Abstract
Phage therapy is being reexamined as a strategy for bacterial control in medical and other environments. As microorganisms often live in mixed populations, we examined the effect of Escherichia coli bacteriophage λW60 and Pseudomonas aeruginosa bacteriophage PB-1 infection on the viability of monoculture and mixed-species biofilm and planktonic cultures. In mixed-species biofilm communities, E. coli and P. aeruginosa maintained stable cell populations in the presence of one or both phages. In contrast, E. coli planktonic populations were severely depleted in coculture in the presence of λW60. Both E. coli and P. aeruginosa developed phage resistance in planktonic culture; however, reduced resistance was observed in biofilm communities. Increased phage titers and reduced resistance in biofilms suggest that phage can replicate on susceptible cells in biofilms. Infectious phage could be released from mixed-culture biofilms upon treatment with Tween 20 but not upon treatment with chloroform. Tween 20 and chloroform treatments had no effect on phage associated with planktonic cells, suggesting that planktonic phage were not cell or matrix associated. Transmission electron microscopy showed bacteriophage particles to be enmeshed in the extracellular polymeric substance component of biofilms and that this substance could be removed by Tween 20 treatment. Overall, this study demonstrates how mixed-culture biofilms can maintain a reservoir of viable phage and bacterial populations in the environment.
Since the early 1900s, bacteriophage have been used in the treatment of bacterial infections (12). The onset of antibiotic resistance has led to a renewed interest in the use of phage as therapeutic antibacterial agents (23). As bacteria grow naturally in mixed-population biofilms (9), the investigation of phage on mixed-culture biofilms is of importance. Bacterium-phage interactions are important in that they play a vital role in recycling nutrients in nature, regulating microbial ecosystems, and facilitating gene transfers among bacterial populations (6, 38). The large numbers of phage found present in ocean water and soil (>106 PFU ml−1) indicate that phage are ubiquitous in nature; however, only a small fraction of phage are infectious to their host at any given time (38). In natural microbial populations, a balance of infected cells and infectious phage is maintained over time. However, in planktonic lab cultures, the presence of phage results in susceptible cells becoming quickly replaced by resistant populations (5). In this context, we explored the ecology of phage-bacterium interactions in mixed-population planktonic and biofilm bacteria.
Biofilms consist of structured, enclosed communities generally attached to a solid substratum and comprise the majority of bacteria in natural ecosystems (37). The sticky matrix enclosing these communities and facilitating cellular arrangement is called extracellular polymeric substance (EPS) (13). The EPS matrix is a major form of protection from extracellular substances, including antimicrobials and bacteriophage (2). Although polysaccharides are a predominate component, EPS contains a variety of other substances, including proteins, extracellular DNA (41), membrane vesicles (36), and other polymers (10). In Escherichia coli, the composition of its EPS includes membrane lipids, proteins, nucleic acids, and various polysaccharides and also β-1,6-N-acetyl-d-glucosamine as a polysaccharide adhesin (17). In Pseudomonas aeruginosa, the composition of its EPS includes the galactose- and mannose-rich Psl polysaccharide, extracellular DNA, proteins, and other molecules (24). The use of bacteriophage to treat biofilm-related infections may be limited by the ability of phage to penetrate the biofilm EPS matrix (18, 21). Depolymerase enzymes are associated on the surfaces of some phage, thus enabling them to penetrate the EPS and gain access to the phage cell surface receptor (30). However, phage depolymerase enzymes are polysaccharide specific (21). The mixtures of polymers occurring in mixed-population biofilms (29) would hinder the action of depolymerase enzymes and their associated phage.
Aside from EPS production, bacteria within biofilms exhibit altered metabolism and gene expression in comparison to their planktonic counterparts. Notable features seen in monoculture biofilms include slow growth, high cell density, and a diversity of chemical microenvironments. These features, predicted by Costerton, Gilbert, and others (8, 16), have been validated by subsequent research. Examples include patterns of global (42) and microenvironment-specific (24) gene expression, cell signaling (11), and biofilm-specific stress response genes (25, 40). We now realize that, analogous to higher organisms, cell development and specialization occur within biofilm communities (33). In contrast, limited studies have been done with mixed-culture biofilms. Of relevance to the present study, there is evidence that a disinfectant (Betadine)-susceptible organism, Pseudomonas putida, can gain protection by associating with resistant organisms in mixed-culture biofilms (43). In this context, we explored the influence of monoculture and mixed-culture growth using P. aeruginosa and E. coli as model organisms in association with their phages PB-1 and λW60. These two bacteria grow together in several environments, notably, the gastrointestinal tract and polluted water (15). As well, phages PB-1 and λW60 have been proposed as therapeutic agents (4, 28).
