Abstract
To enhance food safety and stability, the food industry tends to use natural antimicrobials such as plant-derived compounds as an attractive alternative to chemical preservatives. Nonetheless, caution must be exercised in light of the potential for bacterial adaptation to these molecules, a phenomenon previously observed with other antimicrobials. The aim of this study was to characterize the adaptation of Salmonella enterica serovar Typhimurium to sublethal concentrations of four terpenes extracted from aromatic plants: thymol, carvacrol, citral, and eugenol, or combinations thereof. Bacterial adaptation in these conditions was demonstrated by changes in membrane fatty acid composition showing (i) limitation of the cyclization of unsaturated fatty acids to cyclopropane fatty acids when cells entered the stationary phase and (ii) bacterial membrane saturation. Furthermore, we demonstrated an increased cell resistance to the bactericidal activity of two biocides (peracetic acid and didecyl dimethyl ammonium bromide). The implications of membrane modifications in terms of hindering the penetration of antimicrobials through the bacterial membrane are discussed.
The addition of preservatives is one of the most frequently used approaches for enhancing the microbiological safety of food products. However, recent reports of the potential formation of carcinogenic by-products from chemical preservatives, e.g., nitrosamines from nitrites and nitrates (45), or allergic reactions among sensitive consumers, to sulfites for example (38), show that there is an urgent need for the food industry to develop safer preservation systems. Consumers are increasingly demanding fresher, more natural foods that undergo minimal processing which ensures a high level of product safety. Plants are a natural source of alternative preservatives which can be used to improve the shelf life and safety of foods. Essential oils and volatile compounds extracted from plants have demonstrated antimicrobial properties against bacteria and fungi (7, 19, 23, 24, 26, 36). Most of these compounds are “generally recognized as safe” (GRAS) in the United States and have been registered by the European Commission for use as flavoring compounds in foodstuffs (3). Nonetheless, caution must be exercised in light of the potential for bacterial adaptation to these compounds. It is well established that bacterial cells grown in nonoptimal conditions can adapt and develop resistance to the bactericidal activity of disinfectants or physical treatments (17, 30, 33, 43, 49). This acquired tolerance can be due to various cellular phenomena: genetic changes occurring through plasmid acquisition or mutation (41), synthesis of stress proteins (29), and modifications of lipid membrane composition (32). Adaptability of bacterial fatty acid membrane composition, in particular, determines the survival ability of the cell by maintaining both membrane integrity and functionality, which helps to overcome nonoptimal conditions (11, 44).
Little is known about bacterial adaptation to plant-derived terpenes, notably in the case of Salmonella enterica serovar Typhimurium, a food-borne pathogen that causes salmonellosis, an important public health problem around the world (1, 31). The aim of the present study was to characterize the adaptation of Salmonella Typhimurium during growth in the presence of sublethal concentrations of four compounds extracted from aromatic plants: thymol, carvacrol, citral and eugenol, as well as combinations thereof. First of all, we investigated the impact of these terpenes on the bacterial membrane fatty acid composition. Cells in the exponential and stationary phases were examined in order to improve understanding of membrane adaptation. Second, we tested the bactericidal activity of two commonly used biocides (peracetic acid and didecyl dimethyl ammonium bromide) on cultures grown in the presence of terpenes.
MATERIALS AND METHODS
Strain and growth conditions.
S. enterica subsp. enterica serovar Typhimurium ATCC 13311 was used in the present study. Bacteria were grown in TSB (tryptose soy broth; bioMérieux, Marcy l'Etoile, France) in the presence or absence of thymol (Th), carvacrol (Ca), eugenol (Eu), and citral (Ci), obtained from Sigma-Aldrich Chemicals, St. Louis, MO, as well as combinations thereof. Terpenes were added to the medium after sterilization from ethanol stock solutions with a final ethanol concentration of 0.2% (vol/vol). Three concentrations (A, B, and C) of each antimicrobial were chosen (Table 1) based on previously determined MICs (35) and equivalences in terms of growth inhibition defined between compounds (27). All combinations of two compounds (Th-Ca, Th-Eu, Th-Ci, Ca-Eu, Ca-Ci, and Eu-Ci) or three compounds (Th-Ca-Eu, Th-Ca-Ci, Th-Eu-Ci, and Ca-Eu-Ci) were tested. In these combinations, the lowest concentration of each compound was used. A control culture grown without antimicrobials in the presence of 0.2% (vol/vol) ethanol was set up in parallel. Four levels of antimicrobials were then defined: level 0 is the control culture without antimicrobials, level 1 refers to a culture containing one compound at concentration A, level 2 refers to cultures with one compound at concentration B or two compounds at concentration A, and level 3 refers to cultures with one compound at concentration C or three compounds at concentration A.
