Abstract
Diacetyl (2,3-butanedione) imparts an unpleasant “butterscotch-like” flavor to alcoholic beverages such as beer, and therefore its concentration needs to be reduced below the sensory threshold before packaging. We examined the mechanisms that lead to highly elevated diacetyl formation in petite mutants of Saccharomyces cerevisiae during beer fermentations. We present evidence that elevated diacetyl formation is tightly connected to the mitochondrial import of acetohydroxyacid synthase (Ilv2), the key enzyme in the production of diacetyl. Our data suggest that accumulation of the matrix-targeted Ilv2 preprotein in the cytosol is responsible for the observed high diacetyl levels. We could show that the Ilv2 preprotein accumulates in the cytosol of petite yeasts. Furthermore, expression of an Ilv2 variant that lacks the N-terminal mitochondrial targeting sequence and thus cannot be imported into mitochondria led to highly elevated diacetyl levels comparable to a petite strain. We further show that expression of a mutant allele of the γ-subunit of the F1-ATPase (ATP3-5) could be an attractive way to reduce diacetyl formation by petite strains.
Apart from the raw materials used, the flavor and taste of alcoholic beverages is strongly affected by fermentation by-products generated during yeast alcoholic fermentation. Although some compounds contribute to a positive sensory impression, others have a negative impact on the flavor and taste of alcoholic beverages. One of these undesirable compounds, diacetyl (2,3-butanedione), imparts an unpleasant “butterscotch-like” flavor to beer and its concentration needs to be reduced to below the taste threshold prior to filtration and packaging. Yeasts produce diacetyl especially in the early phase of fermentation and reduce it later during beer maturation into sensory more neutral compounds such as acetoin and 2,3-butanediol (6).
The enzyme acetohydroxyacid synthase (Ilv2) is of central importance for diacetyl formation. It catalyzes the first step in the biosynthesis of branched-chain amino acids, the irreversible decarboxylation of pyruvate and the condensation with a second molecule of pyruvate or 2-ketobutyrate (2). The vicinal diketones diacetyl and pentanedione are formed from these precursors by nonenzymatic oxidative decarboxylation (6).
The biosynthesis of branched-chain amino acids takes place in the mitochondrial matrix (17). The nuclear encoded Ilv2 protein is synthesized in the cytosol as a precursor protein with an N-terminal mitochondrial targeting sequence (MTS), which directs import into the mitochondrial matrix (5). It has been reported that petite mutants of Saccharomyces cerevisiae produce highly elevated levels of diacetyl (4). Petite mutants have lost part (rho−) or all (rho0) of their mitochondrial genome (mtDNA) (10). Since four subunits of the electron transport chain and three subunits of the F0 part of the F0F1-ATPase are encoded in the mitochondrial DNA, rho− mitochondria loose the capacity to generate ATP by oxidative phosphorylation (1). At the same time, the membrane potential across the inner mitochondrial membrane (Δψ) is compromised (16). An energized inner membrane is essential for the import of mitochondrial preproteins, possibly via an electrophoretic effect exerted onto the positively charged presequences (12). In the absence of mtDNA, the membrane potential is maintained through the ADP/ATP carrier (AAC) (3, 9). The exchange of ADP3− for ATP4− is electrogenic, i.e., generates a membrane potential across the inner membrane. ATP hydrolysis by the F1-ATPase increases the ADP concentration in the matrix, which in turn stimulates the exchange activity of the ADP/ATP carrier. However, the membrane potential appears to be lower under these conditions compared to the wild type and for this reason the import of some preproteins is less efficient (16). This seems to be true for Ilv2, since we observed an accumulation of the Ilv2 preprotein in the cytosol in petite mutants. Our data suggest that this cytosolic accumulation of Ilv2 is the reason for the elevated diacetyl production of petite mutants.
MATERIALS AND METHODS
Yeast strains, plasmids, and media.
The yeast strains used are listed in Table 1. All yeast strains are derived form the CEN.PK background. Insertions and deletions were introduced into the yeast genome by one-step gene replacement with PCR-generated cassettes (11). The insertions and deletions were verified by PCR. To construct pRK1107, the ATP3 gene (positions −630 to 1009, ATG = +1) was amplified by PCR from yeast chromosomal DNA and cloned into YCplac33 (8). To generate the ATP3-5 mutation (T297A) (21), pRK1107 was mutagenized by QuikChange mutagenesis (Agilent, Waldbronn, Germany) to give plasmid pRK1108. All cloned genes were sequenced to ensure that no PCR mutations occurred. For standard experiments, yeast cells were grown in YPD medium (1% yeast extract, 2% Bacto peptone, and 2% glucose) or in SD/CAS medium (0.67% yeast nitrogen base without amino acids, 1% casein hydrolysate, and 2% glucose).
