growth (OD600 of 0.65 to 0.75), 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to induce the expression of His6-nagR. Cells were harvested for protein purification after 16 h of growth at 16°C with shaking at 250 rpm. To purify His6-NagR, cells were pelleted by centrifugation at 6,100 × g for 15 min at 4°C and resuspended in 4 ml cold buffer A (25 mM potassium phosphate buffer, pH 7.4, 0.5 M NaCl, 5 mM DTT, 20 mM imidazole). Cells were lysed by two passes through a French press at 20,000 lb/in2 at 4°C, and the resulting lysate was centrifuged at 29,500 × g for 1 h at 4°C to pellet insoluble material. The supernatant was then passed over a 1-ml His-Trap HP column (GE Healthcare) equilibrated with cold buffer A. The column was washed with 4 ml cold buffer B (25 mM potassium phosphate buffer, pH 7.4, 0.5 M NaCl, 5 mM DTT) containing 200 mM imidazole, and His6-NagR was eluted with 4 ml cold buffer B containing 400 mM imidazole. All fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. Western blotting on nitrocellulose membranes was performed with an alkaline phosphatase-conjugated monoclonal anti-polyhistidine His-1 antibody (Sigma) and Western Blue stabilized substrate for alkaline phosphatase (Promega). Purified His6-NagR was desalted with an Amicon Ultra-4 centrifugal filter device (10-kDa cutoff) by successive concentration and dilution in storage buffer (25 mM phosphate buffer, pH 7.4) until the imidazole concentration was <5 mM. Protein concentrations were quantified with the Bio-Rad protein assay (Bio-Rad). Purified His6-NagR was stored in 25 mM phosphate buffer at −80°C.
EMSA.
The primers used to generate probes for electrophoretic mobility shift assays (EMSA) are listed in Table 2. Probes were generated by PCR and gel purified using a QIAquick gel extraction kit (Qiagen). Ten picomoles of probe was labeled with [γ-32P]ATP (Sigma-Aldrich) using a KinaseMax kit (Ambion) according to the manufacturer's instructions. Unincorporated radiolabeled nucleotides were removed using NucAway spin columns (Ambion). An unlabeled probe targeting an intragenic region of nagR was generated using the primers PA3757intF and PA3757intR (Table 2) and used for EMSA competition experiments. For EMSA, 100 nM probe (5,000 cpm) was incubated with 250 nM His6-NagR in 1× DNA binding buffer [20 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM EDTA, 1 mM DTT, 2% glycerol, 100 μg/ml bovine serum albumin, 10 μg/ml poly(dI-dC)] (31, 46). For each cold competition reaction, a 20-fold molar excess of unlabeled probe was added to the binding reaction mixture. All binding reaction mixtures included 250 μM GlcNAc. EMSA reaction mixtures were incubated at 30°C for 30 min prior to separation on 5% native polyacrylamide gels. Gels were prerun at 80 V for 1 h prior to loading EMSA reaction mixtures. Gels were dried in 100% ethanol, exposed to phosphorimager screens overnight, and visualized with a personal molecular imager (Bio-Rad).
Construction of a P. aeruginosa nagA-glmS deletion mutant.
To delete nagA and glmS, the DNA regions upstream of nagA and downstream of glmS were amplified by PCR using the primer pairs 57F-P1/57R-P1 and 60F-P2/60R-P2, respectively (Table 2). The two amplicons were combined using overlap extension PCR to create a DNA fragment in which nagA and glmS were deleted (13). The resulting product was digested with EcoRI and KpnI and ligated into pEX18Tc (14). The resulting plasmid, pEX18Tc-P1P2, was conjugated into P. aeruginosa, and mutants lacking nagA-glmS were selected as described previously (14) and confirmed by PCR. The ability of P. aeruginosa ΔnagA-glmS to grow with GlcNAc as the primary source of carbon and energy was tested on MOPS-V containing 20 mM GlcNAc.
Construction of a nag promoter-lacZ transcriptional fusion.
The promoter region upstream of the putative nag operon was PCR amplified from P. aeruginosa chromosomal DNA using the primers 57promoterF and 57promoterR (Table 2), and its sequence verified. After digestion with EcoRI and XbaI, the 500-bp amplicon was ligated into EcoRI/XbaI-digested pMP220 (51), resulting in pMP220-nag. pMP220-nag contains a transcriptional fusion of the nag operon promoter to lacZ. pMP220-nag and pMP220 were introduced into the wild-type P. aeruginosa and the P. aeruginosa nagR mutant via electroporation as described previously (1).
β-Galactosidase assays.