TABLE 1.
Salmonella Typhimurium MIC and subinhibitory concentrations used to define antimicrobial levels of the four plant-derived terpenes
| Antimicrobial | MIC (mM)a | Concn (mM) |
||
|---|---|---|---|---|
| A | B | C | ||
| Thymol | 1 | 0.3 | 0.6 | 0.9 |
| Carvacrol | 1 | 0.3 | 0.6 | 0.9 |
| Citral | 3.4 | 1 | 2 | 3 |
| Eugenol | 3.2 | 0.8 | 1.2 | 2.4 |
Data are from Nazer et al. (35).
TSB with or without antimicrobials was inoculated at 1% (vol/vol; i.e., approximately 106 CFU ml−1) using a standardized inoculum obtained after three subcultures in TSB. To evaluate the growth rate of Salmonella in the presence of each antimicrobial at level 0, 1, 2 or 3, the cultures were grown at 37°C in an automated Bioscreen C analyzer (Labsystems, Les Ulis, France). Each well contained 200 μl of culture, and the OD600 was measured every 30 min for 72 h. For fatty acid analysis and disinfectant testing, cultures were assessed in 50 ml of TSB with or without antimicrobials and incubated statically at 37°C.
Growth rate determination.
Maximum specific growth rates were estimated from optical density (OD) growth kinetics by fitting the modified Gompertz model (19):
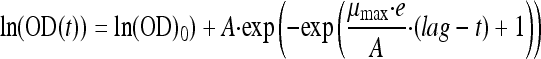 |
(1) |
where t is the time, OD0 is the OD value at time t = 0, A is equal to ln(ODmax) − ln(OD0), ODmax is the maximum OD, lag is the duration of the lag phase, μmax is the maximum growth rate, and e is the exponential of 1. OD0, ODmax, lag, and μmax are model parameters. The logarithmic transformation of OD was performed to stabilize the variance.
Growth kinetics of four independent cultures was assessed for each range of antimicrobial concentrations.
Fatty acids analysis.
Cells from cultures grown with antimicrobials at levels 0, 1, 2, or 3 were harvested in the exponential or stationary phase according to growth curves (data not shown) and washed twice with 150 mM NaCl before testing. Extraction and methylation of fatty acids were carried out according to the method of Méchin et al. (32). Briefly, whole bacterial cells were saponified for 30 min at 100°C with a strong base (NaOH at 3.75 mol/liter in 50% [vol/vol] methanol solution). Fatty acids were then methylated for 10 min at 80°C (HCl at 3.25 mol/liter in 45% [vol/vol] methanol solution), and fatty acid methyl esters were extracted with a mixture of diethyl ether and cyclohexane (1:1 [vol/vol]). The organic phase was then washed with a dilute base (NaOH, 0.3 mol/liter). Analytical gas chromatography of fatty acid methyl esters was carried out on a 6890HP system (Agilent Technologies, Santa Clara, CA) equipped with a DB5 capillary column (Agilent Technologies) and a flame ionization detector. Column temperature was set at 150°C for 4 min and then increased to 250°C at the rate of 4°C/min. The data were acquired by using an HPCORE ChemStation system (Agilent Technologies) and expressed as a percentage of the area of the nine biggest peaks. Fatty acids were identified using fatty acid methyl ester standards. The results are the average of six profiles (two injections of three extractions from independent cultures) for each condition.
Disinfectant testing.