MATERIALS AND METHODS
Bacteria and bacteriophages.
E. coli MG1655 (obtained from D. A. Siegele, Texas A&M University) and P. aeruginosa PAO1 (obtained from V. Deretic, University of New Mexico) were grown in Luria-Bertani (LB) broth (Accumedia Manufacturers, Inc., Lansing, MI) at 37°C in a continually rotating shaker water bath (Max Q 2000; Thermo Scientific, Waltham, MA). High-titer bacteriophage stocks of λW60 (ATCC 97537) (28) and PB-1 (ATCC 15692-B3) (4) were prepared using the agar overlay technique described by Adams (1). Lysed overlays were scraped into 10 ml LB broth and phage was eluted at 4°C for 4 h. Cell debris was pelleted at 3,200 × g (Eppendorf centrifuge 5810R V3.5; Eppendorf International, Westbury, NY), and the supernatant was filtered (0.22 μm; Fisher 25-mm syringe filter; Fisher Scientific Inc., Dublin, Ireland) and stored at 4°C.
Planktonic growth.
E. coli and P. aeruginosa were grown for 18 h in LB broth at 37°C and 60 rpm in a rotating shaker bath (Max Q 2000; Thermo Scientific, Waltham, MA). For monoculture, 10 ml LB broth in a 25-ml Erlenmeyer flask was inoculated with 100 μl of an overnight culture of E. coli or P. aeruginosa at a final cell density of 106/ml. For mixed culture, 10 ml LB broth was inoculated with 100 μl each of E. coli and P. aeruginosa. For phage infection, monocultures and mixed cultures were infected at a multiplicity of infection (MOI) of 10 with λW60 and PB-1. Cultures were incubated in a 37°C horizontally shaking water bath at 60 rpm (Forma Scientific model 2564 shaker bath; Forma Scientific Inc., Marietta, OH). At 24-h intervals, cultures were assayed for both CFU and PFU. Both monocultures and mixed cultures were diluted 1:100 in 10 ml fresh LB broth in new 25-ml Erlenmeyer flasks daily. E. coli and P. aeruginosa were differentiated in mixed cultures by growth on LB medium which contained either 20 μg ml−1 cefsulodin (44) (selects for E. coli [M. M. Weber, W. Boswell, and R. J. C. McLean, unpublished data]) or 100 μg ml−1 ampicillin (selects for P. aeruginosa).
Biofilm growth.
Silicone rubber disks, 7 mm diameter by 1 mm (Dapro Rubber Inc., Tulsa, OK) were placed in 250-ml beakers which contained 50 ml LB broth and inoculated with overnight cultures of E. coli and P. aeruginosa at a final cell density of 106/ml. Monocultures and mixed cultures were incubated in a 37°C horizontally shaking bath at 60 rpm for 2 days at 37°C. During this incubation, the biofilm-colonized disks were transferred to fresh medium after 24 h. Biofilm growth was measured using the sonication and dilution plating protocol described by Corbin et al. (7).
Phage infection.
Disks which contained biofilm growth were gently rinsed with 20 ml phosphate-buffered saline (5 mM K2HPO4, 4.5 mM KH2PO4, 150 mM NaCl, pH 7.2) in sterile petri dishes for 1 min to remove unattached cells and placed into individual 28- by 61-mm scintillation vials (VW74504-20; Kimble Chase Life Science, Vineland, NJ) which contained 10 ml LB broth and incubated at 37°C at 60 rpm in a horizontally shaking water bath. For phage infection, λW60, PB-1, or a mixture of both phages was added to broth in vials at a biofilm density equivalent to an MOI of 10. Infected and uninfected biofilm disks were selected at 24-h intervals and assayed for CFU and PFU by dilution plating (7). At 24-h intervals, 9 ml spent medium was removed and 9 ml fresh medium was added to the remaining biofilm growth vials.
Bacteriophage resistance and bacteriophage selection.
Planktonic and biofilm monocultures and mixed cultures were grown and infected as previously described. Four bacterial isolates were chosen from each monoculture and mixed culture following 5 days of incubation. Each bacterial isolate was grown and used as indicator cells to assay the original infecting phage population. To determine if bacteriophage were selected to infect a resistant cell population, supernatant from planktonic and biofilm 5-day cultures was assayed for infectivity using both the initial bacterial stock and 5-day bacterial isolates as indicator cells.
Infectious centers and free and trapped virions.
Planktonic and biofilm monocultures and mixed cultures were grown and infected as previously described. Planktonic and biofilm media from 5-day cultures were assayed before and following filtration (0.22 μm) to determine infectivity due to infectious phage particles and infectious centers, respectively. Biofilm supernatants were mixed with either 0.5 ml chloroform per ml supernatant to lyse infectious centers or 0.1 ml 0.5% Tween 20 (Sigma Aldrich Inc., Saint Louis, MO) per ml supernatant to release EPS-trapped phage. Treated supernatants were assayed for infectivity as previously described.
