Abstract
Understanding factors that influence persistence of influenza virus in an environment without host animals is critical to appropriate decision-making for issues such as quarantine downtimes, setback distances, and eradication programs in livestock production systems. This systematic review identifies literature describing persistence of influenza virus in environmental samples, i.e., air, water, soil, feces, and fomites. An electronic search of PubMed, CAB, AGRICOLA, Biosis, and Compendex was performed, and citation relevance was determined according to the aim of the review. Quality assessment of relevant studies was performed using criteria from experts in virology, disease ecology, and environmental science. A total of 9,760 abstracts were evaluated, and 40 appeared to report the persistence of influenza virus in environmental samples. Evaluation of full texts revealed that 19 of the 40 studies were suitable for review, as they described virus concentration measured at multiple sampling times, with viruses detectable at least twice. Seven studies reported persistence in air (six published before 1970), seven in water (five published after 1990), two in feces, and three on surfaces. All three fomite and five air studies addressed human influenza virus, and all water and feces studies pertained to avian influenza virus. Outcome measurements were transformed to half-lives, and resultant multivariate mixed linear regression models identified influenza virus surviving longer in water than in air. Temperature was a significant predictor of persistence over all matrices. Salinity and pH were significant predictors of persistence in water conditions. An assessment of the methodological quality review of the included studies revealed significant gaps in reporting critical aspects of study design.
The aim of this review is to summarize findings from experiments that report persistence of influenza virus in the environment. The motivation was to provide better science-based information to inform policies that will impact livestock producers and surrounding communities. The period of time that influenza viruses persist in environmental matrices (e.g., air, soil, feces, water, and fomites) and factors that affect that period should inform many decisions in regulatory livestock disease control. Avian and equine influenza are World Organization for Animal Health (OIE) notifiable diseases, and OIE strongly advises all its members to notify the disease linked with the now-called “2009 pandemic H1N1” virus to the OIE when detected in animals. For avian influenza, control measures include quarantine and depopulation, while for the pandemic H1N1 2009 virus, quarantine may be imposed by the member nation. During outbreaks of highly pathogenic avian influenza (HPAI) in the United States, infected premises are depopulated, and a period of quarantine is imposed before new animals can be introduced (91). Further, legislative initiatives have requested consideration of the distance that viable pathogens associated with animal health, including avian and swine influenza viruses, can travel between infected facilities when establishing guidelines for granting permits for new livestock production facilities, otherwise referred to as setback distances (33). The period of time influenza virus can be reasonably expected to persist in environmental matrices without amplifying hosts should form the basis for these depopulation times and setback distances.
Given the growing importance of influenza viruses and the need for science-informed public policy, the purpose of this review is to summarize the literature reporting the persistence of influenza virus in environmental matrices to better inform these regulatory decisions. Further, as an important aspect of science-informed policy is the accurate discussion of scientific uncertainty, this review evaluates the presence or absence of important features of study design that influence internal and external validity of the research studies included in the review.
Therefore, the objective of this study is to use the systematic review methodology to answer the question, “What is the evidence for an association between humidity, temperature, UV intensity, and medium composition and the persistence of influenza virus in air, soil, feces, water, and fomites?” As part of that review process, the review also aims to explicitly describe the presence or absence of study design features associated with internal and external validity in the studies incorporated into the review.
MATERIALS AND METHODS
The approach to reporting the systematic review follows the guidelines of the PRISMA statement (50), with modifications where needed, as the PRISMA statement refers mainly to intervention studies rather than bench science applications.
Definitions. (i) Study.
A study refers to a manuscript reporting primary research.
(ii) Experiment.
An experiment is a research trial described within a single study.
(iii) Observation.
An observation is a single persistence measurement derived from a complete set of persistence data over time within an experiment. This individual persistence data per time interval (and parameter) or summary outcome for an experiment was the extracted information for this meta-analysis. The raw data were in various formats, including virus concentration per time interval, log10-transformed virus concentration per time interval, the slope of the persistence line, percent recovery from starting concentration after equilibration, and actual half-life calculations.
(iv) Systematic review methodology.
The systematic review methodology is a formalized approach to conducting a critical review of the literature and has been applied to the policymaking process in clinical sciences, social sciences, food safety regulation, and environmental sciences (9, 31, 66, 75, 84, 102). The methodology has several key principles designed to limit the incorporation of biased scientific results or the selective use of particular scientific results into review conclusions: transparency, comprehensiveness, and quality assessment. Transparency refers to the reporting of all aspects of the review to enable the reader to assess the validity of the review process and potential biases. Comprehensiveness refers to a broad approach to identifying the literature to be considered for the review. Quality assessment refers to the evaluation of the primary research for the presence of study design features necessary for valid primary research. Studies failing to report key features are not included in the summation of findings. A consequence of this approach is that well-executed but poorly reported studies cannot be differentiated from poorly executed but accurately reported studies. Systematic reviews have the following four formalized steps: (i) literature search, (ii) relevance screening, (iii) quality assessment and data extraction, and (iv) data analysis and summation. In clinical sciences, some systematic reviews are registered and have published protocols; this review did use a working protocol, but it was not registered, as there is no mechanism for registering reviews outside the clinical sciences.
Literature search.
Electronic literature searches in PubMed (1948 to present), CAB (1910 to present), AGRICOLA (1970 to present), Biosis (1926 to present), and Compendex (1884 to present) were conducted. Terms that described influenza virus and persistence in environmental matrices were identified in the National Agricultural Thesaurus and the PubMed MESH database after consulting review papers (2, 8, 77, 99). The searches were designed to capture the population of interest, i.e., influenza virus, the outcome of interest (i.e., persistence), and the environmental matrices. Boolean terms were used to combine terms within a string (OR) and between strings (AND) (see Supplement S1 to S4 in the supplemental material).
The search used in PubMed for the water matrix was as follows: [influenza OR influenzavirus OR Orthomyxoviridae OR influenzavirus C OR influenza C OR influenza A OR influenzavirus A OR H1N1 OR H2N2 OR H3N2 OR H3N8 OR H2N3 OR H5N2 OR H7N7 OR H9N2 OR (influenza in birds) OR influenza B OR influenzavirus B OR (hemagglutinin glycoproteins) OR human influenza] AND [(virus or viral or microbial or microbe) AND (pathogenicity OR survivability OR survival OR stability OR infectivity OR infection OR infective OR “infective dose” OR infect OR viability OR “environmental stability” OR inactivation OR transmission)] AND [water OR wetland* OR waterway OR watershed OR pH OR manure OR feces OR faeces or fecal shedding OR faecal shedding OR wastewater OR effluent OR irrigation OR drying OR desiccation OR desiccating OR lyophilization OR lyophilized OR water microbiology]. Retrieved citations were stored in reference management software (Reference Manager version 11, Berkeley, CA). Duplicate citations were removed by electronic and hand scanning of the electronic database. When multiple instances of the same citation were identified, the most complete citation was retained. After deduplication, citations were uploaded to a Web-based systematic review software for coordination of the review (SRS version 4, Trial Stat, Ottawa, Ontario, Canada).
Hand searching of the reference lists of relevant papers and previously published narrative reviews was conducted as the review progressed, i.e., after a paper or review was identified as relevant to the review. Two reviewers evaluated the reference list and identified potentially relevant citations. If the electronic search had not captured the citation, it was added to the Web-based systematic review software.
Relevance screening.
The purpose of relevance screening in the systematic review methodology is to rapidly remove citations not relevant to the review, as the literature search process should be highly sensitive, with low specificity. Eligible studies were primary research papers that reported persistence of influenza virus in the environmental matrices.
Two levels of relevance screening were used. For level 1 relevance screening, each citation was reviewed independently by a primary and secondary reviewer. The primary reviewers were a B.V.Sc. with a doctoral degree in epidemiology, a B.V.Sc. with a master's degree in epidemiology, a scientist with a Bachelor of Science degree, and a D.V.M. completing a master's training in epidemiology. The secondary reviewers were doctors of veterinary medicine, three with M.S. degrees and a Ph.D. candidate. The secondary reviewers participated in a 60-min training session about the review process and the aims of the review.
The level 1 relevance screening questions were as follows.
Question 1: Is the full publication written in English? Possible responses were yes, no, and can't tell.
Question 2: What type of publication does the abstract or title describe? Possible responses were primary research, simulation model, review, report, survey, testimonial, editorial, opinion and can't tell.
Question 3: Given that the article is primary research, is influenza virus the focus microbe of the abstract or title? Possible responses were yes, no, can't tell, and not applicable.