TABLE 1.
Yeast strains
| Strain | Genotype | Source or reference |
|---|---|---|
| CEN.PK K29 | MATα his3-Δ1 ura3-52 | M. Ramezani-Rad, Düsseldorf, Germany |
| CEN.PK K61 | MATaleu2-3,112 trp1-289 | M. Ramezani-Rad, Düsseldorf, Germany |
| RKY2139 | MATa/α his3-Δ1/+ ura3-52/+ leu2-3,112/+ trp1-289/+ | This study |
| RKY2315 | MATa/α his3-Δ1/+ ura3-52/+ leu2-3,112/+ trp1-289/+ rho− | This study |
| RKY2399 | MATα his3-Δ1 ura3-52 rho− | This study |
| RKY2491 | MATa/α his3-Δ1/+ ura3-52/+ leu2-3,112/+ trp1-289/+ ILV2-Δ55/+ | This study |
Induction of petites with ethidium bromide.
To generate isogenic rho− mutants of our strains, cells from an overnight YPD culture were diluted 1:50 in YPD and incubated at 30°C for 3 h. Then, 10 μg of ethidium bromide/ml was added to the culture. After 3 h, a 5-ml sample was withdrawn, washed twice with water to remove the ethidium bromide, resuspended in 200 μl of water, and plated on YPD plates. Petites grow on fermentable carbon sources such as glucose but are unable to utilize nonfermentable carbon sources such as ethanol and glycerol. Therefore, the petite phenotype was assayed on YP plates containing 2% ethanol and 2% glycerol as the sole carbon sources.
Fermentations.
Commercially available Muntons dry malt extract (Muntons, Suffolk, United Kingdom) was used to prepare the beer wort for fermentation. The dry malt was dissolved in water (10% [wt/vol]), autoclaved at 121°C for 20 min, stored overnight at 4°C, and then sterilized by filtration. The sugar profile as determined by high-pressure liquid chromatography was typical of a beer wort. Precultures (3 ml) grown overnight at 30°C in malt extract (MEX) medium with shaking were inoculated into 50 ml of MEX medium in a 300-ml Erlenmeyer flask and grown again overnight to stationary phase. These aerobically precultured cells were adjusted to a cell density typical of beer fermentations (107 cells/ml) with MEX medium in a final volume of 275 ml. The flasks were sealed with an airlock. Fermentations were carried out at 30°C for up to 34 h. Samples were drawn at 2-h intervals without opening the flask, sterile filtered, and immediately frozen at −20°C. Diacetyl in the collected samples was measured by headspace gas chromatography (HS-GC) with an electron capture detector and a FS-INNOPEG-1000 column (CS-Chromatographie; Langerwehe). The closed GC vials were heated to 65°C for 90 min before measuring to ensure complete conversion of the precursor α-acetolactate to diacetyl (= total diacetyl). HS-GC was performed with a Perkin-Elmer-8500 instrument and an HS-40 headspace sampler. GC conditions were as follows: sample temperature, 60°C; needle temperature, 90°C; transfer line temperature, 100°C; GC-cycle time, 40 min; equilibration time, 15 min; pressurize time, 1 min; injection time, 0.04 min; withdrawal time, 0.3 min; carrier gas, nitrogen; carrier pressure, 150 kPa; sample size, 5 ml; and internal standard, 2,3-hexanedione (1 mg/liter).
Cell fractionation.