Electron microscopy.
Medium (10 μl) containing phage was placed on 400 μm Formvar/carbon-coated copper grids (SPI Supplies, West Chester, PA) and allowed to dry for 1 min. Excess liquid was removed with no. 1 Whatman filter paper (Whatman, Inc., Florham Park, NJ), and phage was negatively stained with 1% (wt/vol) uranyl acetate for 5 min. Excess stain was removed using Whatman filter paper, and grids were air dried and stored in sterile petri plates prior to viewing. All grids were examined in a JEOL 1200 EXII transmission electron microscope (TEM) at an accelerating voltage of 120 kV.
RESULTS
E. coli and P. aeruginosa growth in the presence and absence of phage.
E. coli and P. aeruginosa were grown in both mixed and monoculture biofilms to determine if each could grow as well in the presence of the another. In order to differentiate these two organisms, we assessed their antibiotic susceptibility using a number of antibiotics before choosing ampicillin and cefsulodin. In preliminary studies, we observed no deleterious effects of cefsulodin on E. coli or ampicillin on P. aeruginosa. As well, conservative estimates of spontaneous cefsulodin-resistant mutations in P. aeruginosa and ampicillin-resistant mutations in E. coli were 10−5 to 10−6 (data not shown), so the selection medium used appeared to work well to differentiate P. aeruginosa PAO1 and E. coli MG1655.
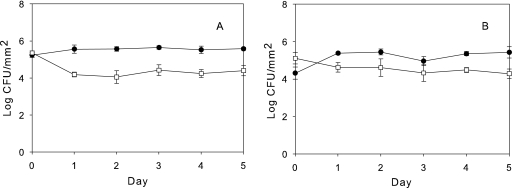
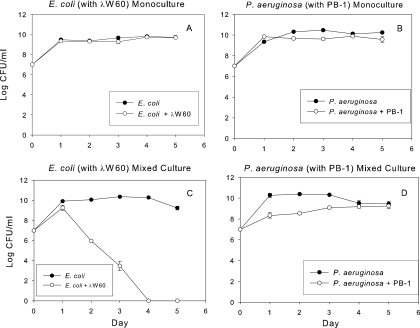
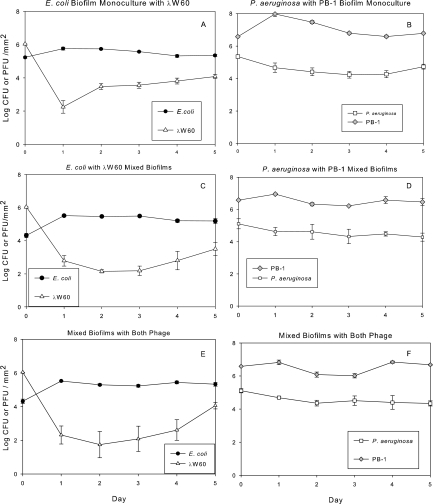
Under the culturing conditions in the present study, in which the medium was replaced daily, these two organisms maintained relatively stable biofilm populations over 5 days in monoculture (Fig. 1 A) and mixed culture (Fig. 1B). Although they were cultured under aerobic conditions with shaking, it is likely that some oxygen depletion occurred in the culture flasks. Oxygen has been recently shown to inhibit the competitive ability of P. aeruginosa (35). Planktonic monoculture populations of E. coli and P. aeruginosa were unaffected by the introduction of their respective phages, λW60 and PB-1 (Fig. 2 A and B), likely due to the onset of phage resistance (Tables 1 and 2). While a population drop due to phage introduction (time zero) undoubtedly occurred, the monoculture planktonic populations had recovered by 24 h due to the growth of phage-resistant populations. In mixed planktonic cultures, E. coli populations dropped considerably in the presence of its phage, λW60 (Fig. 2C), whereas the effect of PB-1 on the P. aeruginosa population was more muted (Fig. 2D). When phage λW60 was introduced into monocultures of E. coli biofilms, the phage titer dropped significantly after 24 h before stabilizing at 2.2 ± 0.62 log10 PFU/mm2 (Fig. 3 A). In contrast, introduction of PB-1 phage to P. aeruginosa biofilm monocultures did not noticeably affect the concentrations of either phage or bacteria (Fig. 3B). Similar patterns of growth were seen under other culture conditions. In mixed bacterial growth (Fig. 3C to F), E. coli and P. aeruginosa levels in biofilms remained relatively constant regardless of whether one or both phages were present (compare Fig. 3C to F with Fig. 1). The E. coli λW60 phage concentrations showed a similar decline in mixed culture after 24 h, as was the case in monoculture E. coli biofilms (compare Fig. 3C and E with A). The P. aeruginosa PB-1 phage remained relatively constant (compare Fig. 3D and F with B). The results suggest mixed-phage infection did not have a synergistic effect on the viability of the biofilm. In addition, the infection of P. aeruginosa with its phage did not have a significant effect on the replication of phage λW60.