Question 4: Given that the article is primary research, does the abstract or title describe a project involving environmental samples, such as, but not limited to, air, feces, fecal slurry, soil, and water? Possible responses were yes, no, can't tell, and not applicable.
Citations advanced to the second relevance screening if the responses of both reviewers were as follows: question 1, yes or can't tell; question 2, primary research or can't tell; question 3, yes or can't tell; and question 4, yes or can't tell.
The second relevance screening was conducted using the full manuscript with two independent primary reviewers (C.K.I. and A.M.O.). The questions for the second level of relevance screening were as follows.
Question 1: Does the manuscript pass level 1 screening questions (English, primary research, about influenza, and includes environmental sampling)? Possible responses were yes or no.
Question 2: Does the manuscript provide at least two observations of the same virus? Possible responses were yes, no, or not applicable, i.e., doesn't pass level 1 screening.
Citations advanced to the next level of the review if the responses to both questions were yes from at least one reviewer.
Quality assessment and data extraction.
The purpose of the quality assessment was to identify primary research that described the key features required in an experiment assessing virus persistence in environmental matrices. To identify these key features, content experts in virology, environmental science, and disease ecology were consulted, and the purpose of the review was described. The key feature identified was the measurement of the virus by using a quantifiable concentration assay. The rationale behind this feature was to enable determination of virus decay. Appropriate concentration assays identified were 50% tissue culture infective dose (TCID50), 50% egg infective dose (EID50), 50% lethal dose (LD50), 50% membrane piece infectivity dose (MP50), PFU, and 50% embryo lethal dose (ELD50). Experiments using hemagglutination assays were considered inadequate, as this assay measures chicken erythrocyte hemagglutination rather than virus activity. Experiments that reported the percentage of dead animals and embryos or the presence or absence of the virus were excluded, as these assays quantitate an infection rather than the persistence of virus. Further, the content experts concluded that each experiment should describe the influenza strain, the virus passages prior to the experiment, the environmental matrix, the method of spiking the environmental matrix with the virus, the study duration and sampling intervals, the environmental parameters (i.e., temperature, relative humidity [RH], salinity, and pH) under which the experiment was conducted, and at least two sample periods where virus continued to be detected. For the manuscripts that passed the second level of relevance screening, the presence of these features was evaluated by two reviewers independently (C.K.I. and A.M.O.). Manuscripts that did not describe these features were not included in the data extraction and summation.
One reviewer (C.K.I.) was responsible for extracting data from the studies that passed quality assessment. When unclear, a second reviewer was consulted. For each experiment, extracted information included the matrix (i.e., air, feces, water, and fomites) and conditions relevant to each matrix (i.e., temperature [°C], pH, and salinity [parts per million of NaCl]). Experiments that described the temperature as room temperature were inferred to have been conducted at 22°C. When relative humidity was reported as room air humidity, this was inferred to be <30% relative humidity. Fresh and tap water were inferred to be 0 ppm NaCl.
Virus concentration was extracted for all time points for all experiments with the exception of aerosol experiments. Based on the recommendation of a content expert, measurements of virus concentration made during the equilibration time were not included in the calculation of virus half-life for aerosolization experiments. For example, if an experiment documented a change in the decay rate from sampling at or before 15 min to a gradual and uniform viral concentration reduction thereafter, the results from the first 15 min were omitted from the calculation of virus half-life as losses due to the differences in droplet sizes and virus settling within the aerosolization chamber. If not reported in the text or tables, data were extracted from graphs when possible.
Data analysis and summation.
The aim of data analysis and summation was to describe the persistence of influenza virus reported in the experiments and the association of environmental matrices with persistence. To compare across experiments, the extracted results were converted to viral half-lives, as this measure was independent of starting viral concentration or unit of measure.
For each experiment, the predicted half-life of the virus was calculated based on the extracted data (C.K.I.). First, a least-squares regression model was used to estimate the decay slope (βpersistence) of the persistence of the virus in the set conditions of the experiment as previously described (10, 81, 82) (equation 1):
 |
(1) |
where y is the concentration of virus in log10 units used in the study, x is the time (days), α is the intercept, βpersistence is the slope of the regression line, and ɛ is the residual error. If the experiment had already calculated the coefficient β (the decay slope), this was used unchanged in further analyses. Using βpersistence from equation 1, the half-life of the virus (t1/2) was calculated using equation 2 (12):
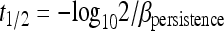 |
(2) |
To describe the association between the explanatory variables and the outcome, log-transformed virus half-life (log10 t1/2), multivariate models were used to obtain adjusted associations for all fixed effects (equations 3, 4, and 5). The multivariate model was a linear mixed regression model (PROC MIXED, SAS version 9.2, SAS Institute Inc. Cary, NC). Additionally, a quad contrast was tested for significance to determine whether there was evidence for nonlinearity in the categories of temperature, salinity, and relative humidity (because pH was a binomial factor, it was not assessed in this fashion). The method of estimation for the variance components was restricted maximum likelihood with a Kenward-Rodger correction for standard errors and degrees of freedom. In all models, environmental variables were included as fixed effects. To account for the nested random effect of study within matrix, as well as the between-study variations of parameters, study and fixed-effect interactions with study were included in each model as random effects (i.e., study × temperature, study × relative humidity, study × water source, study × salinity, study × pH).
For all models, biologically sensible interactions between fixed effects were assessed and removed if the likelihood ratio test indicated that these were not significant with P values of <0.10 or if there was insufficient data representation within levels of the main effects to make valid comparisons between the effect levels. Model assumptions were assessed by evaluating the form of the plot of residual values versus fitted values, a quantile-quantile (Q-Q) plot, and a histogram of the distribution of residuals. The model was determined appropriate if the mean of the plot of the residual values versus fitted values was centered around 0, the Q-Q plot was essentially a positive linear line, and the histogram showed normal distribution around 0.
For all fixed main effects, the null hypothesis was that the main effect was not associated with virus log t1/2. The main effect was evaluated using the type III sum of squares test in PROC MIXED (SAS), and if the P value was less than 0.05 the effect was considered significant. If the main effect was significant, the Tukey-Kramer test for multiple comparisons was used to make pairwise comparisons within that fixed main effect for polychotomous variables. The group mean differences (Δ) were estimated by point estimates, and 95% confidence intervals (CI) and P values adjusted by the Tukey-Kramer method were reported.
Point estimates near zero indicate relative equivalence to the log t1/2 of the referent. For all models, the interpretation of the point estimate within each effect was related to the half-life ratio, where 10Δ estimated the multiplicative affect of each parameter or category of an effect. Values of 10Δ greater than 1 suggest that the response is associated with increased t1/2, and values of 10Δ less than 1 suggest that the response is associated with decreased t1/2. Inclusion of 1 in the 95% confidence interval of 10Δ signified that the P value of the Tukey-Kramer test was >0.05.
Three models were constructed. The first model evaluated virus log t1/2 across matrices; therefore, the explanatory fixed effects were matrix (4-level categorical variable: water, air, feces, fomites) and temperature (°C) categorized into three levels (2 to 12°C, 17 to <27°C, and ≥27°C), which followed a natural grouping from the studies themselves. Temperatures were rounded to the nearest whole number for categorization. Two random effects were included in the overall model: study nested within the matrix and an interaction term between study and temperature (equation 3). The code for the models is included in Supplement S5 in the supplemental material:
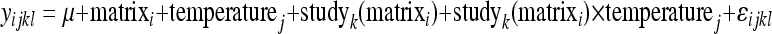 |
(3) |
where yijkl denotes the log of virus half-life (log10 t1/2) for the lth observation of the kth study of the matrix i and temperature j, and the coefficients on the right-hand side of the equation denote the group means, e.g., matrixi denotes the mean response in matrix group i.
The subsequent models were matrix specific. For the analysis evaluating virus log t1/2 in aerosolization experiments, the explanatory fixed effects were temperature (categorized into 7 to 12°C, 17 to <27°C, and ≥27°C) and RH (categorized into <30%, 30 to <70%, ≥70%). Two random effects were included: an interaction term between study and temperature and one between study and RH (equation 4):
 |
(4) |
For the analysis evaluating virus log t1/2 in water experiments, the fixed effects were water source (three-level categorical variable: distilled, buffered, or lake), temperature (categorized as 2 to 12°C, 17 to <27°C, and ≥27°C), pH (categorized as normal [pH 6 to 8] or extreme [pH <6 or ≥9]) and salinity (categorized into 0 to 1 ppm, >1 to <30 ppm, or ≥30 ppm) (58). Like temperature, pH and salinity were rounded to the nearest whole number before categorization. Five random effects were included in the water model: study and the interaction between study and each main effect (i.e., study × water source, study × temperature, study × salinity, study × pH) (equation 5):
 |
(5) |
Describing the presence/absence of design features in studies included in meta-analysis. (i) Identifying key features of study design for evaluation.