Yeast cultures were grown overnight to exponential phase (OD600 ≤ 1.0) in YPD medium. The cells were harvested by centrifugation, and the wet weight of the cell pellet was determined. The cells were washed with an equal volume of 10 mM NaN3 and resuspended in 2 ml of dithiothreitol (DTT) buffer (100 mM Tris-SO4 [pH 9.4], 10 mM DTT)/g of cells. Samples were placed on a shaker at 50 rpm for 30 min at 30°C. The cells were then washed with 5 ml of zymolyase buffer (1.2 M sorbitol, 20 mM KH2PO4 [pH 7.4]) and resuspended in 7 ml of zymolyase buffer/g of cells. Then, 1 mg of zymolyase (100T)/g of cells was added to the samples, followed by incubation for 1 h at 30°C. Spheroplasting was monitored by microscopical examination (cell lysis in H2O), and ≥99% of the cells were spheroplasts. Spheroplasts were carefully washed with cold (4°C) zymolyase buffer by pipetting up and down. The washed cells were resuspended in 7 ml of cold homogenization buffer (0.6 M sorbitol, 10 mM Tris-Cl [pH 7.4], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.2% bovine serum albumin)/g of cells and lysed with a Dounce homogenizer. Cell debris and unbroken spheroplasts were removed by centrifugation at 500 × g for 5 min at 4°C. Then, 0.5 ml of the supernatant was taken as the total fraction (TF). Another sample (0.5 ml) was centrifuged at 100,000 × g for 1 h at 4°C in an S45-A rotor. The supernatant (S100) was transferred to a fresh tube, and the pellet (P100) was resuspended in 0.5 ml of zymolyase buffer. Samples were denatured at 50°C for 15 min with 1 volume of sample buffer.
RESULTS
Enhanced diacetyl production in petite mutants.
It has been reported that respiratory-deficient petite (rho−) mutants accumulate higher diacetyl levels (4), which constitutes a major problem in beer fermentations. This effect could be reproduced in our yeast CEN.PK strain background. Laboratory-scale fermentations were carried out at 30°C with beer wort at cell densities typical of beer fermentations. Samples were collected at time intervals and the diacetyl levels in the samples were determined by headspace gas chromatography with an electron capture detector. For the diploid wild-type strain, very low diacetyl levels were observed with a peak at 8 h after the start of fermentation (0.126 mg/liter) (Fig. 1 A). In contrast, diacetyl levels were much higher in the isogenic petite mutant, again with a peak at 8 h of fermentation (0.847 mg/liter). In addition, the sugar profiles and ethanol formation were determined during the course of fermentation. The profiles were very similar for both strains (Fig. 1B and C). The sugar composition was typical of a beer wort, with a high concentration of maltose and some glucose. Glucose was consumed first, before maltose utilization started.