An oxidizing agent (peracetic acid [PA]; Sigma, St. Louis, MO) and a quaternary ammonium compound (didecyl dimethyl ammonium bromide [DDAB]; Fluka, Buchs, Switzerland) were selected as disinfectants because they have different mechanisms of action (28). Cells from cultures grown with antimicrobials at level 3 were harvested in the stationary phase according to growth curves (data not shown) and washed twice with 150 mM NaCl before testing. The resistance of washed cells to the bactericidal activity of both disinfectants was evaluated according to the European standard EN 1040 (2). Briefly, cells (1 × 108 to 3 × 108 cells/ml) were exposed to 3 ppm of PA or 10 mg of DDAB/liter for 5 min ± 5 s at 20°C. The control was processed with deionized water instead of disinfectant. The action of the biocide was stopped by transfer (1:9) to a quenching solution (3 g of l-α-phosphatidylcholine, 30 g of Tween 80, 5 g of sodium thiosulfate, 1 g of l-histidine, and 30 g of saponin/liter). After 5 min at 20°C, serial dilutions were made in 150 mM NaCl, and survivors were enumerated by the 6×6 drop-count method (9) on tryptose soy agar (bioMérieux). The logarithm reduction is the difference between the log10 survivors after the test with deionized water and the log10 survivors after the test with disinfectant. The results are the mean of at least three experiments performed on independently grown cultures.
Statistics.
Analyses of variance (ANOVA) were performed by using Statgraphics software (Manugistic, Rockville, MD). P values from F-tests were used to test the statistical significance of each factor. When P values are lower than 0.05, the factor has a statistically significant effect at the 95% confidence level.
RESULTS
Influence of sublethal concentrations of terpenes on Salmonella Typhimurium growth kinetics.
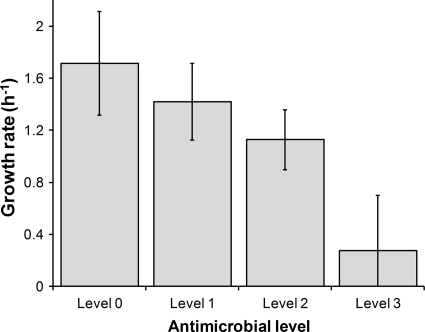
Growth kinetics was determined for the 23 cultures in TSB with antimicrobials at levels 0 to 3. The cultures consisted of one control culture, 12 cultures with one compound (three concentrations for four different terpenes), and 10 combinations (six of two compounds and four of three compounds). In each case, the growth rate was determined from OD curves to obtain a quantitative evaluation of the antimicrobial inhibitory effect (Fig. 1). ANOVA performed on growth rate data revealed significant differences between antimicrobial levels (P < 0.05). Growth rates were highly affected at levels 2 and 3 for all antimicrobials.
FIG. 1.
Growth rate of Salmonella Typhimurium cultivated in the presence of different levels of antimicrobial compounds. Level 0 is the control culture without antimicrobials, level 1 refers to a culture containing one compound at concentration A, level 2 refers to cultures with one compound at concentration B or two compounds at concentration A, and level 3 refers to cultures with one compound at concentration C or three compounds at concentration A (see Table 1 for the numeric values of the concentrations).
Influence of sublethal concentrations of terpenes on Salmonella Typhimurium fatty acid composition.
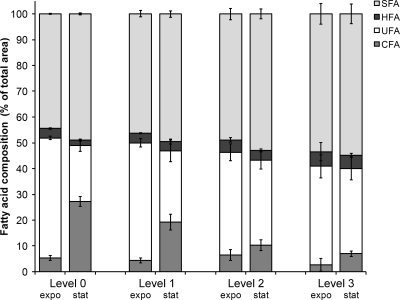
Membrane fatty acid composition of Salmonella Typhimurium cultivated in TSB without antimicrobials was first determined as control. It contained nine main fatty acids (FA) grouped in four classes: saturated fatty acids (SFA), unsaturated fatty acids (UFA), hydroxylated fatty acids (HFA), and cyclopropane fatty acids (CFA). They refer respectively to the sums of (i) lauric acid (C12), myristic acid (C14), palmitic acid (C16), and stearic acid (C18) proportions, (ii) palmitoleic acid (cis9 C16:1) and cis-vaccenic acid (cis11 C18:1) proportions, (iii) 3-hydroxy-myristic acid (3-OH C14) proportion, and (iv) cis-9,10-methylene-hexadecanoic acid (ΔC17) and lactobacillic acid (ΔC19) proportions. The main fatty acids when harvested in the exponential phase were palmitic, palmitoleic, and cis-vaccenic acids. Significant differences were obtained between exponential- and stationary-phase fatty acid compositions (P < 0.05). CFA and C16:0 increased, while UFA decreased when cells entered the stationary phase (Fig. 2, level 0).