For study designs such as randomized controlled trials, diagnostic test evaluations, and observational studies, published guidelines provide the key study features required for a reproducible document, and they are readily available (7, 18, 19, 38, 42, 50, 51, 76, 83, 90, 92, 93, 95, 96). For the laboratory sciences, we were not aware of guidelines for comprehensive reporting; therefore, the key features required for evaluation were determined using a two-step process. First, content experts in virology, environmental sciences, and disease ecology were consulted in a series of group and individual meetings and asked to identify key features that enable reproducibility in an experiment to assess virus persistence in environmental matrices. This group concluded that each experiment should describe the influenza subtype, including the number of virus passages prior to the experiment, the environmental matrix, the method of spiking the environmental matrix with the virus, the study duration and sampling intervals, the environmental parameters (i.e., temperature, relative humidity, salinity, pH) under which the experiment was run, measurement of the virus using a quantifiable concentration assay, and at least two sample periods where virus continued to be detected. The rationale for the last two features was to enable determination of virus decay.
The second set of quality criteria of key design features for assessment were established at the conclusion of this systematic review process, where additional features associated with the reproducibility of the studies, the ability to assess bias, and the ability to extract data were identified. These related mainly to a description of the study protocol and the methods of data handling and analysis. A list of 17 key reporting features was developed (see Supplement S6 in the supplemental material). Of the 17 key reporting features evaluated, 15 were methodological features and two related to descriptions of the results. The 15 methodological features were subdivided into attributes about the study organism, study setting, study protocol, and data handling. The last two concerned data analysis. The features and rationale are reported in Supplement S6 in the supplemental material.
(ii) Assessing the presence of key features.
The unit of concern for the evaluation of reporting was the study. For each study, the presence or absence of the feature in the appropriate section of the manuscript was evaluated. Evaluation for features was conducted by one reviewer (C.K.I.), who consulted with the experts or a coauthor when the information was unclear. Possible responses for the 17 key features were “yes” or “no.” No judgment was made about the correctness of the approach reported. For example, a study reporting the detection limit for the virus quantification assay received a “yes” response regardless of the level of detection and a “no” response if the detection limit was not mentioned. If a study referred the reader to another citation for a method, the response for that feature was presumed to be “yes,” although additional investigation was not pursued. Experimental settings and conditions were expected to be described clearly. Descriptions such as “grown in eggs,” “serial passage,” “in a drawer at room temperature,” or “room humidity” were considered insufficient for replication and resulted in a negative response. Further, the feature was expected to be present in the appropriate section of the manuscript. For example, if unmanipulated or manipulated experimental parameters were not stated in the Methods section of the manuscript, the response for that feature in this review was “no,” even if graphs or narration in the Results section provided this information. When multiple aspects were required for a complete description of a key feature, it was marked “yes” only when all aspects of the description were present. For example, key feature 1 required both a description of the concentration units of the assay and a description of the detection limits for an affirmative response.
RESULTS
Literature search and relevance screening.
The cutoff date for citation searching was 25 January 2008. After deduplication by matrix, 2,118, 8,114, and 8,288 citations remained in the air, soil (includes feces and fomites), and water searches, respectively. After deduplication, 9,760 references were available for relevance screening. Four citations were identified by hand searching (Fig. 1). A total of 132 citations passed the first relevance screening. Reasons for exclusion are included in Supplement S7 in the supplemental material. Of the 132 citations, 92 were excluded at the second relevance level after retrieving the articles, primarily due to a lack of environmental sampling or reporting only discovery and not persistence of the influenza virus. Other citations were excluded, as they reported virus stability in laboratory techniques (1, 39, 61, 63), disinfection (15, 23, 54, 62, 86, 103), persistence in eggs, meat, or carcasses (1, 4, 41, 47, 60, 72), transmission rather than persistence (44, 56, 85), or only one sampling time (24, 27, 45, 69, 101).
FIG. 1.
Flow chart of literature search, relevance screening, and quality assessment process for influenza virus persistence in environmental matrices.
Quality assessment and data extraction.
Forty studies were identified that contained 122 experiments, of which 77 were relevant and evaluated for quality assessment. Fifteen studies reported persistence of influenza virus in air, 15 in water, 10 in soil or feces, and five on fomites (several studies included multiple matrices). Twelve studies published prior to 1970 (13, 22, 28, 29, 32, 40, 43, 49, 59, 70, 74, 100) reported influenza virus persistence in air, while the remaining three were published between 1970 and 1990 (35, 48, 67). Five studies reporting persistence in water were published prior to 1970 (26, 52, 88, 89, 94), three between 1970 and 1990 (68, 98, 104), and seven from 1990 to January 2008 (10, 37, 44, 81, 82, 85, 105). Two studies reporting persistence in feces, wastewater, soil, or compost were published prior to 1970 (78, 94), one between 1970 and 1990 (98), and seven since 1990 (17, 27, 45, 46, 71, 79, 101). Persistence of influenza virus on fomites was investigated twice prior to 1970 (21, 94), once between 1970 and 1990 (4), and twice since 1990 (55, 87).
Of the 77 relevant experiments within the 40 studies, 56 did not describe the key features recommended by the content experts. Ultimately, only 19 studies contained at least one experiment which included the quality criteria. The most common feature missing was a description of virus concentration at two time points. Six of the 15 aerosol studies were excluded because none of the experiments reported results in viral concentration (22, 40, 43, 48, 74, 100), and two studies reported mean persistence in all experiments rather than persistence over time (29, 35). Of the 15 water studies, four studies failed to report virus concentration adequately in all experiments (45, 53, 88, 94), three studies contained experiments which reported mean persistence time at multiple pH measurements (24, 68, 104), several experiments reported only a final persistence time when virus was determined undetectable (27, 101, 104), and one reported all results as persistence over freeze-thaw cycles rather than time (26). No study with experiments reporting on virus persistence in wastewater, soil, compost, or under UV light passed quality assessment (17, 34, 46, 71, 94, 101).
Data analysis and evidence summation.
Twenty-one relevant experiments contained within 19 studies passed quality-assessment review. The detailed characteristics of the 19 studies are provided in Supplement S8 in the supplemental material.
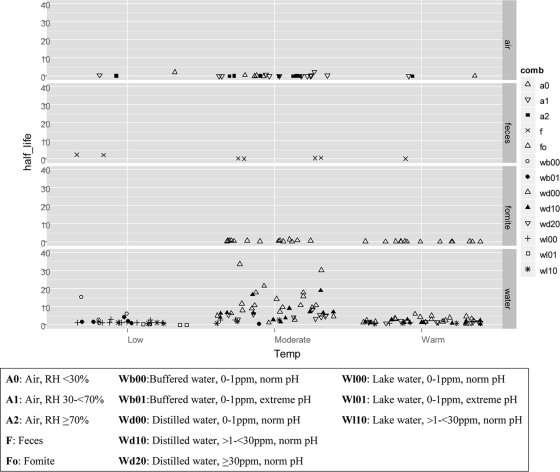
Supplement S9 in the supplemental material describes the number of times it was possible to calculate the virus half-life for each combination of virus and matrix from the 21 experiments. It is notable that no reporting of variation could be performed at the observation level, as none was reported in any experiment evaluated. The description of the observations (converted to half-lives [days]) extracted from the 21 experiments of the 19 studies are depicted in Fig. 2, categorized by matrix, grouped by temperature (low, 2 to 12°C; moderate, 17 to <27°C; and warm, ≥27°C), and identified by varied parameter (e.g., categories of relative humidity, water source, salinity, or pH). The majority of half-life observations (127/191) were available from experiments evaluating virus persistence in water. Table 1 describes the frequency of half-life observations in air, water, and feces evaluated from the 21 experiments. The most common temperature evaluated in aerosol experiments (22/28 half-life observations) evaluated virus persistence at temperatures between 17 and 27°C. The most common humidity evaluated in aerosol experiments (13/28 half-life observations) was 30 to 70%. Most water experiments evaluated low-pathogenicity viruses in buffered, filtered water at freshwater salinity (0 to 1 ppm) and normal pH (6-8). Twenty-eight independent observations of influenza virus half-life on fomites were extracted from the four relevant experiments of three studies. Numerous fomites were represented only in a single study; therefore, a half-life table and reported conditions for each experiment are provided in Supplement S10 in the supplemental material and no summary analysis was attempted for these data. Similarly, the numbers of studies (n = 2), experiments (n = 4), and virus half-life observations (n = 28) that evaluated feces or diluted feces matrices were limited; therefore, the raw data, estimated half-lives, and conditions of each experiment were reported in Supplement S11 in the supplemental material.