P. aeruginosa and the P. aeruginosa nagR mutant carrying pMP220-nag or pMP220 were grown overnight in LB broth containing 50 μg/ml tetracycline. Cells were harvested by centrifugation at 6,100 × g for 15 min, washed with MOPS-B, and inoculated at an OD600 of 0.02 into MOPS-V with 20 mM succinate containing 50 μg/ml tetracycline. Cultures were grown to an OD600 of 0.1, at which time either 1 mM GlcNAc, 1 mM glucose, or 1 mM succinate was added. Cultures were incubated for an additional 3 h, and β-galactosidase levels quantified as previously described (35, 43).
14C-labeled-GlcNAc uptake assays.
For GlcNAc uptake studies, overnight-grown P. aeruginosa strains were starved as described above and resuspended to an OD600 of 0.1 in MOPS-B. Cultures were incubated at 37°C for 5 min, followed by the addition of 100 μM N-acetyl-d-[14C]glucosamine (Amersham). At various time points, 100-μl aliquots were removed and added to 2.9 ml of ice-cold MOPS containing 20 mM unlabeled GlcNAc to quench the reaction. Quenched reaction mixtures were filtered with a 0.2-μm filter and washed with 3 ml of ice-cold MOPS containing 20 mM GlcNAc. Filters were placed in scintillation vials containing 4 ml of Ecolite scintillation fluid and counted in an LS6500 scintillation counter (Beckman-Coulter). To examine the effect of preexposure to GlcNAc on GlcNAc transport, wild-type P. aeruginosa was starved for 1 h in MOPS-B, followed by the addition of 100 μM unlabeled GlcNAc. After incubation for 30 min at 37°C, the cells were washed twice with MOPS-B to remove the unlabeled GlcNAc and resuspended to an OD600 of 0.1. GlcNAc uptake was performed as described above with 50 μM N-acetyl-d-[14C]glucosamine. As a control, heat-killed P. aeruginosa (95°C for 10 min) was utilized.
Peptidoglycan addition experiments.
When used, insoluble S. aureus peptidoglycan was purchased from Sigma Aldrich (catalog number 77140) or purified from S. aureus MN8 (44) as described previously (41). Insoluble peptidoglycan is composed of large peptidoglycan fragments of unknown molecular weight, as well as associated techoic acids. For these experiments, 330 μg/ml insoluble peptidoglycan was used. Based on the molecular weight of peptidoglycan, we estimate that this amount of peptidoglycan corresponds to less than 500 μM GlcNAc.
Pyocyanin analysis.
To quantify pyocyanin, P. aeruginosa strains were inoculated into MOPS-V with 20 mM succinate containing one of the following: 1 mM GlcNAc, 1 mM succinate, 1 mM glucose, or 330 μg/ml insoluble S. aureus peptidoglycan. Cells were grown to an OD600 of 1.5, and pyocyanin was extracted and quantified from 1-ml cultures as previously described (25, 55).
Gram-positive antimicrobial assays.
Lysis assays were performed by thoroughly swabbing a brain heart infusion plate with an overnight culture of S. aureus Xen36 (Xenogen Biosciences) or a MOPS succinate agar plate with an overnight culture of Bacillus licheniformis ATCC 12759. Plates were air dried, followed by placement of a 6-mm filter disc (Whatman) on the agar. Discs were spotted with 5 μl of an overnight culture of wild-type P. aeruginosa or the nagE mutant. Plates were incubated overnight (37°C for S. aureus and 30°C for B. licheniformis), and zones of clearing measured.
Microarray data accession number.
The microarray data are available in the EMBL-EBI data bank (www.ebi.ac.uk/miamexpress) under experiment accession number E-MEXP-2867.
RESULTS AND DISCUSSION
Transcriptome analyses of P. aeruginosa growing in CF sputum in vitro revealed induction of genes putatively involved in GlcNAc catabolism (34), suggesting that GlcNAc or a structurally similar compound was present in CF sputum. Examination of three sputum samples using HPLC confirmed the presence of GlcNAc in CF sputum in micromolar amounts (data not shown). Based on these results and the observation that P. aeruginosa probably encounters GlcNAc in most natural environments it inhabits, we investigated the ability of P. aeruginosa to grow using GlcNAc as the primary source of carbon and energy. P. aeruginosa in a MOPS defined medium containing GlcNAc as the primary carbon and energy source grew with a generation time of 280 min (Fig. 1A), significantly slower than growth with succinate (55 min). Analysis of GlcNAc levels in P. aeruginosa cultures indicated that the increase in cell yield directly corresponded to a decrease in the amount of GlcNAc in the culture medium (Fig. 1B), providing definitive evidence that P. aeruginosa catabolizes GlcNAc. Although vitamins were added to the defined medium used in these experiments, they do not represent a significant carbon and energy source, as P. aeruginosa displayed negligible growth in the absence of GlcNAc or succinate (Fig. 1A).
FIG. 1.