FIG. 1.
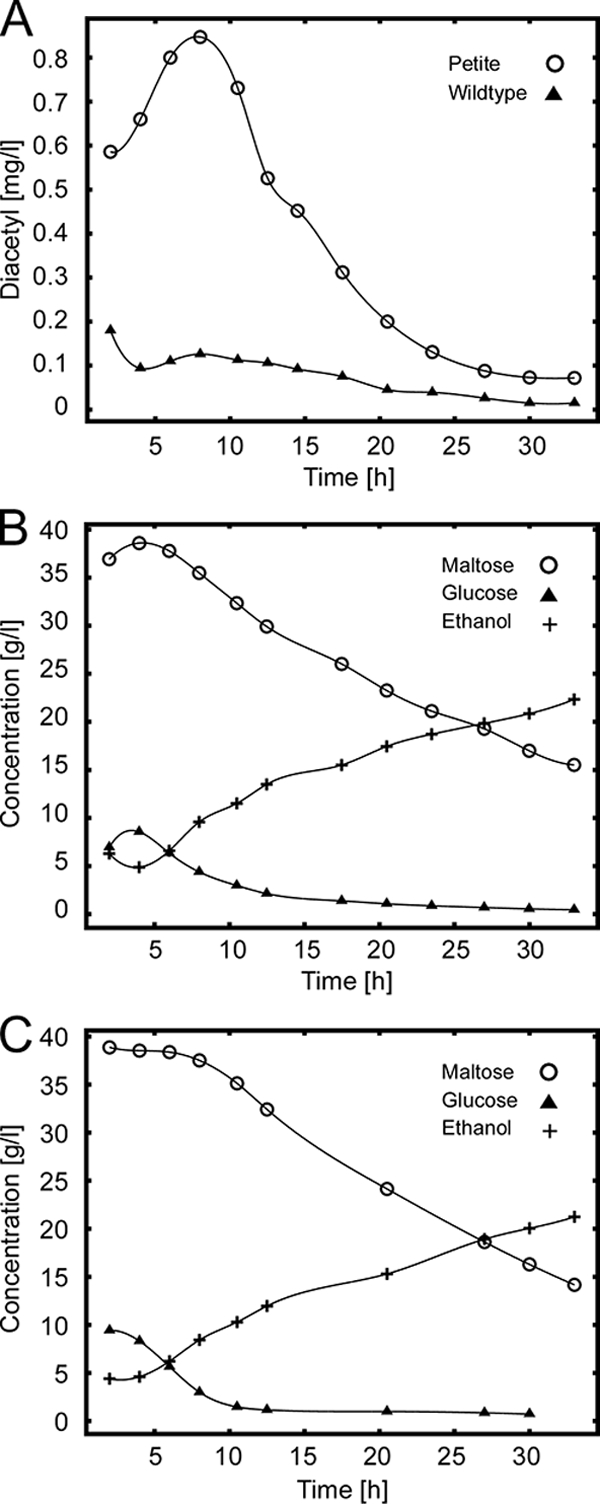
Diacetyl formation in wild-type and petite strains. Beer fermentations were performed in MEX medium at 30°C for the times indicated. (A to C) Diacetyl formation with RKY2139 (WT, ▴) and RKY2315 (rho−, ○) (A) and sugar profiles and ethanol formation with RKY2139 (B) and RKY2315 (C) (the concentrations of maltose [○], glucose [▴], and ethanol [+] are indicated). Experiments were performed at least three times. The results of a representative experiment are shown.
To understand the reason for the difference in diacetyl levels between rho− and wild-type strains, the enzyme responsible for the synthesis of the diacetyl precursor α-acetolactate, acetohydroxyacid synthase (Ilv2), was examined more closely. On Western blots with cell extracts from a normal rho+ wild-type strain, a single Ilv2 band could be observed (Fig. 2 A). Curiously, however, an additional slower migrating band could be observed with cell extracts from the isogenic petite strain. The mitochondrial matrix protein Ilv2 is synthesized in the cytosol as a precursor protein (PP) with an N-terminal mitochondrial targeting sequence, which is removed after import into mitochondria. One obvious possibility therefore was that the additional slower-migrating band corresponds to the Ilv2 precursor protein. To test this prediction, the uncoupling reagent carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added to cell cultures of a rho+ strain before extract preparation. CCCP dissipates the membrane potential (Δψ) across the inner mitochondrial membrane, which is essential for mitochondrial targeting of matrix proteins (13). After CCCP addition, an additional Ilv2 band was observed with the same mobility as the second band in the rho− background (Fig. 2A). We therefore conclude that the slower-migrating band corresponds to the precursor protein.
FIG. 2.
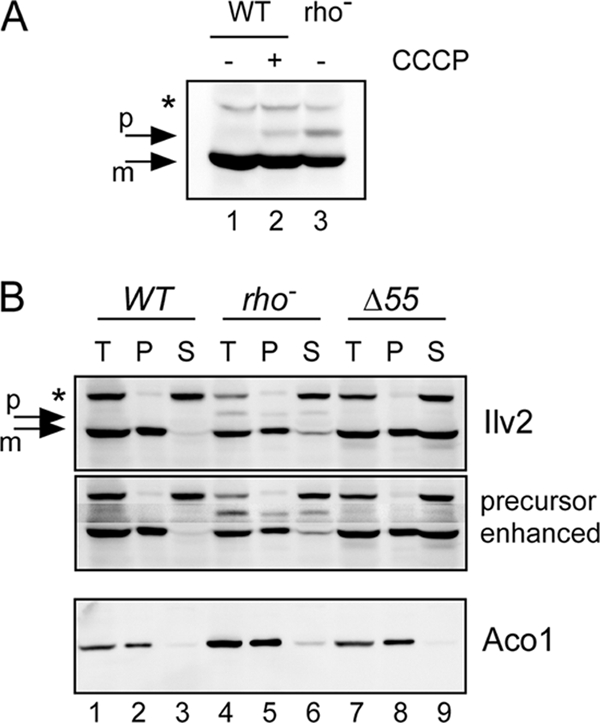
Accumulation of the Ilv2 precursor in the petite mutant. (A) Detection of Ilv2 by Western blotting. Lane 1, RKY2139 (WT); lane 2, RKY2139 (WT+CCCP, 40 μM, 4 h); lane 3, RKY2315 (rho−). (B) Fractionation of Ilv2. Cell extracts of the rho+ strain RKY2139 (lanes 1 to 3), the rho− strain RKY2315 (lanes 4 to 6), and the ILV2/ILV2-Δ55 strain RKY2491 (lanes 7 to 9) were fractionated by centrifugation. T, total fraction (lanes 1, 4, and 7); P, pellet fraction (lanes 2, 5, and 8); S, supernatant fraction (lanes 3, 6, and 9). Proteins were detected by Western blotting with anti-Ilv2 antibodies (upper two panels) and anti-aconitase antibodies (lower panel). In the second panel the region of the Ilv2 precursor was selectively enhanced. p, Ilv2 precursor; m, mature Ilv2. A background band is marked with an asterisk.