FIG. 2.
The 191 observations (converted to t1/2 [days]) sorted by matrix, separated by temperature, and differentiated by various parameters are shown. For better graphic visualization, data points of t1/2 = 75 days in water (low-temperature category) and t1/2 = 120 days in feces (low-temperature category) were excluded.
TABLE 1.
Frequency of matrix conditions from 19 experiments studying the persistence of influenza virus in the environment
| Matrix | Variable reported | Measure reported | No. of virus half-life estimates |
|---|---|---|---|
| Air | Temp | 7-12°C | 3 |
| Temp | 17-<27°C | 22 | |
| Temp | ≥27°C | 3 | |
| Relative humidity | <30% | 6 | |
| Relative humidity | 30-<70% | 13 | |
| Relative humidity | ≥70% | 9 | |
| Water | Water type | Buffered | 86 |
| Water type | Distilled | 11 | |
| Water type | Lake | 30 | |
| Temp | 2-12°C | 30 | |
| Temp | 17-<27°C | 50 | |
| Temp | ≥27°C | 47 | |
| Salinity | 0-1 ppm | 71 | |
| Salinity | >1-<30 ppm | 36 | |
| Salinity | ≥30 ppm | 20 | |
| pH | Normal (pH 6-8) | 117 | |
| pH | Extreme (<6 and ≥9) | 10 | |
| Water clarity | Filtered | 106 | |
| Water clarity | Unfiltered | 10 | |
| Water clarity | Not described | 11 | |
| Feces | Feces type | Dried | 1 |
| Feces type | Moist | 5 | |
| Feces type | In river water | 2 | |
| Temp | 4-12°C | 3 | |
| Temp | 17-<27°C | 4 | |
| Temp | ≥27°C | 1 |
Neither standard deviations nor errors were reported at the experiment level; therefore, it was not possible to assess variance at the experiment or study level nor between studies. With this in mind, the following models were constructed based on the available summary observations reported in each experiment. The results of the overall linear mixed model showed that both main effects in the model, matrix (P < 0.02) and temperature (P = 0.034), were significant. The pairwise comparisons are presented in Table 2. The half-life of influenza virus was predicted to be significantly longer in water than in air; however, the confidence interval after Tukey's adjustment for multiple comparisons was vast (10Δ = 27 times longer half-lives in water than in air; 95% confidence interval [CI] of 2.22 to 336 times). Increasing temperature was associated with a shorter virus half-life, although a significant difference (P = 0.031) was found only between low temperatures (2 to 12°C) and elevated temperatures (≥27°C) (10Δ = 11.6 times longer half-lives in low versus elevated temperatures; 95% CI of 1.28 to 105 times [Table 2]). No other matrix or temperature comparison was significant (Tukey-Kramer test P value of >0.05). The quad contrast for temperature did not identify significant quadratic influence to any model nor did the quad contrast for salinity or relative humidity for the water or air models, respectively. The covariance parameter estimates for the random effects, study nested within matrix, study(matrix) × temperature, and the residual error were 0.17, 0.20, and 0.12, respectively. Although the study(matrix) × temperature component comprised 41% of the variance, the biological significance of this is not clear. We hypothesize that it is related to the diversity of the temperature parameters investigated between the studies in that temperature was the single parameter measured across matrices. It is plausible that although temperature would preferably have been studied as a continuous variable, the extracted data necessitated broad categories to be used instead, possibly causing observations which otherwise would have been spread out to be coalesced into groups.
TABLE 2.
Multivariate, multiple-comparison-adjusted estimates of the association between environmental conditions and influenza virus half-life (log10 t1/2) (n = 191)a
| Multiple comparison | Point estimate of the difference (Δ) | Half-life ratio (10Δ) | 95% CI of 10Δb | Adjusted P valuec |
|---|---|---|---|---|
| Matrix | ||||
| Water vs aerosol | 1.44 | 27.3 | 2.22-336 | 0.010 |
| Feces vs aerosol | 1.04 | 11.0 | 0.43-285 | 0.18 |
| Fomite vs aerosol | 0.63 | 4.22 | 0.19-92 | 0.52 |
| Water vs fomite | 0.81 | 6.46 | 0.30-139 | 0.31 |
| Water vs feces | 0.39 | 2.48 | 0.12-52.7 | 0.81 |
| Feces vs fomite | 0.42 | 2.61 | 0.06-109 | 0.87 |
| Temp (°C) | ||||
| 2-12 vs ≥27 | 1.06 | 11.6 | 1.28-105 | 0.03 |
| 2-12 vs 17-<27 | 0.79 | 6.12 | 0.73-51.1 | 0.09 |
| 17-≥27 vs ≥27 | 0.28 | 1.90 | 0.28-13.1 | 0.63 |
Full model: log10 t1/2 = μ + matrix + temperature + study(matrix) + study (matrix) × temperature.
95% confidence intervals that include 1 show no significance at α = 0.05.
P values from Tukey-Kramer adjustment for multiple comparisons.
Seven studies containing seven relevant experiments reported persistence of influenza virus in aerosols. Table 1 and Supplement S8 in the supplemental material illustrate the diversity evaluated by the 28 observations within those seven experiments passing quality assessment. The main effects for the aerosol model were temperature (P = 0.003) and RH (P = 0.15). The pairwise comparison suggested that the half-life of influenza virus decreased as temperature increased (Table 3). For example, virus half-life was predicted to be approximately 16.5 times longer at temperatures between 7°C and 12°C then at temperatures of ≥27°C (95% CI, 4.88 to 56 times). The covariance parameter estimates for the random effects, study, study × temperature, study × RH, and the residual error were 0.33, 0.007, 0.10, and 0.08, respectively.
TABLE 3.
Pairwise-adjusted estimates of the change of virus half-life (log10 t1/2) and environmental conditions in air (n = 28)a
| Multiple comparison of temp (°C) | Point estimate of the difference (Δ) | Half-life ratio (10Δ) | 95% CI of 10Δb | Adjusted P valuec |
|---|---|---|---|---|
| 7-12 vs 17-<27 | 0.70 | 4.99 | 1.59-15.67 | 0.02 |
| 7-12 vs ≥27 | 1.22 | 16.5 | 4.88-55.96 | 0.0002 |
| 17-<27 vs ≥27 | 0.52 | 3.31 | 1.05-10.39 | 0.099 |
Full model: log10 t1/2 = μ + temperature + RH + study + study × temperature + study × RH.
95% confidence intervals that include 1 show no significance at α = 0.05.
P values from Tukey-Kramer adjustment for multiple comparisons.
Seven studies with eight relevant experiments described influenza virus persistence in water. The main effects for the water model were water source (P = 0.37), temperature (P = 0.12), salinity (P = <0.0001), and pH (P = 0.04). Increased salinity was a significant deterrent to influenza virus persistence, with the persistence in both freshwater (0 to 1 ppm) (having the longest persistence) and brackish water (>1 to <30 ppm) significantly longer than that in salt water (≥30 ppm) (2.31 times longer [P < 0.0001] and 1.49 times longer [P = 0.006], respectively). Table 4 provides the pairwise comparison for salinity. pH was also a significant main effect, where influenza virus persisted an estimated 6.89 times longer (95% CI, 1.12 to 42.2 times) in pH 6 to 8 than in extreme pH (<6 and ≥9). The covariance estimates for the random effects of study, study × water source, study × temperature, study × salinity, study × pH, and residuals were 0, 0.087, 0.064, 0, 0.049, and 0.043, respectively.
TABLE 4.
Pairwise-adjusted estimates of the change of virus half-life (log10 t1/2) and environmental conditions of water (n = 127)a
| Multiple comparison | Point estimate of the difference (Δ) | Half-life ratio (10Δ) | 95% CI of 10Δb | Adjusted P valuec |
|---|---|---|---|---|
| Salinity (ppm) | ||||
| 0-1 vs >1-30 | 0.19 | 1.55 | 1.19-2.01 | 0.0004 |
| >1-<30 vs ≥30 | 0.17 | 1.49 | 1.06-2.09 | 0.016 |
| 0-1 vs ≥30 | 0.36 | 2.31 | 1.66-3.22 | <0.0001 |
| pH 6-8 vs pH <6 or ≥9 | 0.84 | 6.89 | 1.12-42.2 | 0.043 |
Full model: log10 t1/2 = μ + water source + temperature + salinity + pH + study + study × water source + study × temperature + study × salinity + study × pH.