Growth of P. aeruginosa with GlcNAc as the primary carbon and energy source. (A) Growth of P. aeruginosa in MOPS defined medium (MOPS-V) containing 20 mM GlcNAc, 20 mM succinate, or no additional carbon. Bacteria were grown with shaking (250 rpm) at 37°C and sampled during exponential growth (OD600 of 0.45 to 0.50) for Affymetrix GeneChip analysis. Representative growth curves are shown. (B) P. aeruginosa growth and GlcNAc consumption in MOPS-V containing 5 mM GlcNAc.
Having determined that GlcNAc can serve as a carbon and energy source, transcriptome analysis using Affymetrix GeneChips was performed to examine P. aeruginosa gene expression during growth on GlcNAc. Growth on GlcNAc resulted in the up-regulation of 186 genes, while 70 genes were down-regulated (at a >5-fold cutoff), compared to their expression in glucose-grown cells (see Table S1 in the supplemental material). As expected, genes annotated to play a role in GlcNAc catabolism were induced between 27- and 34-fold (Table 3). The putative P. aeruginosa GlcNAc catabolism genes appear to be organized into an operon (referred to as the nag operon) (Fig. 2A) composed of a putative transcriptional regulator (PA14_15830), a putative GlcNAc-6-phosphate deacetylase (PA14_15820, nagA), a putative aminotransferase (PA14_15810, glmS), and two genes that comprise the EIIA (PA14_15790, nagF) and EIIB (PA14_15780, nagE) components of a putative GlcNAc phosphotransferase system (PTS) transporter. Interestingly, some genes encoding virulence factors in P. aeruginosa were also induced by GlcNAc (Table 3). These included genes involved in phenazine biosynthesis, as well as genes encoding the extracellular proteases LasA and LasB. While these extracellular factors are important for pathogenesis in multiple models (23, 38, 39, 52, 56), phenazines and LasA also display antimicrobial activity against some prokaryotes (2, 17). Thus, in addition to genes putatively involved in GlcNAc catabolism, genes encoding factors important for killing eukaryotic and prokaryotic cells were induced during growth with GlcNAc as the primary carbon and energy source.
TABLE 3.
Select P. aeruginosa genes upregulated during growth on GlcNAc
| Function and open reading framea | Genea | Category or classa | Fold upregulationb |
|---|---|---|---|
| GlcNAc catabolism | |||
| PA3757 | nagR | Probable transcriptional regulator | 27 |
| PA3758 | nagA | N-Acetylglucosamine-6-phosphate deacetylase | 31 |
| PA3759 | glmS | Probable aminotransferase | 30 |
| PA3760 | nagF | Probable phosphotransferase protein | 34 |
| PA3761 | nagE | Probable phosphotransferase system protein | 33 |
| Phenazine biosynthesis | |||
| PA1901 | phzC | Phenazine biosynthesis protein | 59 |
| PA1902 | phzD | Phenazine biosynthesis protein | 39 |
| PA1903 | phzE | Phenazine biosynthesis protein | 30 |
| PA1904 | phzF | Probable phenazine biosynthesis protein | 41 |
| PA1905 | phzG | Probable pyridoxamine 5-phosphate oxidase | 37 |
| PA4210 | phzA | Probable phenazine biosynthesis protein | 52 |
| PA4211 | phzB | Probable phenazine biosynthesis protein | 50 |
| Extracellular enzymes | |||
| PA1871 | lasA | LasA protease precursor | 14 |
| PA3724 | lasB | LasB elastase | 12 |
From the Pseudomonas Genome Database (www.pseudomonas.com).
Fold upregulation of genes expressed during P. aeruginosa growth on GlcNAc compared to that on glucose.
FIG. 2.
Genes of the putative nag operon are cotranscribed. (A) Chromosomal organization of nag operon. Genes are designated as described at the Pseudomonas Genome Database (www.pseudomonas.com), except for PA14_15830, which we have designated nagR. Numbered horizontal bars represent regions amplified by primer sets overlapping coding regions of putative cotranscribed genes (Table 2). (B) RT-PCR analysis of nag operon. P. aeruginosa was grown with GlcNAc and harvested for RNA isolation. RNA was used as a template for synthesis of cDNA, and cDNA was subsequently used as a template for PCR with primer sets overlapping coding regions represented by the numbered horizontal bars above. P. aeruginosa genomic DNA (gDNA) and P. aeruginosa RNA were used as positive and negative controls, respectively. Bands are ∼500 bp.
An in silico analysis of the P. aeruginosa PA14 genome revealed that the P. aeruginosa GlcNAc catabolism genes were probably organized as an operon (Fig. 2A) (40). To test this prediction, RT-PCR was performed using RNA isolated from P. aeruginosa grown in MOPS defined medium (34) containing 20 mM GlcNAc. Primer sets were designed to amplify the overlapping coding regions of the putative cotranscribed genes, shown in Fig. 2A. The results indicate that, as predicted, an operon containing PA14_15830, nagA, glmS, nagF, and nagE is present in GlcNAc-grown P. aeruginosa (Fig. 2B).