FIG. 2.
Cyclopropane fatty acid (CFA), unsaturated fatty acid (UFA), hydroxylated fatty acid (HFA), and saturated fatty acid (SFA) proportions of Salmonella Typhimurium grown in the absence or presence of plant-derived terpenes. Level 0 is the control culture without antimicrobials, level 1 refers to a culture containing one compound at concentration A, level 2 refers to cultures with one compound at concentration B or two compounds at concentration A, and level 3 refers to cultures with one compound at concentration C or three compounds at concentration A (see Table 1 for the numeric values of the concentrations).
The FA composition of Salmonella Typhimurium was then analyzed based on the antimicrobial levels (0, 1, 2, or 3), the growth phase (exponential or stationary), and the type of terpene (thymol, carvacrol, citral, and eugenol) (Fig. 2). Membrane SFA proportion was highly dependent on all of these factors (P < 0.05). When the antimicrobial level increased, SFA increased in both the exponential and the stationary phases. No significant differences were found between the presence of a single compound or combinations of compounds at the same overall level. For each antimicrobial level, SFA increased from the exponential phase to the stationary phase, but the increase was smaller in level 3 cultures because of their already high proportions in the exponential phase. Among the antimicrobial compounds used singly, eugenol led to the highest proportions of SFA (mean of 51.7%), followed by thymol (mean of 50.3%), carvacrol (mean of 50.2%), and citral (mean of 48.6%).
Antimicrobial level and growth phase were significant factors in CFA analyses (P < 0.05) (Fig. 2). In the stationary phase, CFA proportions were highly dependent on the level of antimicrobials, while that was not the case in the exponential phase. In level 0 and 1 cultures, CFA increased substantially when cells entered the stationary phase, contrary to cultures with higher levels of antimicrobials. The type of antimicrobial compound was not a significant factor for CFA proportions (means between 12.5 and 13.5%). Antimicrobial level and growth phase were significant factors in UFA analyses (P < 0.05) (Fig. 2). UFA proportions were highly dependent on the level of antimicrobials. In level 0 and 1 cultures, UFA decreased significantly when cells entered the stationary phase; a smaller decrease was recorded in level 2 and 3 cultures. The decrease in UFA offset the CFA increase, which occurred simultaneously. The type of antimicrobial compound was not a significant factor for UFA proportions (means between 31.3 and 33.6%). Since the HFA proportion relates solely to 3-hydroxy-myristic acid, which represents <5% of the total FA composition, the slight modifications observed in HFA proportion were not taken into account (Fig. 2).
Influence of sublethal concentrations of terpenes on Salmonella Typhimurium tolerance to bactericidal activity of disinfectants.
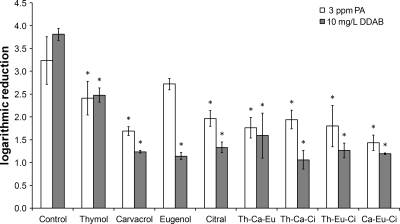
Tolerance to bactericidal activities of disinfectants (PA and DDAB) was determined for Salmonella Typhimurium previously grown without antimicrobials (control) or with plant-derived terpenes at level 3. Decimal logarithmic reductions obtained after 5 min of application of 3 ppm of PA or 10 mg of DDAB/liter to the nine cultures are presented in Fig. 3. Tolerance to both disinfectants of cells cultivated with sublethal concentrations of terpenes increased significantly (P < 0.05) (averages of +1.3- and +2.4-log reductions compared to the control for PA and DDAB, respectively).
FIG. 3.
Bactericidal activities of 3 ppm of peracetic acid and 10 mg of didecyl dimethyl ammonium bromide (DDAB)/liter against Salmonella Typhimurium previously grown without antimicrobials (control) or with antimicrobial compounds at level 3 (one compound alone at concentration C or combinations of three compounds at concentration A). Th refers to thymol, Ca to carvacrol, Ci to citral, and Eu to eugenol. An asterisk indicates a significant difference from the control with respect to log reduction.