The Ilv2 precursor accumulates in the cytosol.
These findings suggest that the accumulation of Ilv2 precursor protein in the cytosol could be responsible for the enhanced diacetyl production observed in petite mutants. To prove that the precursor is indeed localized to the cytosol and not to the mitochondrial matrix, a cell fractionation experiment was performed. Yeast cells were spheroplasted and gently lysed with a Dounce homogenizer. The cell extract was centrifuged at 100,000 × g and separated into a mitochondrion containing P100 pellet and an S100 supernatant containing the soluble cytosolic proteins. To test for the integrity of mitochondria, the distribution of the mitochondrial matrix protein aconitase (Aco1) was examined (Fig. 2B). The protein was almost exclusively found in the pellet fraction. However, we consistently detected a small amount of mature Aco1 in the supernatant fraction (ca. 5% of the total protein). Evidence has been presented that a small fraction of Aco1 is localized to the cytosol (15). Therefore, it is difficult to decide whether this small amount of Aco1 in the supernatant results from the breakage of mitochondria or whether it represents a genuinely cytosolic Aco1 pool. The fractionation pattern of mature Ilv2 was very similar to the fractionation pattern of Aco1. The Ilv2 precursor, however, displayed a different fractionation behavior. The majority of the Ilv2 precursor was detected in the supernatant fraction (ca. 60%). Since the fraction of the Ilv2 precursor in the supernatant is much higher compared to mature Ilv2 or Aco1, we conclude that the Ilv2 precursor is to a large part localized to the cytosol.
If cytosolic Ilv2 is responsible for the high diacetyl levels in petite mutants, then expression of an import-incompetent Ilv2 variant should also lead to elevated diacetyl levels. To test this prediction, fermentations were performed with a diploid (rho+) yeast strain carrying one copy of the wild-type IlV2 gene and one copy of a truncated version missing the first 55 codons. The truncated protein Ilv2Δ55 expressed from the mutant gene is missing the mitochondrial presequence and should not be imported into mitochondria. To prove that the Ilv2Δ55 protein is indeed localized to the cytosol, a cell fractionation experiment was performed (Fig. 2B). In contrast to the diploid wild-type strain, where only a small fraction of mature Ilv2 could be detected in the supernatant (7% of total protein), more than half of the processed/truncated Ilv2 protein (ca. 60% of total protein) was now found in the supernatant fraction. Thus, the fractionation experiment demonstrates that the truncated Ilv2Δ55 protein is not imported into mitochondria. (Since the Ilv2 precursor is presumably cleaved by MPP between amino acids 55 and 56 in the mitochondrial matrix, the processed and the truncated forms cannot be distinguished based on their gel mobility.)
With the ILV2/ILV2-Δ55 strain, diacetyl levels comparable to a rho− strain were detected (Fig. 3). This experiment, therefore, supports the notion that cytosolic localization of Ilv2 is responsible for the high diacetyl levels produced by petite mutants.
FIG. 3.