To identify important DNA regulatory sites controlling the expression of the nag operon, the promoter region upstream of the nag operon was mapped using primer extension. To map the transcriptional start site, a nonradioactive primer extension assay, previously used to identify Helicobacter pylori and P. aeruginosa transcriptional start sites (4, 21), was employed. Briefly, a fluorescently labeled primer antisense to the coding strand of PA14_15830 was used to generate cDNA from GlcNAc-grown P. aeruginosa RNA. The size and quantity of the cDNA product was determined using a standard DNA sequencer (Fig. 3A) and used to map the transcriptional start site of the nag operon. This analysis revealed that the primary nag operon transcriptional start site is an adenosine located 37 bp upstream from the PA14_15830 translational start codon (Fig. 3B).
FIG. 3.
Identification of the nag operon promoter by primer extension analysis. Primer extension was performed using a fluorescently (6-carboxyfluorescein) labeled primer as previously described (4, 21, 31). (A) Sizes and fluorescence values for the primer extension cDNA fragments. The fluorescence value indicates the relative quantity of the cDNA product, and the values represented were obtained with Primer 2. Peaks obtained were analyzed using Peak Scanner software from Applied Biosystems. (B) The nag operon promoter region based on primer extension data. The transcriptional start site is bold, and a putative −35 promoter region is underlined. The boxed regions indicate the target sequences for the two oligonucleotide primers used for primer extension.
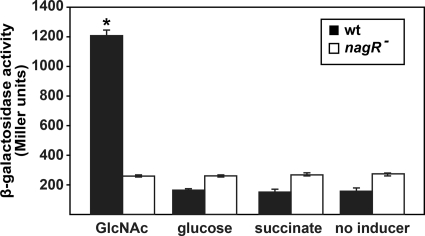
To examine the regulation of the nag operon, a transcriptional fusion of the nag promoter to lacZ was constructed (pMP220-nag) and introduced into P. aeruginosa. β-Galactosidase activity was assessed in the presence of GlcNAc, glucose, or succinate. The addition of GlcNAc resulted in an approximately 6-fold induction in β-galactosidase activity, indicating that, as expected from the GeneChip studies (Table 3), the nag operon is inducible by GlcNAc (Fig. 4). The first gene in the nag operon, PA14_15830 (PA3757 in strain PAO1), encodes a protein with 77% identity (E value, 10−78 using BLASTp) to the Collimonas fungivorans chitinolysis transcriptional regulator NagQ. To determine if this putative transcriptional regulator controlled the expression of the nag operon, pMP220-nag was introduced into an isogenic P. aeruginosa strain containing a transposon insertion in PA14_15830. The addition of GlcNAc to the P. aeruginosa PA14_15830 mutant carrying pMP220-nag had no effect on β-galactosidase activity (Fig. 4), indicating that the protein encoded by PA14_15830 (hereinafter referred to as NagR) regulates, either directly or indirectly, the transcription of the nag operon.
FIG. 4.
The P. aeruginosa nag operon is inducible by GlcNAc and is controlled by NagR. The β-galactosidase activities of wild-type P. aeruginosa (wt) and the isogenic nagR mutant (nagR−) carrying pMP220-nag were assessed in the absence (no inducer) and presence of GlcNAc, glucose, and succinate. For these experiments, cells were grown in MOPS-V with 20 mM succinate to an OD600 of 0.1, at which point the cultures were supplemented with GlcNAc, glucose, succinate, or water. After 3 h of growth, β-galactosidase activity was measured. β-Galactosidase activity of P. aeruginosa carrying the empty vector (pMP220) was 30 Miller units. Values represent the average results of four separate experiments, and error bars show standard errors of the means. *, P < 0.001 by Student's t test compared independently to all other measurements.
To examine whether NagR binds directly to the nag promoter, P. aeruginosa nagR was cloned into the expression vector pET15b to create N-terminally His6-tagged NagR. His6-NagR was purified using a nickel column, resulting in a single band at 27 kDa on Coomassie-stained SDS-PAGE gel (Fig. 5A). Western blot analysis with anti-His6 antibody confirmed that this protein was His6-NagR (data not shown). The binding of His6-NagR to the nag promoter was examined by electrophoretic mobility shift assay (EMSA). The DNA region containing the nag promoter was amplified by PCR and 5′-end-labeled with 32P for use as an EMSA probe. The labeled nag promoter was incubated with purified His6-NagR and 250 μM GlcNAc to assess binding. His6-NagR bound to and retarded the mobility of the nagR promoter in the presence of GlcNAc (Fig. 5B), indicating that His6-NagR binds the nag operon promoter. To confirm the specificity of the binding, excess nonradioactive (cold) specific and nonspecific competitor DNA was added to the EMSA binding reaction mixtures. The presence of cold specific DNA eliminated binding of His6-NagR to the promoter, while a nonspecific DNA competitor had no effect on His6-NagR binding (Fig. 5B). These results suggest that NagR is a transcriptional regulator that positively regulates the nag operon by binding directly to the nag promoter. Of note, GlcNAc was added to all EMSA reaction mixtures, as it enhanced His6-NagR binding to the nag promoter.