DISCUSSION
Terpenes extracted from plant essential oils are recognized as being among the most efficient natural antimicrobials against numerous pathogens (5, 19, 47). Thymol, carvacrol, eugenol, and citral are examples of these plant-derived monoterpenes. The first three are phenolic compounds. We sought to characterize the adaptation of Salmonella to these terpenes in terms of modifications of membrane fatty acid composition and possible acquired resistance to biocides commonly used in the food industry.
Our results showed that growth in the presence of plant-derived terpenes induced significant alteration of the fatty acid composition of S. enterica serovar Typhimurium ATCC 13311: CFA are significantly undersynthesized when cells enter the stationary phase, whereas SFA are overproduced. Similar results were obtained with an undomesticated strain of Salmonella Typhimurium recently isolated from a cutting board (data not shown). Many studies report the implication of phospholipid modifications in the adaptation of bacteria to several sublethal stresses (11, 42), including toxic chemicals (21, 50). This appears to be a primary response of bacteria in order to maintain both membrane integrity and functionality. Different modulation mechanisms have been described depending on the bacterial strains, the bacterial state (growing or resting cells) and the type of compound (11). Polar solvents such as acetone or ethanol caused an enrichment in UFA, whereas incubation with apolar solvents such as benzene, chloroform, or toluene resulted in an increase of SFA synthesis in Escherichia coli (22) and Pseudomonas putida (20) membranes. Similarly, concentrations of phenol and 4-chloro-phenol were found to increase the degree of saturation of E. coli membrane lipids (25). Some Pseudomonas strains react to high concentrations of phenolic compounds by converting cis-UFA into the corresponding trans-isomer (13). Other findings include the conversion of UFA to CFA in E. coli during acid habituation (6) and the oversynthesis of HFA in Pseudomonas aeruginosa after adaptation to quaternary ammonium compounds (18, 32). In the present study, the principal FA modifications observed in cells when grown with high levels of plant-derived terpenes consisted of a significant decrease in CFA synthesis in the stationary phase. CFA are known to be preferentially synthesized when cells enter the stationary phase (10, 15). They are produced by the addition of a methyl group from S-adenosyl methionine across a UFA cis-double bond (6). A similar decrease in CFA synthesis has been reported in the stationary phase for Lactobacillus plantarum grown in the presence of ferulic or caffeic acids, which are both phenolic compounds such as thymol, carvacrol, and eugenol (39). One study also reported a larger increase of UFA content in Salmonella Typhimurium harvested in the stationary phase after growth in the presence of four essential oil compounds (14). Although CFA were not quantified in that study, the observed increase of UFA is certainly related to the concomitant decrease in cyclopropane synthesis. The gene of the cyclopropane fatty acyl phospholipid synthase cfa was shown to be downregulated but not inhibited in the presence of cranberry concentrate, which contains phenolic compounds (51). Similarly, in our case, the cyclization pathway was not completely inhibited because ΔC17 and ΔC19 were still detected in level 3 cultures, albeit in small amounts.
Membrane FA composition of Salmonella grown in the presence of terpenes can be explained by considering the properties of these compounds, which are hydrophobic with octanol-water partition coefficient (log Po/w) values of about 3 (3.64, 3.30, 2.49, and 3.45 for thymol, carvacrol, eugenol, and citral, respectively). Weber and deBont (50) reported that compounds with a log Po/w value higher than 3 partition deeply in the membrane, occupying more than the normal amount of space between two fatty acid chains. The plant-derived terpenes used in the present study were thus expected to partition within the bacterial membrane lipids, damaging their structural and functional properties (7). Consequently, the higher the antimicrobial concentration, the greater the amount of compound that should partition in the membrane, and the greater the physical disturbance to the stability of the phospholipid bilayer. We can therefore hypothesize that terpenes, which accumulate between acyl chains of fatty acids, limit the accessibility of S-adenosylmethionine to the UFA cis-double bond. As a result, cyclization should rarely occur even if the pathway is still operational. To compensate for the resulting physical membrane modifications, Salmonella reacted by producing a larger amount of saturated, high-melting-point fatty acids during growth.