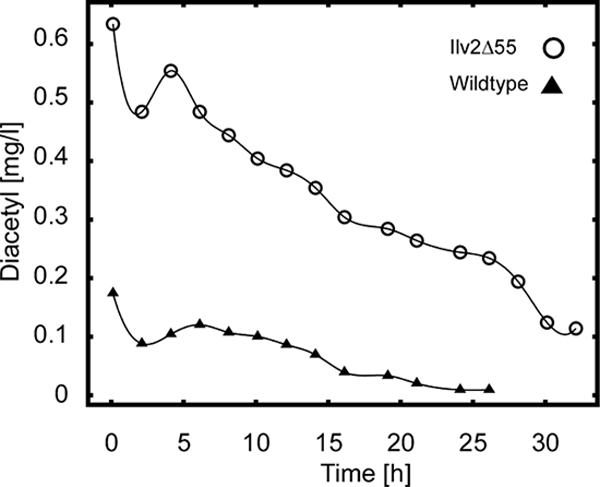
High diacetyl formation with N-terminal truncated Ilv2. Beer fermentations were performed in MEX medium at 30°C with RKY2139 (WT, ▴) and RKY2491 (Ilv2Δ55, ○) for the times indicated. The sugar profiles and ethanol formation were similar to Fig. 1 (not shown). Experiments were performed at least three times. The results of a representative experiment are shown.
Reduced diacetyl formation by ATP3-5 expression.
Since cytosolic accumulation of the Ilv2 precursor appears to be responsible for enhanced diacetyl production of petite yeasts, we were looking for a way to improve the efficiency of mitochondrial import of the precursor to reduce diacetyl production. The main reason for the reduced import efficiency of petite yeasts seems to be a lowered membrane potential (Δψ). In rho− yeasts, the membrane potential is maintained by exchange of matrix ADP3− for ATP4− by the ADP/ATP carrier. The ADP concentration in the mitochondrial matrix is raised by ATP hydrolysis by the F1-ATPase, which stimulates the exchange activity of the ADP/ATP carrier. There is evidence that enhanced ATP hydrolysis by a mutation in the γ-subunit of the F1-ATPase (ATP3-5) can boost Δψ. This notion is based on the finding that the dominant ATP3-5 mutation suppresses the petite-negative phenotype of a Δyme1 mutant (21). Presumably, due to a negative effect on mitochondrial import, the Δyme1 mutation is not compatible with the petite state.
To test the effect of ATP3-5 on diacetyl formation of petite strains, a rho− strain was transformed with a plasmid expressing ATP3-5 or as a control with a plasmid expressing wild-type ATP3. Fermentations were carried out in SD/CAS medium with selection for the plasmid and in MEX medium without plasmid selection. In SD/CAS medium a clear reduction in diacetyl levels down to 40% of the wild-type control was observed with the ATP3-5 transformant (Fig. 4 A). In MEX medium, the effect was less pronounced but was nevertheless clearly detectable (reduction to 70% of control levels) (Fig. 4B). These experiments show that introduction of the ATP3-5 allele into brewing yeast strains could be an interesting possibility to reduce diacetyl levels during beer fermentation.
FIG. 4.
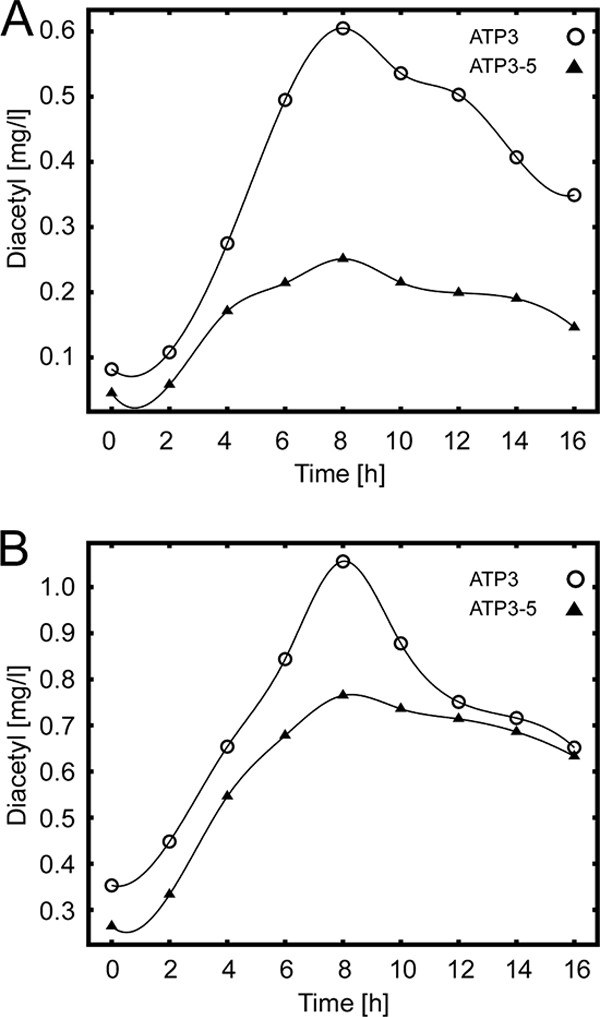
Reduction of diacetyl levels by expression of ATP3-5. Fermentations were carried out at 30°C with the rho− strain RKY2399 transformed with plasmid pRK1107 (containing the ATP3 gene, ○) or plasmid pRK1108 (containing the ATP3-5 gene, ▴). Diacetyl levels were determined at the times indicated. (A) SD/CAS medium; (B) MEX medium. Experiments were performed at least three times. The results of a representative experiment are shown.