FIG. 5.
His6-NagR binds to the nag operon promoter. (A) His6-NagR was purified using a HisTrap nickel column, separated on a 12% SDS-PAGE gel, and visualized after staining with Coomassie blue. The lanes contained desalted NagR used for EMSA (NagR), molecular weight ladder (L), cell lysate (Lys), pellet obtained after ultracentrifugation of lysate (P), soluble crude extract (Sup), column flowthrough after soluble cell extract addition (Ft), wash with buffer B containing 200 mM imidazole (W1), and elution of His6-NagR with buffer B containing 400 mM imidazole (W2). (B) EMSA analysis of His6-NagR binding to the nag operon promoter region. The lanes contain the following: 32P-labeled nag operon promoter probe (probe); 32P-labeled nag operon promoter probe with 250 nM His6-NagR and 250 μM GlcNAc (NagR); 32P-labeled nag operon promoter probe with 250 nM His6-NagR, 250 μM GlcNAc, and a 20-fold molar excess of unlabeled probe (specific); and 32P-labeled nag operon promoter probe with 250 nM His6-NagR, 250 μM GlcNAc, and a 20-fold molar excess of unlabeled nonspecific DNA competitor (nonspecific).
Immediately downstream of nagR are two genes, nagA and glmS, that encode proteins with 79% identity (E value, 10−130 using BLASTp) to the GlcNAc-6-phosphate deacetylase from Collimonas fungivorans and 61% identity (E value, 10−72 using BLASTp) to the glucosamine-fructose-6-phosphate aminotransferase from Xanthomonas campestris pv. Campestris, respectively. As these genes are predicted to encode the first two steps in GlcNAc catabolism, conversion of GlcNAc-6-phosphate to fructose-6-phosphate, we hypothesized that deleting these genes would eliminate the ability to grow with GlcNAc as the primary carbon and energy source. Surprisingly, the deletion of both nagA and glmS had no effect on GlcNAc growth, indicating that NagA and GlmS are not essential for GlcNAc catabolism. The most obvious explanation for this phenotype is that other genes on the P. aeruginosa chromosome encode enzymes that functionally complement NagA and GlmS. Indeed, P. aeruginosa possesses several potential deacetylases and aminotransferases, including a putative UDP-3-O-acyl-N-acetylglucosamine deacetylase (PA14_57260) and a second GlmS homolog (PA14_73170).
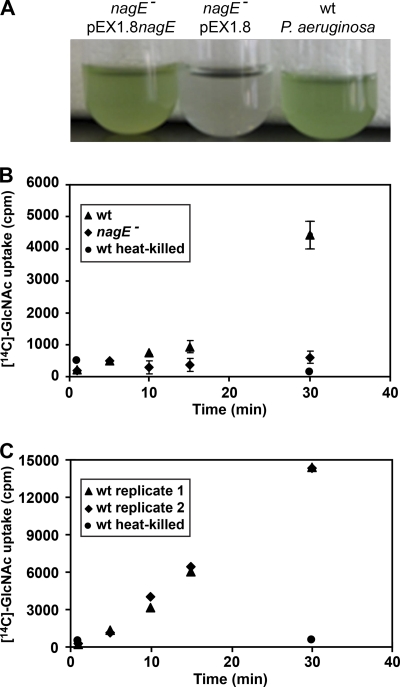
The last two genes in the nag operon, nagF and nagE, encode proteins with high homology to the EIIA and EIIB components of a PTS that is putatively responsible for the transport of GlcNAc into P. aeruginosa (16, 40). In support of this prediction, insertional inactivation of nagE eliminated the ability of P. aeruginosa to grow with GlcNAc (Fig. 6A). Growth was restored by the addition of nagE in trans (Fig. 6A), indicating that the lack of growth in the nagE mutant was due to inactivation of nagE and not to polar effects on surrounding genes. To directly assess the importance of nagE for GlcNAc transport, we performed uptake assays with [14C]-labeled GlcNAc. For these experiments, wild-type P. aeruginosa and the nagE mutant were starved in MOPS-B, followed by incubation with [14C]-labeled GlcNAc. Samples were removed from the cultures at various times, and cell associated radioactivity measured. Wild-type P. aeruginosa displayed very little uptake of [14C]-labeled GlcNAc over the first 15 min after addition; however, high levels of [14C]-labeled GlcNAc were associated with cells after 30 min (Fig. 6B). No uptake was observed in the P. aeruginosa nagE mutant, even after 30 min, indicating that NagE is required for the transport of GlcNAc (Fig. 6B). It was intriguing that significant uptake of [14C]-labeled GlcNAc was not observed until 30 min; however, the P. aeruginosa cells used for these experiments had not been grown in the presence of GlcNAc. Since the nag operon is highly induced by GlcNAc (Table 3 and Fig. 4), we reasoned that the poor uptake of [14C]-labeled GlcNAc by P. aeruginosa during the first 15 min was due to low-level expression of the nag operon. To test this, starved P. aeruginosa cells were preincubated with unlabeled GlcNAc, washed, and then incubated with [14C]-labeled GlcNAc. As expected, no lag in [14C]-labeled GlcNAc uptake was observed (Fig. 6C), indicating that preexposure to GlcNAc enhances the uptake of [14C]-labeled GlcNAc.