The tolerance of cells to the bactericidal activity of two biocides was evaluated after adaptation of Salmonella to sublethal concentrations of plant-derived terpenes. It has long been known that bacteria can develop tolerance when exposed to harsh environmental conditions (29, 33), including sublethal concentrations of some chemicals such as quaternary ammonium compounds, phenolics, salicylanilides, or diamidines (32, 40, 48). Cross-adaptation to chemically related biocides often occurs and cross-adaptation to unrelated biocides has occasionally been found. For example, the acid tolerance response is a well-known phenomenon in which cells cultivated in the presence of acid develop adaptation to further acid shock (52). Cells grown in the presence of acid can also develop acquired resistance to disinfectant testing (33). Our results showed that Salmonella Typhimurium cultivated in the presence of plant-derived terpenes was more tolerant to bactericidal activities of both PA and DDAB. These compounds have different modes of action. PA is an oxidizing agent which denatures proteins and enzymes (28). DDAB is a quaternary ammonium compound that alters cytoplasmic membrane integrity by electrostatic interaction with phospholipids (12). In this context, we hypothesized that the tolerance of cells grown in the presence of terpenes would not be induced by specific mechanisms. First of all, a slow growth rate could partly explain the higher tolerance to these biocides: slow growth is known to induce further tolerance to acid stress (37) or mild heat (4) and is involved in the multilayered mechanism of biofilm resistance (46). In the present study, bacterial growth rates at level 3 were significantly lower than for the control. The accumulation of terpenes and the high SFA contents in the membrane could also help to increase resistance by hindering the penetration of biocides (regardless of their mode of action) through the membrane. Furthermore, it should be noted that resistance increased, whereas CFA oversynthesis—considered an important resistance factor in acid stresses (8)—did not occur.
We have shown that, like numerous other antimicrobials, plant-derived terpenes can induce bacterial adaptation and acquired tolerance to inactivation processes. Caution must therefore be exercised when sublethal concentrations of natural antimicrobials are used, especially in applying the hurdle concept, which holds that many inhibitory factors can be highly efficient at sublethal levels when used in combination (16, 34).
Acknowledgments
We thank Virginie Thiry, Stéphane Durieux, and Elodie Aglioni for their technical assistance.
Footnotes
Published ahead of print on 3 December 2010.
REFERENCES
- 1.Anonymous. 2004. Morbidité et mortalité dues aux maladies infectieuses d'origine alimentaire en France. Institut de Veille Sanitaire, Agence Française de Sécurité Sanitaire des Aliments, Paris, France.
- 2.Anonymous. 1997. Chemical disinfectants and antiseptics: basic bactericidal activity. Test method and requirements (phase 1). European Standard NF EN 1040. AFNOR, Paris, France.
- 3.Anonymous. 1999. Commission decision of 23 February 1999 adopting a register of flavoring substances used in or on foodstuffs drawn up in application of regulation (EC) No 2232/96 of the European Parliament and of the Council of 28 October 1996 (1999/217/EC). Off. J. Eur. Commun. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:084:0001:0137:EN:PDF.
- 4.Berney, M., H. U. Weilenmann, J. Ihssen, C. Bassin, and T. Egli. 2006. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl. Environ. Microbiol. 72:2586-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuchat, L. R., and D. A. Golden. 1989. Antimicrobials occurring naturally in foods. Food Technol. 43:134-142. [Google Scholar]
- 6.Brown, J. L., T. Ross, T. A. McMeekin, and P. D. Nichols. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163-173. [DOI] [PubMed] [Google Scholar]
- 7.Burt, S. 2004. Essential oils: their antibacterial properties and potential applications in foods: a review. Int. J. Food Microbiol. 94:223-253. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. Y., and J. E. Cronan. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. Y., G. W. Nace, and P. L. Irwin. 2003. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 55:475-479. [DOI] [PubMed] [Google Scholar]