95% confidence intervals that include 1 show no significance at α = 0.05.
P values from Tukey-Kramer adjustment for multiple comparisons.
Quality review of the 19 studies of this systematic review.
Figure 3 describes the frequency of reporting of the 17 key features (see Supplement S6 in the supplemental material) in the 19 studies, and the frequency of reporting by matrix (air, water, feces, and fomites) and publication year category (<1970, 1970 to 1990, and ≥1990) are tabulated in Supplement S12 in the supplemental material. It is notable that no study reported all 17 key features.
FIG. 3.
Frequency of reporting the 17 key design features (see Supplements S6 and S12 in the supplemental material) in studies reporting the persistence of influenza virus in the environment.
Attributes of the virus (key features 1 to 3).
All 19 studies described the virus assay, but only 21% (4/19) provided the limit of detection for the assay prior to reporting the results.
Eleven of 19 studies (58%) provided complete descriptions of the influenza virus; however, six of eight studies with incomplete descriptions were published prior to 1977, and these studies provided descriptions which included colloquial terms (e.g., PR8, Melbourne strain, Dutch East Indies fowl plague virus) but no H (hemagglutinin) or N (neuraminidase) subtype information. All studies published prior to 1970 also lacked H and N subtype characterization. After investigating, we found a WHO memorandum released in 1971 (3, 16) recommending revisions to the methods of influenza nomenclature to include the H and N antigenic characteristics of influenza viruses, which explains this observation. The majority of studies reported the method of virus propagation (16/19), but only 37% (7/19) detailed the propagation method and described virus passages.
Attributes of the setting (key features 4 to 9).
All studies provided a complete description of the matrix. Fifteen of the 19 studies described the experimental baseline data, i.e., the nonmanipulated conditions of the laboratory. Four of seven studies published from 1970 to 1990 contained the sought information; however, two of the eight published in or after 1990 failed to include it. Sixteen of 19 studies provided the specific details of the investigator-manipulated parameters; however, none described the sensitivity of the equipment (i.e., the sensitivity of sensors for relative humidity, salinity, or temperature). The methods of inoculating the primary suspension and the matrix were consistently well reported. The majority of studies (14/19) reported the concentration of the replicate after inoculation, and often this was the first sampling time (or series of samplings, i.e., aerosol studies), but it was sometimes unclear in resultant graphs whether the author intentionally included equilibration time as part of the decay curve.
Study protocol (key features 10 to 13).
Only 11 of the 19 studies described the study duration in the Methods section of scientific manuscripts, although the duration of a study could often be determined by looking at tabulated or graphical results. A description of the sampling intervals was also infrequently present in the Methods section (seven of the 19 studies), although 11 of the 12 which failed to discuss the sampling intervals in the Methods section did have them reported in tables or graphs in the Result section. Only two studies clearly stated the number of true replicates used in the study (one of three fomite studies and one of seven water studies). Of these two studies, one was published after 1990 and the other between 1970 and 1990. Both stated multiple replicates. Seven other studies provided either a range of replicates used in the experiments or pictorially described two presumable replicates in graphed or tabulated results, but because the descriptions required interpretation, they did not meet the criteria for reproducibility. In seven of the 19 studies, the number of samples per replicate was stated or it was interpreted that the sample equaled the replicate, using terms like “aliquots were removed each time period” and “each time [a] sample was removed.” Of these seven, only three reported more than one sample per replicate.
Attributes of data handling and analysis (key features 14 and 15).
No study completely addressed how sample or replicate data were summarized at each interval, because none included all three components of the criteria: a description of the statistics used to summarize the data from sampling intervals (mean and standard deviation or range), a description of the statistics used to summarize the replicates, and the methods describing any necessary transformation of data. One study did state the mean was the summary statistic (28); however, this study did not provide measures of variation for the mean, nor did it state the number of replicates or samples taken per replicate; therefore, there was no description of statistics used to summarize data for either samples or replicates. Another study reported the summary result as “The best fit was estimated by eye” (67) but again contained insufficient information about the number of replicates or samples per replicate the study used. Neither of the two studies with multiple replicates described the method of summarizing replicate data, though Bean et al. (4) did describe the statistical procedures used to summarize the final outcomes by fomite. Eight studies did not log transform data because their results remained in virus titers or were percent recovery values. Seven studies did transform data according to graphs in the Results section but did not mention the transformation in the Methods section. Only four studies stated that some type of transformation of outcomes was performed for results reporting, and one was Schaffer et al. (67), where the visual estimate of percent recovery was transformed to half-lives. Only one other study mentioned half-life calculations (82). Four of the 19 studies reported the statistical methods used to assess the outcomes.
Reporting attributes of data analysis (key features 16 and 17).
All 19 studies provided descriptive results, typically in graphic or tabular form. However, none of the summarized outcomes also provided estimates of variance. It is noteworthy that the preliminary experiment of Bean et al. (4) provided confidence intervals for recovered concentrations of virus immediately after matrix inoculation; however, no additional reporting of variance in the following persistence experiments was stated.
Three studies created univariate linear regression models for overall persistence at each investigator-manipulated environmental parameter by influenza virus subtype (10, 81, 82); however, none described the variation within the slope estimates of each of those models (i.e., confidence intervals) nor model fit. Only one calculated half-lives from the persistence outcomes but without variance (82).
DISCUSSION
The aim of this review was to summarize the findings from experiments that report persistence of influenza virus in the environment and to convey information about the quality of reporting for the body of work considered. The motivation was to provide better science-based information to advise policies that will impact livestock producers and surrounding communities. For example, to establish that a production site is free of influenza virus prior to repopulation, it may be necessary to sample the premises. The available literature should be able to inform which environmental matrices are associated with longer persistence and therefore should be targeted for testing for influenza virus. Recent outbreaks of avian influenza as well as the interest in the novel 2009 pandemic H1N1 influenza virus suggest that the need for high-quality information about the persistence of influenza virus in livestock environments will only increase.
The data, although limited, suggest that the half-life of influenza virus is significantly shorter in air than in other matrices and that in air, as in other matrices, persistence of influenza virus is longer at lower temperatures. Theoretically, this information and the accompanying estimates of virus half-life could be combined with estimates of virus concentration to predict aerosol dispersion between facilities. Such approaches have been used to predict aerosol transmission of other livestock pathogens, such as foot-and-mouth disease virus and porcine reproductive and respiratory syndrome virus (5, 6, 30, 37). However, although general associations can be described from the data, the estimates obtained from the review of virus half-life have wide confidence intervals (Tables 2, 3, and 4). This limitation highlights the need for more applicable primary research into the feasibility of facility-to-facility transmission of influenza virus.
The data summation also suggests that influenza virus has an increased half-life in water compared with that in feces and fomites (Table 2) and that persistence may be longer in cool, clean water than in buffered or lake water (P = 0.0015). The application of this information is that in a depopulation situation, to understand whether influenza virus remains in a barn, water testing would appear to be the more sensitive evaluation, and sampling water from clean water sources, such as troughs or nipples, would be better than testing manure, waste, or contaminated water in the barn. Weber and Stilianakis (97) also concluded that water might be considered a reservoir for influenza virus, given the similar data evaluated.
These conclusions are consistent with others (73) regarding prolonged persistence at low temperatures and shortened persistence at extreme pHs and salinities. However, other studies have not previously tried to quantitatively summarize the magnitude of differences across multiple studies. More recent studies continue to demonstrate similar temperature and pH associations with influenza virus (11, 25). Weber and Stilianakis (97) discussed the apparent short duration of persistence of influenza virus in the airborne state as well, particularly in low to moderate temperatures and low RH, although this statement was based on human transmission models, which may not be appropriate to apply to airborne persistence in the field between barns of pigs or poultry.
One potential source of bias in our summarized analysis was the number of studies ultimately evaluated, which may have resulted in correlations between results of the same study. The use of a nested random effect was incorporated to adjust for this issue; however, statistical adjustment post hoc is likely a poor substitute for more studies with greater variation. This particularly applies to the water data set, where, after adjusting for the between-study variation in the random effect (i.e., study × temperature), temperature was no longer a significant variable, likely due to the large discrepancy between observation contributions from each study (e.g., one of the seven water studies alone contributed 63 to the total 127 observations) (see Supplement S8 in the supplemental material). For the water model, if study was included as a main effect along with water source, temperature, salinity and pH, all main effects but water source became significant at P values of <0.0001.