FIG. 1.
Mean biofilm bacterial density without bacteriophage. (A) E. coli (•) and P. aeruginosa (□) in monoculture biofilms. (B) E. coli (•) and P. aeruginosa (□) in mixed-culture biofilms. Error bars in all figures represent standard error. Values (±SE) depict log10 CFU mm−2 for bacteria and log10 PFU mm−2 for phages.
FIG. 2.
Planktonic populations of monoculture and mixed-culture E. coli and P. aeruginosa populations in the presence and absence of their respective phage. Values (±SE) depict log10 CFU mm−2 for bacteria and log10 PFU mm−2 for phages.
TABLE 1.
E. coli resistance to λW60 bacteriophage
| Culture type | % of colonies isolated found resistant to original t0 phage |
||
|---|---|---|---|
| E. coli monoculture with λW60 | Mixed culture with λW60 | Mixed culture with both phages (λW60) | |
| Planktonic | 100 | 100 | 100 |
| Biofilm | 0 | 0 | 25 |
TABLE 2.
P. aeruginosa resistance to PB-1 bacteriophage
| Culture type | % of colonies isolated found resistant to original t0 phage |
||
|---|---|---|---|
| P. aeruginosa monoculture with PB-1 | Mixed culture with PB-1 | Mixed culture with both phages (PB-1) | |
| Planktonic | 100 | 100 | 100 |
| Biofilm | 75 | 50 | 75 |
FIG. 3.
Monoculture and mixed-culture E. coli and P. aeruginosa biofilm density in the presence of phages λW60 and PB-1. (A) E. coli and λW60 in monoculture biofilms. (B) P. aeruginosa and λW60 in monoculture biofilms. (C) Mixed-culture E. coli and P. aeruginosa in the presence of λW60. (D) Mixed-culture E. coli and P. aeruginosa in the presence of PB-1. (E) Mixed-culture E. coli and λW60 in the presence of both phages. (F) Mixed-culture P. aeruginosa and PB-1 in the presence of both phages. Values (±SE) depict log10 CFU mm−2 for bacteria and log10 PFU mm−2 for phages.
Bacteriophage resistance.
To determine bacteriophage resistance in host E. coli and P. aeruginosa cultures, individual colonies were isolated from planktonic and biofilm cultures that were exposed to phage for 5 days (referred to as t5 phage), and phage from initial inoculations (t0) were assayed using 5-day cells as indicators. The results demonstrate both E. coli and P. aeruginosa in planktonic growth developed high levels of resistance to phage, whereas, biofilm cultures developed limited levels of resistance (Tables 1 and 2). Overall, in both mixed and monoculture biofilms, E. coli developed less resistance compared to P. aeruginosa. The development of increased phage resistance by P. aeruginosa suggests P. aeruginosa is more susceptible to phage infection than is E. coli in biofilms.
Phage selection.
To determine if phage were selected to infect resistant cells, phage were isolated from planktonic and biofilm cultures, which were exposed to bacteriophage for 5 days, and t5 phage were assayed using 5-day cells as indicators. The results show t5 phage from planktonic cultures could infect t0 cells but not t5 cells (Table 3). However, t5 phage from biofilm cultures could infect both t0 cells and t5 cells. The results of planktonic growth indicate phage were not selected to infect a population of resistant cells (Table 3). In biofilms, however, phage populations were selected which infect resistant cells (Table 4). P. aeruginosa biofilm grown cells show no significant difference in resistance to the t5 phage population compared to t0 phage (Table 1). E. coli biofilm grown cells showed reduced resistance, indicating either susceptibility to new t5 phage populations or the lack of development of phage resistance.
TABLE 3.
λW60 phage selection to infect resistant E. coli
| Culture type | % of colonies isolated found susceptible to t5 phage |
||
|---|---|---|---|
| E. coli monoculture with λW60 | Mixed culture with λW60 | Mixed culture with both phages λW60 and PB-1 | |
| Planktonic | 0 | 0 | 0 |
| Biofilm | 100 | 100 | 100 |
TABLE 4.