DISCUSSION
We examined here the mechanisms that lead to increased diacetyl production by petite strains during beer fermentation. Our experiments suggest that diacetyl production is intimately linked to the mitochondrial import of acetohydroxyacid synthase, Ilv2. We could show that in petite mutants, which give rise to high diacetyl levels, the Ilv2 precursor protein accumulates in the cytosol. We propose that this cytosolic accumulation is the reason for the high diacetyl levels observed with petite yeasts. This notion is supported by the finding that expression of an Ilv2 variant with truncated signal sequence, which cannot be imported into mitochondria, leads to high diacetyl levels comparable to the levels observed with petite mutants. Also, it has been shown recently that cytosolic overexpression of the acetohydroxyacid reductoisomerase (Ilv5), the enzyme that functions immediately downstream of Ilv2 in the synthesis of branched-chain amino acids, reduces diacetyl levels (14).
Why should cytosolic localization of Ilv2 lead to elevated diacetyl levels in beer fermentations? In the case of cytosolic production, it may be easier for the diacetyl precursor α-acetolactate to reach the surrounding culture medium, since it only has to be transported across one membrane, the plasma membrane, while in the case of production in the mitochondrial matrix, it would have to cross three different membranes (mitochondrial inner and outer membrane and the plasma membrane). The transporter responsible for α-acetolactate membrane transport is not known. Maybe, there is no such transporter in the mitochondrial membranes. Another explanation could be that in the mitochondrial matrix the Ilv2 product α-acetolactate is quickly consumed by the next enzyme in the Ilv pathway, the acetohydroxyacid reductoisomerase Ilv5. In the cytosol, Ilv5 may not be present under normal conditions; thus, α-acetolactate could accumulate without being consumed by Ilv5. Yet another possibility is that Ilv2 activity in the mitochondrial matrix is negatively regulated by the regulatory subunit Ilv6. Again, Ilv6 may not be present in the cytosol; thus, Ilv2 activity could be higher than in the mitochondrial matrix under our growth conditions (rich medium containing valine, leucine, and isoleucine).
Ilv2 therefore appears to be another example of an enzyme with a dual localization that may be able to function in two different cellular compartments. Dual distribution between cytosol and mitochondria has been demonstrated for yeast fumarase (Fum1) (19). A fraction of fumarase returns to the cytosol after being processed in mitochondria. Rapid folding into an import-incompetent conformation seems to be crucial for retrograde movement (18). A similar competition between spontaneous folding to an import-incompetent conformation and mitochondrial import also leads to a dual localization of yeast adenylate kinase (Adk1/Aky2) (20). A dual localization may not always be easily discernible. In the case of mitochondrial aconitase (Aco1) only minute amounts were detected in the cytosol, and yet this activity appears to be physiologically relevant (15). Cytosolic accumulation of matrix-targeted proteins in petite mutants is not a general phenomenon but appears to be connected to specific features of the protein. In this context, it is interesting that a conserved subclass of preproteins known to reside in internal mitochondrial compartments (including Ilv2) was detected in the mitochondrial outer membrane fraction (22). This specific localization may also be linked to site-specific localization and transcription of messenger RNAs on the surfaces of mitochondria (7).
It appears that a lowered membrane potential across the inner mitochondrial membrane is the reason for the elevated diacetyl levels observed with petite yeast strains. We show here that boosting the membrane potential by expressing the ATP3-5 allele in yeast strains could be a way to reduce diacetyl formation by petite strains during beer fermentations.
Acknowledgments
We thank Thomas Brune for his assistance with some of the experiments.
Footnotes
Published ahead of print on 3 December 2010.