Another source of potential bias was the diversity in measurements of viral concentration (i.e., TCID50, EID50, ELD50, PFU, and MP50). We used conversion of all assays to viral half-life as a method to obtain a measure of persistence independent of specific assay; however, there was little overlap between measurement units even within the same matrix, unless an author provided continuity between papers (10, 81, 82). Unless the research community agrees upon a standard method for quantification of virus, this issue will continue to arise for those needing to summarize results across studies.
Potentially, the most significant findings of the review were ancillary findings about data quantity and quality. The review documents the paucity of experiments reporting quantitative assays to assess the persistence of influenza virus in environmental matrices found in livestock facilities, a finding determined by Stallknecht and Brown (80) as well. The application of systematic review principles to reviewing literature is not as widespread in the bench sciences as clinical sciences; however, others have applied similar approaches to the evaluation of the information about influenza virus and reached similar conclusions about the paucity and disparity of data (97). To our knowledge, this systematic review is the first to also evaluate the quality of studies regarding influenza virus. Shahid et al. (73) investigated inactivation rather than virus persistence in a narrative discussion, but they likewise noted that the aim of their review was to add evidence to the scant information available for biosecurity recommendations for poultry facilities. In this investigation, we had anticipated that persistence of influenza virus on surfaces and in feces and feces-like matrices would have generated more primary research; however, statistical synthesis of virus half-life on fomites and in feces was not possible, as so few observations were available (see Supplements S8, S10, and S11 in the supplemental material). Similarly, since no soil or compost study reported key features of a persistence study, it was not possible to report on the persistence of influenza virus in common methods of livestock mortality removal. More recent work has evaluated the persistence of avian influenza virus in land disposal (25).
The lack of data may partly be a function of the systematic review methodology which uses predetermined parameters and criteria for the evaluation of citations for relevance, and these criteria are followed sequentially and strictly. As a consequence of this approach, relevant experiments would not be considered if the title or abstract did not discuss the pertinent topic of the persistence of influenza virus or were not evidently primary research. However, the potential for this bias seems unlikely, as few relevant studies were identified outside the electronic search, the search was comprehensive, and others have reported the paucity of data.
Further, in the experiments conducted, the variation in parameters assessed was narrow. Illustrative of the lack of range assessed is that only 30 observations in water, three observations for feces or diluted feces, and three observations in air were available at or below 12°C. This lack of data is particularly relevant, as low temperatures may occur in livestock facilities or manure storage units. Data on the persistence of influenza virus at extreme values of pH or salinity are of less importance, since it is likely the range of pH and salinity observed in livestock facilities is narrow.
The study designs and methods of reporting were also extremely heterogeneous and often limiting. Several studies were performed at room temperature, and descriptions of the sensitivity of the equipment were uniformly absent; therefore, there was significant interpretation necessary regarding the parameter values reported. Because of this, it was unfortunate but necessary to categorize naturally continuous variables like temperature, salinity, pH, and relative humidity. The continuous nature of these parameters may impact viral half-life in a progressive manner, and this could have been lost by our wide groupings. Likewise, even within the categories, there was insufficient representation to examine interactions between temperature and humidity, or temperature and pH, for example, and these are common questions about influenza virus persistence.
The evaluation of reporting quality is not as widespread in the bench sciences as clinical sciences, but it is useful to identify strengths and weaknesses in study reporting. In clinical research, there has been an increased focus in recent years on the quality of reporting and how closely reports adhere to the concept of reproducibility. Many studies have provided empirical evidence that clinical trials and observational studies frequently fail to report sufficient information for reproduction, assessment of bias, and research synthesis (14, 20, 36, 64, 65). Articles or editorials have described poor reporting of statistical methods (57); otherwise, there appears to be little empirical evaluation of the quality of reporting in the laboratory sciences.
This review suggests that, as has been documented in other fields, the reporting of these studies may be less than ideal to meet the requirements for a reproducible description of an executed study. Authors consistently failed to report sufficient information to fully understand the experiment design, execution, and results. Beyond looking at the reporting methods, this review identified what appeared to be common flaws in design execution as well. For example, sampling and replication, fundamental concepts and requirements for proper statistical assessment and reporting of standard deviation, were clearly insufficient in the studies under review to confidently extrapolate results to field application. Multiple replicates and samples per sampling time enable the expression of normal variation for estimates of continuous outcomes, and they improve confidence in parameter estimates for statistically meaningful results. Further detailed discussion of reporting gaps and replicate and sample numbers can be found in Supplements S13 and S14 in the supplemental material. Because of the absence of replicates, the uncertainty within studies clearly impacts the uncertainty when synthesizing information between studies for this review, as evidenced by the large confidence intervals around the persistence estimates (Tables 2, 3, and 4).
Additional key areas that require considerable improvement in reporting are the descriptions of environmental conditions and the statistical methods, including data transformation. For baseline environmental conditions that did not vary during the experiment, such as temperature, pH, salinity, or RH, improved and more detailed descriptions are imperative to enable comparisons between studies. In this review, terms such as “room temperature” or “fresh water” were interpreted and estimates were assumed, because of lack of descriptions, to incorporate results into the cumulative data set. Similarly, experiments which portrayed data only graphically were interpreted and estimated to enable their inclusion in the review, and this estimation is not as accurate as data extracted from experiments presenting numerical results or statistical outcomes with well-described methodologies.
Finally, it was unexpected to find so few studies reporting results as decay rates or half-lives of the virus. Virus titer, percent virus remaining, and duration of persistence are not easily applicable to the field, as they can be useful only when exact starting concentrations are repeated. Alternatively, results reported as decay rates or half-lives have significantly more utility, as they can be applied to any starting concentration and therefore are able to be used in existing environmental settings and can be applied to any known starting concentration of virus.
The results of this study draw attention to potential needs for improved reporting of design and methods in the current scientific literature concerning influenza. Similar studies showing empirical evidence of poor reporting have provided the motivation for reporting guidelines in other areas of scientific research. There are numerous examples of disciplines in which guidelines have been published, where evidence of systemic problems with design execution and reporting in multiple fields led to guideline development (7, 51, 83, 84, 93). The methodological assessment of the 19 studies included in the review confirms the need for additional but significantly improved studies regarding influenza virus persistence in the environment, the need for more transparency, with more focus on detailed reporting within sampling, and the need for attention to replication, to provide more robust outcome information to support decision-making and policy formation.
Ultimately, this review revealed that, although there is a significant amount of published literature regarding influenza virus, there are very few studies that can be used to support decision-making and policy formation. Although this study was comprehensive, the resultant data extracted for this synthesis leave a great deal of uncertainty for field application or management decisions and are outdated for certain matrices. Future work should use improved reporting of study designs and outcomes to enable a more thorough and robust meta-analysis of environmental persistence of influenza virus.
Supplementary Material
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agranovski, I. E., et al. 2004. Inactivation of viruses in bubbling processes utilized for personal bioaerosol monitoring. Appl. Environ. Microbiol. 70:6963-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, D. J. 1982. Ecological aspects of influenza A viruses in animals and their relationship to human influenza: a review. J. R. Soc. Med. 75:799-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assaad, F. A., et al. 1980. A revision of the system of nomenclature for influenza viruses: a WHO memorandum. Bull. World Health Organ. 58:585-591. [PMC free article] [PubMed] [Google Scholar]
- 4.Bean, B., et al. 1982. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146:47-51. [DOI] [PubMed] [Google Scholar]
- 5.Bessell, P. R., D. J. Shaw, N. J. Savill, and M. E. Woolhouse. 2010. Statistical modeling of holding level susceptibility to infection during the 2001 foot and mouth disease epidemic in Great Britain. Int. J. Infect. Dis. 14:e210-e215. [DOI] [PubMed] [Google Scholar]
- 6.Bessell, P., D. Shaw, N. Savill, and M. E. J. Woolhouse. 2008. Geographic and topographic determinants of local FMD transmission applied to the 2001 UK FMD epidemic. BMC Vet. Res. 4:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossuyt, P., et al. 2003. Towards complete and accurate reporting of studies of diagnostic accuracy; the STARD initiative. BMJ 326:41-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brankston, G., L. Gitterman, Z. Hirji, C. Lemieux, and M. Gardam. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7:257-265. [DOI] [PubMed] [Google Scholar]
- 9.Brooks, J. S., M. A. Franzen, C. M. Holmes, M. N. Grote, and M. Borgerhoff Mulder. 2006. Development as a conservation tool: evaluating ecological, economic, attitudinal, and behavioral outcomes. Collaboration for environmental evidence, systematic review no. 20, p. 1-32. Centre for Evidence-Based Conservation, University of California, Davis, CA.