PB-1 phage selection to infect resistant P. aeruginosa
| Culture type | % of colonies isolated found susceptible to t5 phage |
||
|---|---|---|---|
| P. aeruginosa monoculture with PB-1 | Mixed culture with PB-1 | Mixed culture with both phages PB-1 and λW60 | |
| Planktonic | 0 | 0 | 0 |
| Biofilm | 33 | 50 | 33 |
Infectivity.
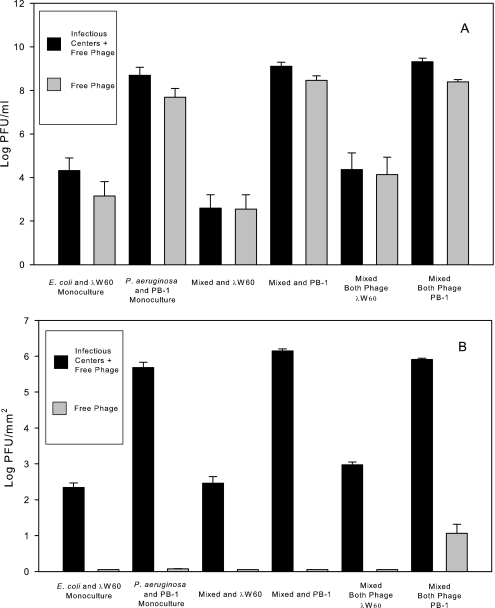
To determine if phage infectivity was present as either virions or infectious centers, planktonic and biofilm cultures were assayed for infectivity on t0 bacteria, both before and after filtration (0.22 μm) to remove infectious centers. The results show that a majority of PFU in planktonic culture are present as infectious virions (Fig. 4 A). In contrast, infectivity within biofilm populations decreased to <10 PFU/ml after filtration. The results suggest that infectivity is present in biofilms as infectious centers and not free virions (Fig. 4B).
FIG. 4.
Infectivity. Phage infectivity in monoculture and mixed-culture planktonic populations (A) was due to free virions (grey). In contrast, the bulk of the infectivity in biofilm populations (B) was due to infectious centers (black). For details, please refer to the text. Values (±SE) depict log10 CFU mm−2 for bacteria and log10 PFU mm−2 for phages.
Effect of chloroform and Tween 20 on infectivity.
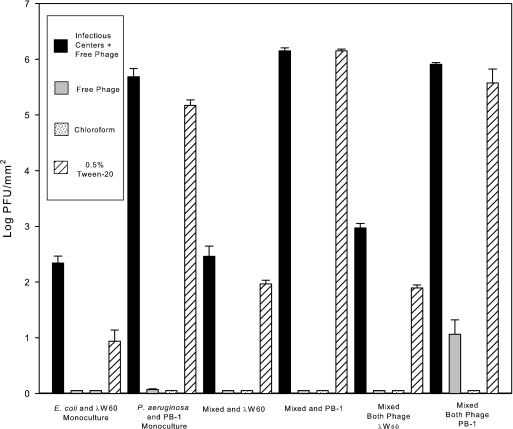
To determine if infectivity in biofilms is indeed infectious centers or infectious virus trapped in EPS, biofilm supernatants were treated with either chloroform to lyse membrane-bound infectious centers or a 0.5% Tween 20 solution to dissolve EPS, filtered (0.22 μm), and assayed for infectivity. Results show that treatment with chloroform did not result in an increase in infectivity (Fig. 5). Treatment with 0.5% Tween 20 resulted in an increase in infectivity similar to that seen in planktonic infected cells. The data indicate λW60 and PB-1 infectious phages are trapped in biofilm EPS.
FIG. 5.
Effect of chloroform and 0.5% (v/v) Tween 20 on infectious phage release from biofilms. Infectious centers within biofilm populations (from Fig. 3) could be released upon treatment with 0.5% Tween 20, but not by chloroform. For details, please refer to the text. Values (±SE) depict log10 CFU mm−2 for bacteria and log10 PFU mm−2 for phages.
Electron microscopy.
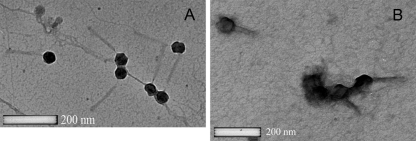
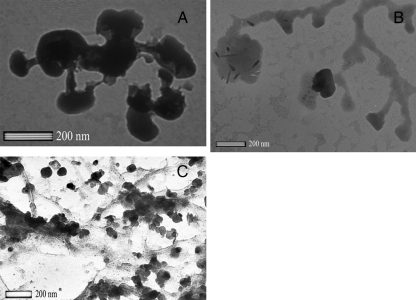
We employed a TEM to see whether phage could be visualized in infected biofilms. Filtered (0.22 μm) and Tween 20-treated biofilm supernatants were negatively stained with uranyl acetate and examined using transmission electron microscopy and compared to phage from planktonic cultures (Fig. 6 and 7). Although the planktonic-grown phage were relatively clean (Fig. 6), biofilm grown phage (Fig. 7A and B) were associated with organic material (presumably EPS). This material could be removed by treatment with Tween 20 (Fig. 7C).