- 10.Cronan, J. E. 1968. Phospholipid alterations during growth of Escherichia coli. J. Bacteriol. 95:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denich, T. J., L. A. Beaudette, H. Lee, and J. T. Trevors. 2003. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 52:149-182. [DOI] [PubMed] [Google Scholar]
- 12.Denyer, S. P., and G. Stewart. 1998. Mechanisms of action of disinfectants. Int. Biodeter. Biodegrad. 41:261-268. [Google Scholar]
- 13.Dieffenbach, R., H.-J. Heipieper, and H. Keweloh. 1992. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl. Microbiol. Biotechnol. 38:382-387. [Google Scholar]
- 14.Di Pasqua, R., N. Hoskins, G. Betts, and G. Mauriello. 2006. Changes in membrane fatty acids composition of microbial cells by addition of thymol, carcavrol, limonene, cinnamaldhehyde, and eugenol in growing media. J. Agric. Food Chem. 54:2745-2749. [DOI] [PubMed] [Google Scholar]
- 15.Dubois-Brissonnet, F., C. Malgrange, L. Guérin-Méchin, B. Heyd, and J. Y. Leveau. 2001. Changes in fatty acid composition of Pseudomonas aeruginosa ATCC 15442 induced by growth conditions: consequences on resistance to quaternary ammonium compounds. Microbios 106:97-110. [PubMed] [Google Scholar]
- 16.Gould, G. W. 1996. Method for preservation and extension of shelf life. Int. J. Food Microbiol. 33:51-64. [DOI] [PubMed] [Google Scholar]
- 17.Greenacre, E. J., S. Lucchini, J. C. D. Hinton, and T. F. Brocklehurst. 2006. The lactic acid-induced acid tolerance response in Salmonella enterica serovar Typhimurium induces sensitivity to hydrogen peroxide. Appl. Environ. Microbiol. 72:5623-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guérin-Méchin, L., F. Dubois-Brissonnet, B. Heyd, and J. Y. Leveau. 2000. Quaternary ammonium compounds stresses induce specific variations in fatty acid composition of Pseudomonas aeruginosa. Int. J. Food Microbiol. 55:157-159. [DOI] [PubMed] [Google Scholar]
- 19.Guillier, L., A. Nazer, and F. Dubois-Brissonnet. 2007. Growth response of Salmonella Typhimurium in presence of natural and synthetic antimicrobials: estimation of MICs from three different models. J. Food Prot. 70:2243-2250. [DOI] [PubMed] [Google Scholar]
- 20.Heipieper, H. J., and J. A. M. Debont. 1994. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl. Environ. Microbiol. 60:4440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. De Bont. 1994. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12:409-415. [Google Scholar]
- 22.Ingram, L. O. 1977. Changes in lipid composition of Escherichia coli resulting from growth with organic solvents and with food additives. Appl. Environ. Microbiol. 33:1233-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juneja, V. K., and M. Friedman. 2007. Carvacrol, cinnamaldhehyde, oregano oil, and thymol inhibit Clostridium perfringens spore germination and outgrowth in ground turkey during chilling. J. Food Prot. 70:218-222. [DOI] [PubMed] [Google Scholar]
- 24.Karapinar, M., and S. E. Aktug. 1987. Inhibition of food-borne pathogens by thymol, eugenol, menthol, and anethole. Int. J. Food Microbiol. 4:161-166. [Google Scholar]
- 25.Keweloh, H., R. Diefenbach, and H. J. Rehm. 1991. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch. Microbiol. 157:49-53. [DOI] [PubMed] [Google Scholar]
- 26.Kim, J. M., M. R. Marshall, J. A. Cornell, J. F. Preston, and C. I. Wei. 1995. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella Typhimurium in culture medium and on fish cubes. J. Food Sci. 60:1364-1374. [Google Scholar]
- 27.Kobilinsky, A., A. Nazer, and F. Dubois-Brissonnet. 2007. Modeling the inhibition of Salmonella Typhimurium growth by combination of food antimicrobials. Int. J. Food Prot. 115:95-109. [DOI] [PubMed] [Google Scholar]
- 28.MacDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey, B. M., and C. Derrick. 1990. Heat shock protein synthesis and thermotolerance in Salmonella typhimurium. J. Appl. Bacteriol. 69:373-383. [DOI] [PubMed] [Google Scholar]