- 10.Brown, J. D., D. E. Swayne, R. J. Cooper, R. E. Burns, and D. E. Stallknecht. 2007. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 51:285-289. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J., G. Goekjian, R. Poulson, S. Valeika, and D. Stallknecht. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 136:20-26. [DOI] [PubMed] [Google Scholar]
- 12.Bryan, M., J. J. Zimmerman, and W. Berry. 1990. The use of half-lives and associated confidence intervals in biological research. Vet. Res. Commun. 14:235-240. [DOI] [PubMed] [Google Scholar]
- 13.Buckland, F. E., and D. A. Tyrrell. 1962. Loss of infectivity on drying various viruses. Nature 195:1063-1064. [DOI] [PubMed] [Google Scholar]
- 14.Chan, A. W., A. Hróbjartsson, M. T. Haahr, P. C. Gøtzsche, and D. G. Altman. 2004. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 291:2457-2465. [DOI] [PubMed] [Google Scholar]
- 15.Chang, H., P. Li, and H. Wang. 1954. Experiments on the use of chemicals for the disinfection of air against influenza A virus. Chin. Med. J. 72:325-335. [PubMed] [Google Scholar]
- 16.Chanock, R. M., et al. 1971. A revised system of nomenclature for influenza viruses. Bull. World Health Organ. 45:119-124. [PMC free article] [PubMed] [Google Scholar]
- 17.Chumpolbanchorn, K., N. Suemanotham, N. Siripara, B. Puyati, and K. Chaichoune. 2006. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian J. Trop. Med. Public Health 37:102-105. [PubMed] [Google Scholar]
- 18.Davidoff, F., P. Batalden, D. Stevens, G. Ogrinc, and S. Mooney. 2008. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual. Saf. Health Care 17(Suppl. 1):i3-i9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond, M., A. Manca, and M. Sculpher. 2005. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int. J. Technol. Assess. Health Care 21:165-171. [PubMed] [Google Scholar]
- 20.Dwan, K., et al. 2008. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 3:e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edward, D. G. F. 1941. Resistance of influenza virus to drying and its demonstration on dust. Lancet 238:664-666. [Google Scholar]
- 22.Edward, D. G. F., W. J. Elford, and P. P. Laidlaw. 1943. Studies on air-borne virus infections. I. Experimental technique and preliminary observations on influenza and infectious ectromelia. J. Hyg. (Lond.) 43:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edward, D. G. F., and O. M. Lidwell. 1943. Studies on air-borne virus infections. III. The killing of aerial suspensions of influenza virus by hypochlorous acid. J. Hyg. (Lond.) 43:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elford, W. J., et al. 1948. Physical properties of the viruses of Newcastle disease, fowl plague and mumps. Br. J. Exp. Pathol. 29:590-599. [Google Scholar]
- 25.Graiver, D., C. Topliff, C. Kelling, and S. Bartelt-Hunt. 2009. Survival of the avian influenza virus (H6N2) after land disposal. Environ. Sci. Technol. 43:4063-4067. [DOI] [PubMed] [Google Scholar]
- 26.Greiff, D., H. Blumenthal, M. Chiga, and H. Pinkerton. 1954. The effects on biological materials of freezing and drying by vacuum sublimation. II. Effect on influenza virus. J. Exp. Med. 100:89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan, Y., et al. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper, G. J. 1961. Airborne microorganisms: survival tests with four viruses. J. Hyg. (Lond.) 59:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmes, J. H., K. C. Winkler, and S. M. Kool. 1962. Virus survival as a seasonal factor in influenza and poliomylitis. Antonie Van Leeuwenhoek 28:221-233. [DOI] [PubMed] [Google Scholar]
- 30.Hermann, J., C. Muñoz, and J. Zimmerman. 2009. A method to provide improved dose-response estimates for airborne pathogens in animals: an example using porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 133:297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins, J., and S. Green. 2008. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration and John Wiley and Sons Ltd., West Sussex, England.
- 32.Hood, A. M. 1963. Infectivity of influenza virus aerosols. J. Hyg. (Lond.) 61:331-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iowa General Assembly. 24 October 2007. and 28 November 2007. 2007 committee briefings: Livestock Odor Study Committee. Iowa General Assembly, Des Moines, IA. http://www.legis.state.ia.us/lsadocs/BriefOnMeetings/2008/BMDLA001.PDF.
- 34.Jakab, G. J. 1982. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am. Rev. Respir. Dis. 126:778-782. [DOI] [PubMed] [Google Scholar]
- 35.Jakab, G. J., and M. E. Knight. 1981. Decreased influenza virus pathogenesis by infection with germicidal UV-irradiated airborne virus. Environ. Int. 8:415-418. [Google Scholar]
- 36.Jefferson, T., et al. 2009. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 339:b3675-b3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keeling, M., et al. 2001. Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science 294:813-817. [DOI] [PubMed] [Google Scholar]
- 38.Kelley, K., B. Clark, V. Brown, and J. Sitzia. 2003. Good practice in the conduct and reporting of survey research. Int. J. Qual. Health Care 15:261-266. [DOI] [PubMed] [Google Scholar]
- 39.Knight, C. A. 1944. The stability of influenza virus in the presence of salts. J. Exp. Med. 79:285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lester, W., Jr. 1948. The influence of relative humidity on the infectivity of air-borne influenza A virus (PR8 strain). J. Exp. Med. 88:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Y., et al. 2006. Detection of Hong Kong 97-like H5N1 influenza viruses from eggs of Vietnamese waterfowl. Arch. Virol. 151:1615-1624. [DOI] [PubMed] [Google Scholar]
- 42.Liberati, A., et al. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151:W65-W94. [DOI] [PubMed] [Google Scholar]
- 43.Loosli, C. G., H. M. Lemon, O. H. Robertson, and E. Appel. 1943. Experimental air-borne influenza infection. I. Influence of humidity on survival of virus in air. Proc. Soc. Exp. Biol. Med. 53:205-206. [Google Scholar]
- 44.Lowen, A. C., S. Mubareka, J. Steel, and P. Palese. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 3:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu, H., et al. 2003. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 47:1015-1021. [DOI] [PubMed] [Google Scholar]
- 46.Lucio-Forster, A., D. D. Bowman, B. Lucio-Martinez, M. P. Labare, and M. A. Butkus. 2006. Inactivation of the avian influenza virus (H5N2) in typical domestic wastewater and drinking water treatment systems. Environ. Eng. Sci. 23:897-903. [Google Scholar]
- 47.Mase, M., et al. 2005. Isolation of a genotypically unique H5N1 influenza virus from duck meat imported into Japan from China. Virology 339:101-109. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell, C. A., and L. F. Guerin. 1972. Influenza A of human, swine, equine and avian origin: comparison of survival in aerosol form. Can. J. Comp. Med. 36:9-11. [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell, C. A., L. F. Guerin, and J. Robillard. 1968. Decay of influenza A viruses of human and avian origin. Can. J. Comp. Med. 32:544-546. [PMC free article] [PubMed] [Google Scholar]
- 50.Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman, and the PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151:264-269. [DOI] [PubMed] [Google Scholar]
- 51.Moher, D., K. F. Schultz, D. G. Altman, and the Consort Group. 2001. The Consort statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357:1191-1194. [PubMed] [Google Scholar]
- 52.Moses, H. E., C. A. Brandly, and E. E. Jones. 1947. The pH stability of viruses of Newcastle disease and fowl plague. Science 105:477-479. [DOI] [PubMed] [Google Scholar]
- 53.Muhmmad, K. 2001. Effect of physico-chemical factors on survival of avian influenza virus (H7N3 type). Int. J. Agric. Biol. 3:416-418. [Google Scholar]
- 54.Neighbor, N. K., et al. 1994. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult. Sci. 73:1511-1516. [DOI] [PubMed] [Google Scholar]
- 55.Noyce, J. O., H. Michels, and C. W. Keevil. 2007. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 73:2748-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamatsu, M., et al. 2007. Low pathogenicity H5N2 avian influenza outbreak in Japan during the 2005-2006. Vet. Microbiol. 124:35-46. [DOI] [PubMed] [Google Scholar]
- 57.Olsen, C. 2003. Review of the use of statistics in infection and immunity. Infect. Immun. 71:6689-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osweiler, G. 1996. Toxicology. National veterinary medical series. Wiley-Blackwell, Hoboken, NJ.