REFERENCES
- 1.Attardi, G., and G. Schatz. 1988. Biogenesis of mitochondria. Annu. Rev. Cell Biol. 4:289-333. [DOI] [PubMed] [Google Scholar]
- 2.Duggleby, R. G., and S. S. Pang. 2000. Acetohydroxyacid synthase. J. Biochem. Mol. Biol. 33:1-36. [Google Scholar]
- 3.Dupont, C. H., J. P. Mazat, and B. Guerin. 1985. The role of adenine nucleotide translocation in the energization of the inner membrane of mitochondria isolated from rho+ and rho0 strains of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 132:1116-1123. [DOI] [PubMed] [Google Scholar]
- 4.Ernandes, J. R., J. W. Williams, I. Russell, and G. G. Stewart. 1993. Respiratory deficiency in brewing yeast strain-effects on fermentation, flocculation, and beer flavor components. ASBC J. 51:16-20. [Google Scholar]
- 5.Falco, S. C., K. S. Dumas, and K. J. Livak. 1985. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 13:4011-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fix, G. J. 1993. Diacetyl: formation, reduction, and control. Brewing Techniques 1:20. http://www.brewingtechniques.com/library/backissues/issue1.2/fix.html. [Google Scholar]
- 7.Garcia, M., T. Delaveau, S. Goussard, and C. Jacq. 2010. Mitochondrial presequence and open reading frame mediate asymmetric localization of messenger RNA. EMBO Rep. 11:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 9.Giraud, M. F., and J. Velours. 1997. The absence of the mitochondrial ATP synthase δ subunit promotes a slow-growth phenotype of rho− yeast cells by a lack of assembly of the catalytic sector F1. Eur. J. Biochem. 245:813-818. [DOI] [PubMed] [Google Scholar]
- 10.Goldring, E. S., L. I. Grossman, D. Krupnick, D. R. Cryer, and J. Marmur. 1970. The petite mutation in yeast: loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J. Mol. Biol. 52:323-335. [DOI] [PubMed] [Google Scholar]
- 11.Longtine, M. S., et al. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 12.Martin, J., K. Mahlke, and N. Pfanner. 1991. Role of an energized inner membrane in mitochondrial protein import: Δψ drives the movement of presequences. J. Biol. Chem. 266:18051-18057. [PubMed] [Google Scholar]
- 13.Nelson, N., and G. Schatz. 1979. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc. Natl. Acad. Sci. U. S. A. 76:4365-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omura, F. 2008. Targeting of mitochondrial Saccharomyces cerevisiae Ilv5p to the cytoplasm and its effect on vicinal diketone formation in brewing. Appl. Microbiol. Biotechnol. 78:503-513. [DOI] [PubMed] [Google Scholar]
- 15.Regev-Rudzki, N., S. Karniely, N. N. Ben-Haim, and O. Pines. 2005. Yeast aconitase in two locations and two metabolic pathways: seeing small amounts is believing. Mol. Biol. Cell 16:4163-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid, G. A., and G. Schatz. 1982. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J. Biol. Chem. 257:13056-13061. [PubMed] [Google Scholar]
- 17.Ryan, E. D., and G. B. Kohlhaw. 1974. Subcellular localization of isoleucine-valine biosynthetic enzymes in yeast. J. Bacteriol. 120:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sass, E., S. Karniely, and O. Pines. 2003. Folding of fumarase during mitochondrial import determines its dual targeting in yeast. J. Biol. Chem. 278:45109-45116. [DOI] [PubMed] [Google Scholar]
- 19.Stein, I., Y. Peleg, R. S. Even, and O. Pines. 1994. The single translation product of the FUM1 gene (fumarase) is processed in mitochondria before being distributed between the cytoplasm and mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:4770-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strobel, G., A. Zollner, M. Angermayr, and W. Bandlow. 2002. Competition of spontaneous protein folding and mitochondrial import causes dual subcellular location of major adenylate kinase. Mol. Biol. Cell 13:1439-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber, E. R., R. S. Rooks, K. S. Shafer, J. W. Chase, and P. E. Thorsness. 1995. Mutations in the mitochondrial ATP synthase gamma subunit suppress a slow-growth phenotype of yme1 yeast lacking mitochondrial DNA. Genetics 140:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahedi, R. P., et al. 2006. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol. Biol. Cell 17:1436-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]