FIG. 6.
NagE is a component of the P. aeruginosa GlcNAc transporter and is required for growth with GlcNAc. (A) NagE is required for P. aeruginosa growth with GlcNAc as a carbon and energy source. Shown from right to left are wild-type (wt) P. aeruginosa, the nagE mutant (nagE−) carrying the plasmid pEX1.8, and the nagE mutant carrying the complementation plasmid pEX1.8nagE. Growth was assessed in MOPS-V with 20 mM GlcNAc. The blue-green color is due to production of pyocyanin. (B) Uptake of 14C-labeled GlcNAc by P. aeruginosa. Starved wt P. aeruginosa, P. aeruginosa nagE mutant, and heat-killed P. aeruginosa were incubated with [14C]-labeled GlcNAc (N-acetyl-d-[14C]glucosamine). Samples were collected at indicated time points, and cell-associated radioactivity measured by scintillation counting. Error bars show standard errors of the means and in some cases are too small to be seen. (C) Uptake of [14C]-labeled GlcNAc by wt P. aeruginosa and heat-killed P. aeruginosa following preincubation with unlabeled GlcNAc. The ▴ and ⧫ symbols represent two independent replicates of wt P. aeruginosa uptake.
In addition to genes in the nag operon, our transcriptome analysis of GlcNAc-grown P. aeruginosa revealed the induction of genes required for biosynthesis of phenazines (Table 3). To determine if this increase in transcription resulted in increased levels of phenazines, wild-type P. aeruginosa was grown in MOPS medium containing 20 mM succinate, and individual cultures were supplemented with GlcNAc, glucose, or succinate. Extraction of the phenazine pyocyanin revealed that the addition of glucose or succinate had no effect on pyocyanin levels, while the addition of GlcNAc resulted in a significant increase in pyocyanin levels (Fig. 7). These data indicate that P. aeruginosa increases the production of the antimicrobial pyocyanin in the presence of GlcNAc.
FIG. 7.
P. aeruginosa produces increased levels of pyocyanin in the presence of GlcNAc and peptidoglycan. Pyocyanin was extracted from wild-type (wt) P. aeruginosa and the P. aeruginosa nagE mutant (nagE−). Cells were grown in MOPS-V with 20 mM succinate containing 1 mM glucose, 1 mM succinate, 1 mM GlcNAc, or 330 μg/ml peptidoglycan. Values represent average results of three separate experiments, and error bars show standard errors of the means. *, P < 0.001 by Student's t test compared to (i) wt P. aeruginosa grown in glucose or succinate and (ii) the nagE mutant grown under all conditions. Abs520, absorbance at 520 nm.
While GlcNAc is clearly present in the CF lung and the natural environment, most of the GlcNAc in these environments is likely present in polymers, such as chitin and peptidoglycan. Peptidoglycan is particularly interesting, as it has been shown to be shed in large amounts by Gram-positive bacteria (9, 26, 27), and thus, P. aeruginosa would probably be exposed to this polymer in most polymicrobial environments. To assess whether a GlcNAc-containing polymer could also affect pyocyanin levels, P. aeruginosa grown in MOPS medium containing 20 mM succinate was supplemented with insoluble S. aureus peptidoglycan and pyocyanin levels quantified. Interestingly, wild-type P. aeruginosa grown in the presence of peptidoglycan displayed a significant increase in pyocyanin (Fig. 7), indicating that P. aeruginosa increases the production of an antimicrobial in the presence of extracellular peptidoglycan.
To determine if the ability to transport GlcNAc is required for the increase in pyocyanin observed with GlcNAc and peptidoglycan, the P. aeruginosa nagE mutant was grown in MOPS medium containing succinate supplemented with succinate, glucose, GlcNAc, or peptidoglycan. Unlike wild-type P. aeruginosa, the addition of GlcNAc and peptidoglycan had no effect on pyocyanin levels in the nagE mutant, indicating that GlcNAc must be transported inside the cell to induce pyocyanin (Fig. 7). At this point, it is not clear whether GlcNAc catabolism is required for pyocyanin induction, as we have not been able to obtain a P. aeruginosa strain that will transport GlcNAc but not catabolize it.