FIG. 6.
Examination with a TEM of negatively stained phages λW60 (A) and PB-1 (B) from planktonic cultures.
FIG. 7.
Examination with a TEM of negatively stained phages λW60 (A) and λW60 plus PB-1 (B) obtained directly from single and mixed-biofilm-infected cultures, showed matrix-associated material with the phage. After treatment with 0.5% Tween 20 (C), most of this material had been removed.
DISCUSSION
This study reports the effect of mixed-phage infection on the density of two-species biofilm bacterial communities. The effect of single phage infection on monoculture and two-species planktonic bacterial communities has been reported (7, 19). Our rationale was that chronic infections can occur as mixed-species biofilms (9) and in that context it would be prudent to target multiple organisms. In the absence of phage and with daily transfers to fresh media, both E. coli and P. aeruginosa grew as well in mixed cultures as in monocultures (Fig. 1 and 2). During prolonged (>72 h) batch cultures in LB broth, P. aeruginosa outcompetes E. coli (M. M. Weber and R. J. C. McLean, unpublished observations). In planktonic monocultures, infection with either or both λW60 and PB-1 bacteriophages resulted in phage multiplication with no significant effect on the cell density of either bacterium (Fig. 2A and B). In mixed planktonic culture, the presence of λW60 caused a dramatic decline in E. coli populations (Fig. 2C). There was also an initial decline in P. aeruginosa populations when this organism was cocultured with E. coli in the presence of its phage, PB-1 (Fig. 2D). These findings are similar to those of Harcombe and Bull (19) who observed that planktonic E. coli grown in coculture with Salmonella enterica did not survive challenge with E. coli phage T5 or T7, due to the combined stresses of lytic phage infection and bacterial competition. Harcombe and Bull considered nutrient competition to be the basis for S. enterica outcompeting E. coli (19). In contrast to S. enterica, P. aeruginosa has been shown to use a number of toxic molecules, often enveloped in outer membrane “predatory vesicles” (3, 26), during microbial competition.
In contrast to the planktonic culture results (Fig. 2), monoculture and mixed-culture biofilm cultures maintained stable populations in the presence of phage (Fig. 3). Although mixed-phage infection did not have a long-term effect on bacterial densities our results show bacterial biofilms support phage multiplication and may act as reservoirs for infectious phage. Phage multiplication in biofilms may act to limit spread of biofilm cells and maintain cells at a density low enough to allow the immune response to clear the infection.
In monoculture planktonic (Fig. 2) and biofilm (Fig. 3) cultures, λW60 and PB-1 were not effective in maintaining low densities of either E. coli or P. aeruginosa. Harcombe and Bull (19) demonstrated that E. coli but not Salmonella species in two-species planktonic cultures experienced competition in the presence of their phage. We observed a similar effect in E. coli exposure to λW60 in planktonic coculture with P. aeruginosa (Fig. 2C). This difference may be due to the particular phages used by Harcombe and Bull (T7 and T5) and the types of bacteria resistance that they selected. Indeed, preliminary data (T. C. Erwin and G. M. Aron, unpublished) show T4 to be effective in maintaining low densities of E. coli in mixed planktonic culture with P. aeruginosa. Thus, the potential use of phage treatment depends in part on the type of phages selected. Our findings are in agreement with others which show that phage infection does not have a long-term effect on the growth of cell density in planktonic culture (19).
The infectivity of both phages maintained high levels in planktonic culture, whereas, in biofilms PB-1 infectivity was lower and λW60 infectivity decreased substantially. Possible explanations include (i) lower phage multiplication and apoptosis, (ii) shedding of phage receptors, and (iii) phage penetration and entrapment of released phage particles in the biofilm EPS matrix. Phage multiplication has been shown to occur at a reduced rate in biofilm cells which are metabolically less active than planktonic cells (37). In addition, apoptosis of phage-infected cells in biofilm communities may lower the number of bacteriophage released into the environment (32). E. coli MG1655 typically sheds its outer membrane during biofilm development and may act as an inhibiting adsorbent for the λW60 J protein receptor which binds to lamB porin. (14). Shedding of lamB-maltose specific proteins were increased in E. coli biofilms, including lamB, malG and malE transporter proteins (14, 34). Lipopolysaccharide (LPS)-containing membrane vesicles are produced by many Gram-negative bacteria, including P. aeruginosa (27), and are found as a component in biofilm matrices (36). Smooth LPS is a specific receptor for bacteriophages E79 and PB-1, and LPS fragments have been shown to inactivate phage particles in suspension (22). Extracellular LPS particles can act as a neutralizing absorbent in the EPS matrix, binding the tail receptors of PB1-like phages.