- 30.Mattick, K. L., J. F. J. D. Legan, H. M. Lappin-Scott, and T. J. Humphrey. 2000. Habituation of Salmonella spp. at reduced water activity and its effect on heat tolerance. Appl. Environ. Microbiol. 66:4921-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mead, P. S., et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:609-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Méchin, L., F. Dubois-Brissonnet, B. Heyd, and J. Y. Leveau. 1999. Adaptation of Pseudomonas aeruginosa ATCC 15442 to didecyldimethylammonium bromide induces changes in membrane fatty acid composition and in resistance of cells. J. Appl. Microbiol. 86:859-866. [DOI] [PubMed] [Google Scholar]
- 33.Naitali, M., F. Dubois-Brissonnet, G. Cuvelier, and M. N. Bellon-Fontaine. 2009. Effects of pH and oil-in-water emulsions on growth and physico-chemical cell surface properties of Listeria monocytogenes: impact on tolerance to bactericidal activity of disinfectants. Int. J. Food Microbiol. 130:101-107. [DOI] [PubMed] [Google Scholar]
- 34.Najjar, M. B., D. Kashtanov, and M. L. Chikindas. 2007. e-Poly-l-lysine and nisin A act synergistically against Gram-positive food-borne pathogens Bacillus cereus and Listeria monocytogenes. Lett. Appl. Microbiol. 45:13-18. [DOI] [PubMed] [Google Scholar]
- 35.Nazer, A., A. Kobilinsky, J. L. Tholozan, and F. Dubois-Brissonnet. 2005. Combinations of food antimicrobials at low levels to inhibit the growth of Salmonella sv. Typhimurium: a synergistic effect? Food Microbiol. 22:391-398. [Google Scholar]
- 36.Nychas, G.-J. E. 1995. Natural antimicrobials from plants, p. 58-89. In G. Gould (ed.), New methods of food preservation. Aspen Publishers, New York, NY.
- 37.Patchett, R. A., N. Watson, P. S. Fernandez, and R. G. Kroll. 1996. The effect of temperature and growth rate on the susceptibility of Listeria monocytogenes to environmental stress conditions. Lett. Appl. Microbiol. 22:121-124. [DOI] [PubMed] [Google Scholar]
- 38.Roller, S. 2003. Natural antimicrobials for minimal processing of foods. Woodhead Publishing, Ltd., Cambridge, United Kingdom.
- 39.Rozes, N., and C. Peres. 1998. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 49:108-111. [Google Scholar]
- 40.Russell, A. D. 2004. Bacterial adaptation and resistance to antiseptics, disinfectants, and preservatives is not a new phenomenon. J. Hosp. Infect. 57:97-104. [DOI] [PubMed] [Google Scholar]
- 41.Russell, A. D. 1999. Bacterial resistance to disinfectants: present knowledge and future problems. J. Hosp. Infect. 43:S57-S68. [DOI] [PubMed] [Google Scholar]
- 42.Russell, N. J., et al. 1995. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28:255-261. [DOI] [PubMed] [Google Scholar]
- 43.Ryu, J. H., and L. R. Beuchat. 1999. Changes in heat tolerance of Escherichia coli O157:H7 after exposure to acidic environments. Food Microbiol. 16:317-324. [Google Scholar]
- 44.Sajbidor, J. 1997. Effect of some environmental factors on the content and composition of microbial membrane lipids. Crit. Rev. Biotechnol. 17:87-103. [DOI] [PubMed] [Google Scholar]
- 45.Scotter, M. J., and L. Castle. 2004. Chemical interactions between additives in foodstuffs: a review. Food Addit. Contam. 21:93-124. [DOI] [PubMed] [Google Scholar]
- 46.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 47.Tiwari, B. K., et al. 2009. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 57:5987-6000. [DOI] [PubMed] [Google Scholar]
- 48.To, M. S., S. Favrin, N. Romanova, and M. W. Griffiths. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 68:5258-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Schaik, W., C. G. M. Gahan, and C. Hill. 1999. Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J. Food Prot. 62:536-539. [DOI] [PubMed] [Google Scholar]
- 50.Weber, F. J., and J. A. M. deBont. 1996. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta Rev. Biomembr. 1286:225-245. [DOI] [PubMed] [Google Scholar]
- 51.Wu, V. C. H., X. J. Qiu, B. G. de los Reyes, C. S. Lin, and Y. P. Pan. 2009. Application of cranberry concentrate (Vaccinium macrocarpon) to control Escherichia coli O157:H7 in ground beef and its antimicrobial mechanism related to the downregulated slp, hdeA, and cfa. Food Microbiol. 26:32-38. [DOI] [PubMed] [Google Scholar]
- 52.Yuk, H. G., and D. L. Marshall. 2004. Adaptation of Escherichia coli O157:H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid. Appl. Environ. Microbiol. 70:3500-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]