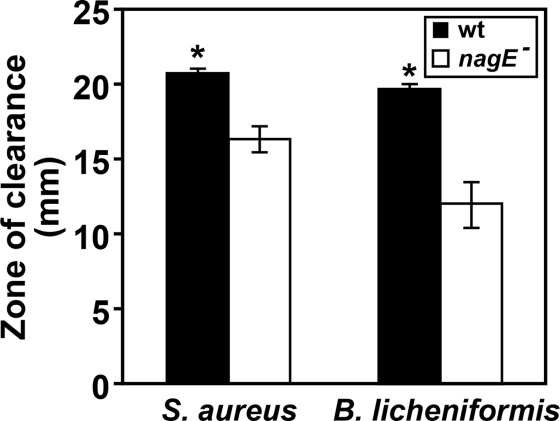
Based on the importance of NagE for enhanced pyocyanin production in the presence of peptidoglycan, we hypothesized that the nagE mutant would display decreased antimicrobial activity, particularly for Gram-positive bacteria which shed peptidoglycan from their cell walls (9, 26, 27). To test this hypothesis, wild-type P. aeruginosa and the P. aeruginosa nagE mutant were examined for the ability to inhibit the growth of the Gram-positive bacteria Staphylococcus aureus and Bacillus licheniformis. For these experiments, S. aureus or B. licheniformis cells were spread on the surface of agar petri dishes, and P. aeruginosa was added to paper discs on the agar surface. The petri dishes were incubated overnight, and P. aeruginosa antimicrobial activity was assessed by measuring the zone of clearing around the discs (Fig. 8). Wild-type P. aeruginosa exhibited significant antimicrobial activity; however, the nagE mutant displayed markedly reduced zones of clearing (Fig. 8). These data implicate the transport of GlcNAc as a critical factor contributing to the antimicrobial activity of P. aeruginosa against Gram-positive bacteria.
FIG. 8.
NagE is required for enhanced lysis of Gram-positive bacteria by P. aeruginosa. S. aureus Xen36 and B. licheniformis ATCC 12759 were swabbed on the surface of an agar plate, followed by the addition of 5 μl of either wild-type (wt) P. aeruginosa or the nagE mutant (nagE−) to a sterile disc in the center of the plate. Plates were incubated overnight, and zones of clearance measured. Values represent the average results of three separate experiments, and error bars show standard errors of the means. *, P < 0.05 by Student's t test.
The goal of this study was to expand on previous studies aimed at defining the effects of specific carbon sources on P. aeruginosa physiology. The characterization of the nag operon expands our understanding of the catabolic potential of P. aeruginosa and provides new insight into the genetic loci that are critical for GlcNAc uptake. We also provide evidence that GlcNAc induces genes involved in the production of several extracellular factors, including the antimicrobial pyocyanin. The most interesting finding in this study was that the addition of peptidoglycan also induced the production of pyocyanin, a phenotype that requires the GlcNAc transporter NagE. This finding may have implications for P. aeruginosa growing in polymicrobial communities, where peptidoglycan will likely be present in the extracellular environment due to shedding by Gram-positive members of the community. These data suggest a scenario in which P. aeruginosa senses peptidoglycan and enhances the production of a potent antimicrobial, thereby implicating peptidoglycan as a cue allowing P. aeruginosa to monitor and respond to the eubacterial constituents of its surroundings.
Supplementary Material
Acknowledgments
This work was funded by a grant from the NIH (5R01AI075068 to M.W.). M.W. is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Disease.
Footnotes
Published ahead of print on 17 December 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ausubel, F., et al. 1997. Short protocols in molecular biology: a compendium of methods from Current Protocols in Molecular Biology.John Wiley & Sons, Inc., New York, NY.
- 2.Baron, S. S., and J. J. Rowe. 1981. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 20:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumler, A. J., J. G. Kusters, I. Stojiljkovic, and F. Heffron. 1994. Salmonella typhimurium loci involved in survival within macrophages. Infect. Immun. 62:1623-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulette, M. L., et al. 2009. Characterization of alanine catabolism in Pseudomonas aeruginosa and its importance for proliferation in vivo. J. Bacteriol. 191:6329-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, S. A., K. L. Palmer, and M. Whiteley. 2008. Revisiting the host as a growth medium. Nat. Rev. Microbiol. 6:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, D. E., et al. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 101:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Dekker, J., P. H. Aelmans, and G. J. Strous. 1991. The oligomeric structure of rat and human gastric mucins. Biochem. J. 277(Pt. 2):423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle, R. J., J. Chaloupka, and V. Vinter. 1988. Turnover of cell walls in microorganisms. Microbiol. Rev. 52:554-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. U. S. A. 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahring, L. C., F. Heffron, B. B. Finlay, and S. Falkow. 1990. Invasion and replication of Salmonella typhimurium in animal cells. Infect. Immun. 58:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison, F. 2007. Microbial ecology of the cystic fibrosis lung. Microbiology 153:917-923. [DOI] [PubMed] [Google Scholar]
- 13.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 14.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 15.Hoiby, N. 1998. Pseudomonas in cystic fibrosis: past, present, and future. Cystic Fibrosis Trust, Bromley, United Kingdom.