Anionic EPS components may physically block or through electrostatic repulsion to negatively charged phage proteins prevent phage access to cell surface receptors (39). Phage depolymerases permit ingress through the bacterial EPS layer (30). Phage F116 and GL1 migration through P. aeruginosa biofilms is facilitated by a reduction in alginate viscosity brought about by enzymatic degradation. The phage utilizes a bacterial enzyme by attaching it to its outer coat proteins to assist penetration of biofilm exopolysaccharide (18). Phage PB-1 may have been able to penetrate and therefore replicate within P. aeruginosa biofilms more easily than λW60 in E. coli biofilms. However, phage depolymerases are highly specific to target EPS chemistry (18). In mixed-culture biofilms, the diversity of polymers present and the heterogeneous distribution of bacteria and their polymers throughout a biofilm (29) would be expected to thwart the action of polysaccharide-specific phage depolymerase enzymes (21). λW60 and PB-1 particles bound to EPS were infectious upon release with Tween 20 treatment (Fig. 4), but not chloroform treatment. Tween 20 is a surfactant that dissociates biofilm EPS (20) (Fig. 6), whereas chloroform disrupts cell membranes. The data suggest binding of infectious phage particles to the biofilm matrix, rather than cell entrapment. Similar findings were reported by Resch et al. which showed Staphylococcus aureus phage SA113 eluting from S. aureus biofilms (31). The inability of phage to fully penetrate biofilms by entrapment in EPS may be beneficial by providing a population of susceptible cells. Uninfected biofilm cells can act as a phage host reservoir and lead to new phage replication as susceptible cells are released from the biofilm. As described previously, P. aeruginosa can compete with other organisms through the production of predatory membrane vesicles (3, 26). Since membrane vesicles are an integral component of biofilm matrices (36), one might anticipate competition to be enhanced in biofilms. As is the case with phage, the EPS component of mixed-population biofilm matrices may also inhibit access of P. aeruginosa membrane vesicles to the E. coli cell surface. Regardless of its competitive ability under laboratory conditions, E. coli is a very successful organism in its natural gastrointestinal environment (15) wherein it is exposed to competition from multiple microorganisms, the host, and also phage. In this context, it is certainly conceivable that the biofilm mode of growth might explain part of the ecological success of E. coli.
Planktonic cells develop complete phage resistance due to mutation of cell surface receptor genes, altered growth physiology, and natural selection (19). The rapid onset of resistance is readily observed in planktonic cultures but less so in biofilm populations (Tables 1 and 2). In a natural ecosystem, a lack of susceptible host cells as suggested by our planktonic results (Tables 1 and 2) might be expected to lead to an extinction of the phage as individual virions become physically or chemically inactivated. As well, multiple stressors, including phage predation and bacterial completion, might be expected to have a deleterious effect on planktonic populations. In this context, the biofilm mode of growth provides a mechanism whereby the component bacteria can persist in spite of lytic phage (Fig. 3), as the phage are entrapped in the biofilm matrix (Fig. 4 and 6). From the phage perspective, biofilms can serve as a phage reservoir and provide a continuous supply of host organisms as susceptible cells are released by the biofilms (33) (Fig. 3). Thus, biofilm ecology has relevance to microbial competition, phage therapy (12, 39), and persistence of phage (38) and bacteria in the environment.
Acknowledgments
This project was funded through the Homer Prince Microbiology Endowment to G.M.A. and a grant from the Norman Hackerman Advanced Research Program of the Texas Higher Education Coordinating Board to R.J.C.M. (003615-0037-2007).
We thank N. Dharmasiri, J. D. McCracken, I. J. Molineux, T. Newman, M. M. Weber, and L. Wiggins for their helpful advice.
Footnotes
Published ahead of print on 3 December 2010.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York, NY.