- 59.Parker, E. R., W. B. Dunham, and W. J. MacNeal. 1944. Resistance of the Melbourne strain of influenza virus to desiccation. J. Lab. Clin. Med. 29:37-42. [Google Scholar]
- 60.Purchase, H. S. 1931. Experiments on the viability of the virus of fowl-plague under trade conditions. Vet. Rec. 11:644-648. [Google Scholar]
- 61.Pyankov, O. V., et al. 2007. Using a bioaerosol personal sampler in combination with real-time PCR analysis for rapid detection of airborne viruses. Environ. Microbiol. 9:992-1000. [DOI] [PubMed] [Google Scholar]
- 62.Rice, E. W., et al. 2007. Chlorine inactivation of highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 13:1568-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roepke, D. C., D. A. Halvorson, S. M. Goyal, and C. J. Kelleher. 1989. An adsorption-elution technique for the recovery of influenza virus from water. Avian Dis. 33:649-653. [PubMed] [Google Scholar]
- 64.Sargeant, J. M., et al. 2009. Quality of reporting in clinical trials of preharvest food safety interventions and associations with treatment effect. Foodborne Pathog. Dis. 6:989-999. [DOI] [PubMed] [Google Scholar]
- 65.Sargeant, J. M., M. E. Torrence, A. Rajic, A. M. O'Connor, and J. Williams. 2006. Methodological quality assessment of review articles evaluating interventions to improve microbial food safety. Foodborne Pathog. Dis. 3:447-456. [DOI] [PubMed] [Google Scholar]
- 66.Sargeant, J., A. Rajic, S. Read, and A. Ohlsson. 2006. The process of systematic review and its application in agri-food public-health. Prev. Vet. Med. 75:141-151. [DOI] [PubMed] [Google Scholar]
- 67.Schaffer, F. L., M. E. Soergel, and D. C. Straube. 1976. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch. Virol. 51:263-273. [DOI] [PubMed] [Google Scholar]
- 68.Scholtissek, C. 1985. Stability of infectious influenza A viruses to treatment at low pH and heating. Arch. Virol. 85:1-11. [DOI] [PubMed] [Google Scholar]
- 69.Schulman, J. L., and E. D. Kilbourne. 1962. Airborne transmission of influenza virus infection in mice. Nature 195:1129-1130. [DOI] [PubMed] [Google Scholar]
- 70.Schulman, J. L. 1967. Experimental transmission of influenza virus infection in mice. IV. Relationship of transmissibility of different strains of virus and recovery of airborne virus in the environment of infector mice. J. Exp. Med. 125:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Senne, D. A., B. Panigrahy, and R. L. Morgan. 1994. Effect of composting poultry carcasses on survival of exotic avian viruses: highly pathogenic avian influenza (HPAI) virus and adenovirus of egg drop syndrome-76. Avian Dis. 38:733-737. [PubMed] [Google Scholar]
- 72.Serena, B. M., C. Terregino, G. Cattoli, and I. Capua. 2006. Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy. Avian Pathol. 35:400-403. [DOI] [PubMed] [Google Scholar]
- 73.Shahid, M., A. Muhammad, H. Sajid, and H. Shamsul. 2009. Avian influenza virus (H5N1); effects of physico-chemical factors on its survival. Virol. J. 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shechmeister, I. 1950. Studies on the experimental epidemiology of respiratory infections. III. Certain aspects of the behavior of type A influenza virus as an air-borne cloud. J. Infect. Dis. 87:128-132. [DOI] [PubMed] [Google Scholar]
- 75.Sherman, E., and B. Primack. 2009. What works to prevent adolescent smoking? A systematic review of the National Cancer Institute's research-tested intervention programs. J. Sch. Health 79:391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiffman, R., et al. 2003. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann. Intern. Med. 139:493-498. [DOI] [PubMed] [Google Scholar]
- 77.Shoham, D. 1993. Biotic-abiotic mechanisms for long-term preservation and reemergence of influenza type A virus genes. Prog. Med. Virol. 40:178-192. [PubMed] [Google Scholar]
- 78.Shope, R. E. 1941. The swine lungworm as a reservoir and intermediate host for swine influenza virus. II. The transmission of swine influenza virus by the swine lungworm. J. Exp. Med. 74:49-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shortridge, K. F., et al. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 80.Stallknecht, D. E., and J. D. Brown. 2009. Tenacity of avian influenza viruses. Rev. Sci. Tech 28:59-67. [DOI] [PubMed] [Google Scholar]
- 81.Stallknecht, D. E., M. T. Kearney, S. M. Shane, and P. J. Zwank. 1990. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 34:412-418. [PubMed] [Google Scholar]
- 82.Stallknecht, D. E., S. M. Shane, M. T. Kearney, and P. J. Zwank. 1990. Persistence of avian influenza viruses in water. Avian Dis. 34:406-411. [PubMed] [Google Scholar]
- 83.Stone, S., et al. 2007. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect. Dis. 7:282-288. [DOI] [PubMed] [Google Scholar]
- 84.Stroup, D., et al. 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008-2012. [DOI] [PubMed] [Google Scholar]
- 85.Sturm-Ramirez, K. M., et al. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas, F. C., T. Ouwerkerk, and P. McKercher. 1982. Inactivation by gamma irradiation of animal viruses in simulated laboratory effluent. Appl. Environ. Microbiol. 43:1051-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tiwari, A., D. P. Patnayak, Y. Chander, M. Parsad, and S. M. Goyal. 2006. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 50:284-287. [DOI] [PubMed] [Google Scholar]
- 88.Tolba, M., and J. Eskarous. 1959. pH stability patterns of some strains of Newcastle disease and fowl-plague viruses. Arch. Mikrobiol. 34:333-338. [DOI] [PubMed] [Google Scholar]
- 89.Tolba, M., and J. Eskarous. 1962. Effect of temperature on the hemagglutination activities and infectivity to chick embryos of different strains of Newcastle disease and fowl-plague viruses. Arch. Mikrobiol. 43:234-244. [DOI] [PubMed] [Google Scholar]
- 90.Tong, A., P. Sainsbury, and J. Craig. 2007. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 19:349-357. [DOI] [PubMed] [Google Scholar]
- 91.USDA APHIS, VS, and Emergency Management and Diagnostics. 2007. Summary of the national highly pathogenic avian influenza (HPAI) response plan, p. 1-86. United States Department of Agriculture, Washington, DC.
- 92.Vandenbroucke, J. P., et al. 2007. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann. Intern. Med. 147:W163-W194. [DOI] [PubMed] [Google Scholar]
- 93.von Elm, E., et al. 2007. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 147:573-577. [DOI] [PubMed] [Google Scholar]
- 94.Vrtiak, O. J. 1967. Study of fowl plague virus resistance in biological and technical material. Bull. Off. Int. Epizoot. 67:969-988. [PubMed] [Google Scholar]
- 95.Wager, E., E. Field, and L. Grossman. 2003. Good publication practice for pharmaceutical companies. Curr. Med. Res. Opin. 19:149-154. [DOI] [PubMed] [Google Scholar]
- 96.Wang, R., S. Lagakos, J. Ware, D. Hunter, and J. Drazen. 2007. Statistics in medicine—reporting of subgroup analyses in clinical trials. N. Engl. J. Med. 357:2189-2194. [DOI] [PubMed] [Google Scholar]
- 97.Weber, T., and N. Stilianakis. 2008. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 57:361-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Webster, R. G. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wells, W. F., and H. W. Browne. 1936. Recovery of influenza virus suspended in air and its destruction by ultraviolet radiation. Am. J. Hyg. 24:407-413. [Google Scholar]
- 101.Yadav, M. P. 1993. Physico-chemical and biological characterization of A/Equi-2 virus isolated from 1987 equine influenza epidemic in India. Int. J. Anim. Sci. 8:93-98. [Google Scholar]
- 102.Yen, I., Y. Michael, and L. Perdue. 2009. Neighborhood environment in studies of health of older adults: a systematic review. Am. J. Prev. Med. 37:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yilmaz, A., U. Heffels-Redmann, and T. Redmann. 2004. Evaluation of the virucidal efficacy of two chemical disinfectants against avian influenza virus A at different temperatures. Arch. Gefluegelkunde 68:50-56. [Google Scholar]
- 104.Zaharia, C. N., S. M. Dumitrescu, and A. Petrescu. 1986. Influence of organic solvents and pH on some characteristics of influenza virus A(H1N1). Note 2. Effect of pH on some spectral and structural characteristics of influenza virus A(H1N1). Virologie 37:55-60. [PubMed] [Google Scholar]
- 105.Zarkov, I. S. 2006. Survival of avian influenza viruses in filtered and natural surface, waters of different physical and chemical parameters. Rev. Med. Vet. 157:471-476. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.