- 16.Johnson, D. A., et al. 2008. High-throughput phenotypic characterization of Pseudomonas aeruginosa membrane transport genes. PLoS Genet. 4:e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler, E., M. Safrin, J. C. Olson, and D. E. Ohman. 1993. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 268:7503-7508. [PubMed] [Google Scholar]
- 18.Lee, J. K., and M. Pierce. 1995. Purification and characterization of human serum N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase. Arch. Biochem. Biophys. 319:413-425. [DOI] [PubMed] [Google Scholar]
- 19.Li, X., and S. Roseman. 2004. The chitinolytic cascade in Vibrios is regulated by chitin oligosaccharides and a two-component chitin catabolic sensor/kinase. Proc. Natl. Acad. Sci. U. S. A. 101:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati, N. T., et al. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd, A. L., B. J. Marshall, and B. J. Mee. 2005. Identifying cloned Helicobacter pylori promoters by primer extension using a FAM-labelled primer and GeneScan analysis. J. Microbiol. Methods 60:291-298. [DOI] [PubMed] [Google Scholar]
- 22.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 24.Mashburn, L. M., A. M. Jett, D. R. Akins, and M. Whiteley. 2005. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 187:554-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422-425. [DOI] [PubMed] [Google Scholar]
- 26.Mauck, J., L. Chan, and L. Glaser. 1971. Turnover of the cell wall of Gram-positive bacteria. J. Biol. Chem. 246:1820-1827. [PubMed] [Google Scholar]
- 27.Mauck, J., and L. Glaser. 1970. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem. Biophys. Res. Commun. 39:699-706. [DOI] [PubMed] [Google Scholar]
- 28.Meibom, K. L., et al. 2004. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. U. S. A. 101:2524-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Ohman, D. E., and A. M. Chakrabarty. 1982. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect. Immun. 37:662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer, G. C., K. L. Palmer, P. A. Jorth, and M. Whiteley. 2010. Characterization of the Pseudomonas aeruginosa transcriptional response to phenylalanine and tyrosine. J. Bacteriol. 192:2722-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer, K. L., L. M. Aye, and M. Whiteley. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189:8079-8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer, K. L., S. A. Brown, and M. Whiteley. 2007. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 189:4449-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer, K. L., L. M. Mashburn, P. K. Singh, and M. Whiteley. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187:5267-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson, J. P., et al. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. U. S. A. 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pier, G. B. 2002. CFTR mutations and host susceptibility to Pseudomonas aeruginosa lung infection. Curr. Opin. Microbiol. 5:81-86. [DOI] [PubMed] [Google Scholar]
- 38.Preston, M. J., et al. 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 65:3086-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahme, L. G., et al. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. U. S. A. 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reizer, J., et al. 1999. Novel phosphotransferase systems revealed by bacterial genome analysis: the complete repertoire of pts genes in Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 1:289-293. [PubMed] [Google Scholar]
- 41.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235:253-285. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 44.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 45.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuster, M., M. L. Urbanowski, and E. P. Greenberg. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. U. S. A. 101:15833-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh, P. K., et al. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 48.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 49.Socransky, S. S., J. L. Dzink, and C. M. Smith. 1985. Chemically defined medium for oral microorganisms. J. Clin. Microbiol. 22:303-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Son, M. S., W. J. Matthews, Jr., Y. Kang, D. T. Nguyen, and T. T. Hoang. 2007. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect. Immun. 75:5313-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spaink, H. P., J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 52.Tan, M. W., and F. M. Ausubel. 2000. Caenorhabditis elegans: a model genetic host to study Pseudomonas aeruginosa pathogenesis. Curr. Opin. Microbiol. 3:29-34. [DOI] [PubMed] [Google Scholar]
- 53.Wagner, S., et al. 1998. Morphological and molecular characterization of human gastric mucous cells in long-term primary culture. Pflugers Arch. 436:871-881. [DOI] [PubMed] [Google Scholar]
- 54.Weissmann, B., K. Meyer, P. Sampson, and A. Linker. 1954. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J. Biol. Chem. 208:417-429. [PubMed] [Google Scholar]
- 55.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wretlind, B., and O. R. Pavlovskis. 1983. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev. Infect. Dis. 5(Suppl. 5):S998-S1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.