- 2.Azeredo, J., and I. W. Sutherland. 2008. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 9:261-266. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge, T. J., S. A. Makin, J. L. Kadurugamuwa, and Z. Li. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, D. E., and D. Robinson. 1968. The structure and infective process of a contractile Pseudomonas aeruginosa bacteriophage. J. Gen. Virol. 3:247-254. [DOI] [PubMed] [Google Scholar]
- 5.Cairns, B. J., A. R. Timms, V. A. Jansen, I. F. Connerton, and R. J. Payne. 2009. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog. 5:e1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chibani-Chennoufi, S., A. Bruttin, M. L. Dillmann, and H. Brüssow. 2004. Phage-host interactions: an ecological perspective. J. Bacteriol. 186:3677-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbin, B. D., R. J. C. McLean, and G. M. Aron. 2001. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can. J. Microbiol. 47:680-684. [PubMed] [Google Scholar]
- 8.Costerton, J. W., et al. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 10.Davey, M. E., N. C. Caiazza, and G. A. O'Toole. 2003. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Kievit, T. R., R. Gillis, S. Marx, C. Brown, and B. H. Iglewski. 2001. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 67:1865-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d'Herelle, F. 1931. Bacteriophage as a treatment in acute medical and surgical infections. Bull. N. Y. Acad. Med. 7:329-348. [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eboigbodin, K. E., and C. A. Biggs. 2008. Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromolecules 9:686-695. [DOI] [PubMed] [Google Scholar]
- 15.Fabich, A. J., et al. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, P., J. Das, and I. Foley. 1997. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 11:160-167. [DOI] [PubMed] [Google Scholar]
- 17.Goller, C., X. Wang, Y. Itoh, and T. Romeo. 2006. The cation-responsive protein NhaR of Escherichia coli activates pgaABCD transcription, required for production of the biofilm adhesin poly-beta-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 188:8022-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanlon, G. W., S. P. Denyer, C. J. Olliff, and L. J. Ibrahim. 2001. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 67:2746-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harcombe, W. R., and J. J. Bull. 2005. Impact of phages on two-species bacterial communities. Appl. Environ. Microbiol. 71:5254-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heineman, R. H., and J. J. Bull. 2007. Testing optimality with experimental evolution: lysis time in a bacteriophage. Evolution 61:1695-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes, K. A., I. W. Sutherland, and M. V. Jones. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039-3047. [DOI] [PubMed] [Google Scholar]
- 22.Jarrell, K. F., and A. M. Kropinski. 1981. Pseudomonas aeruginosa bacteriophage phi PLS27-lipopolysaccharide interactions. J. Virol. 40:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, T. K., and J. J. Collins. 2009. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. U. S. A. 106:4629-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma, L., et al. 2009. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5:e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mah, T. F., et al. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 26.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422-425. [DOI] [PubMed] [Google Scholar]
- 27.Mashburn-Warren, L. M., et al. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69:491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merril, C. R., et al. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U. S. A. 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neu, T. R., and J. R. Lawrence. 1997. Development and structure of microbial biofilms in river water studied by confocal laser scanning microscopy. FEMS Microbiol. Ecol. 24:11-25. [Google Scholar]
- 30.Petter, J. G., and E. R. Vimr. 1993. Complete nucleotide sequence of the bacteriophage K1F tail gene encoding endo-N-acylneruaminidase (endo-N) and comparison to an endo-N homolog in bacteriophage PK1E. J. Bacteriol. 175:4354-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resch, A., B. Fehrenbacher, K. Eisele, M. Schaller, and F. Gotz. 2005. Phage release from biofilm and planktonic Staphylococcus aureus cells. FEMS Microbiol. Lett. 252:89-96. [DOI] [PubMed] [Google Scholar]
- 32.Rice, K. C., and K. W. Bayles. 2008. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 72:85-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schembri, M. A., K. Kjærgaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-267. [DOI] [PubMed] [Google Scholar]
- 35.Schertzer, J. W., S. A. Brown, and M. Whiteley. 2010. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol. Microbiol. 77:1527-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schooling, S. R., and T. J. Beveridge. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoodley, P., D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 38.Suttle, C. A. 2007. Marine viruses-major players in the global ecosystem. Nat. Rev. Microbiol. 5:801-810. [DOI] [PubMed] [Google Scholar]
- 39.Teplitski, M., and K. Ritchie. 2009. How feasible is the biological control of coral diseases? Trends Ecol. Evol. 24:378-385. [DOI] [PubMed] [Google Scholar]
- 40.Weber, M. M., C. L. French, M. B. Barnes, D. A. Siegele, and R. J. C. McLean. 2010. A previously uncharacterized gene, yjfO (bsmA) influences Escherichia coli biofilm formation and stress response. Microbiology 156:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 42.Whiteley, M., et al. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 43.Whiteley, M., J. R. Ott, E. A. Weaver, and R. J. C. McLean. 2001. Effects of community composition and growth rate on aquifer biofilm bacteria and their susceptibility to betadine disinfection. Environ. Microbiol. 3:43-52. [DOI] [PubMed] [Google Scholar]
- 44.Zhao, W. H., Z. Q. Hu, and T. Shimamura. 2008. Potency of IMP-10 metallo-beta-lactamase in hydrolysing various antipseudomonal beta-lactams. J. Med. Microbiol. 57:974-979. [DOI] [PubMed] [Google Scholar]