Abstract
Terrestrial rocks, petroleum reservoirs, faults, coal seams, and subseafloor gas hydrates contain an abundance of diverse methanoarchaea. However, reports on the isolation, purification, and characterization of methanoarchaea in the subsurface environment are rare. Currently, no studies investigating methanoarchaea within fault environments exist. In this report, we succeeded in obtaining two new methanogen isolates, St545MbT of newly proposed species Methanolobus chelungpuianus and Methanobacterium palustre FG694aF, from the Chelungpu fault, which is the fault that caused a devastating earthquake in central Taiwan in 1999. Strain FG694aF was isolated from a fault gouge sample obtained at 694 m below land surface (mbls) and is an autotrophic, mesophilic, nonmotile, thin, filamentous-rod-shaped organism capable of using H2-CO2 and formate as substrates for methanogenesis. The morphological, biochemical, and physiological characteristics and 16S rRNA gene sequence analysis revealed that this isolate belongs to Methanobacterium palustre. The mesophilic strain St545MbT, isolated from a sandstone sample at 545 mbls, is a nonmotile, irregular, coccoid organism that uses methanol and trimethylamine as substrates for methanogenesis. The 16S rRNA gene sequence of strain St545MbT was 99.0% similar to that of Methanolobus psychrophilus strain R15 and was 96 to 97.5% similar to the those of other Methanolobus species. However, the optimal growth temperature and total cell protein profile of strain St545MbT were different from those of M. psychrophilus strain R15, and whole-genome DNA-DNA hybridization revealed less than 20% relatedness between these two strains. On the basis of these observations, we propose that strain St545MbT (DSM 19953T; BCRC AR10030; JCM 15159) be named Methanolobus chelungpuianus sp. nov. Moreover, the environmental DNA database survey indicates that both Methanolobus chelungpuianus and Methanobacterium palustre are widespread in the subsurface environment.
The strictly anaerobic, methane-producing archaea exhibit marked habitat diversity. They have been isolated from virtually every habitat in which anaerobic biodegradation of organic compounds occurs, including freshwater and marine sediments, digestive and intestinal tracts of animals, and anaerobic waste digesters (6, 12, 20, 60). Methanogens have also been isolated from extreme environments such as geothermal springs, both shallow and deep-sea hydrothermal vents, cold waters of Antarctica, and salty solar salterns (5, 16, 19, 38, 51).
Subsurface ecosystems have been postulated to possess more than 70% of the Earth's biomass (59). Evidence suggests that viable methanogenic archaea are present deep underground in rocks and surrounding groundwater (44). In this habitat, methanogens may represent chemoautolithotrophic organisms that initiate food chains in the oligotrophic deep subsurface environment at the expense of geologically produced molecular hydrogen. Although methanoarchaea comprise a major group of microorganisms that live deep underground, few have been characterized. The first methanogen isolated from the deep subterranean biosphere was Methanobacterium subterraneum, which was isolated from deep granitic groundwater at depths of 68, 409, and 420 m (27). PCR-amplified 16S rRNA gene sequence studies have indicated that Methanobacterium spp. dominate in the 4- to 5-kilometer-deep fault at Drietontein Consolidated Mine in Johannesburg, South Africa (37).
Investigations of microbial communities have used 16S rRNA gene sequence analysis to characterize archaea in the deep subsurface such as in terrestrial rocks (45, 52), petroleum reservoirs (8, 9, 18, 39, 40, 42, 58), faults (37, 49, 57), coal seams (11, 48), gas fields (36), and subseafloor gas hydrates (17, 33, 35). These studies have demonstrated the abundance and diversity of methanoarchaea. However, reports of the isolation, purification, and characterization of methanoarchaea in the subsurface environment are rare. Currently, no studies investigating methanoarchaea within fault environments exist.
Taiwan is located at a boundary between the Philippine Sea Plate and the Eurasian Plate. Continuous convergence between these two plates has resulted in an arc-continent collision that has been further inducing the uplift of the Eocene-to-Pleistocene strata above the sea level along a series of east-dipping thrusts for the past 5 million years (54). On 21 September 1999, the magnitude 7.7 Chi-Chi earthquake devastated central Taiwan. The severity of this earthquake triggered a geostructural survey of the faults of Taiwan and the initiation of the Taiwan Chelungpu Drilling Project (TCDP; details in www.idsp-online.de/sites/chelungpu/news/news.html). Coring was used to retrieve samples from the active fault zone that triggered the Chi-Chi earthquake (56). The coring penetrated through Cholan, Chinshui, and Kueichulin formations to a depth of 2,000 m below land surface (mbls). These rock formations are mainly composed of sandstone and shale in different proportions. Two major fracture zones were encountered: one was located within the Chinshui formation at around 1,110 mbls and the other at the boundary between the Kueichulin formation and the underlying Cholan formation at around 1,750 mbls. At depths greater than 1,750 mbls, the Cholan formation was displaced underneath the Kueichulin formation by overthrusting along the deeper fracture zone (57).
This drilling project provided a great opportunity to investigate subsurface microbiology. In this report, we succeeded in obtaining two new methanogen isolates, St545MbT (DSM 19953; JCM 15159; BCRC AR10030) of newly proposed species Methanolobus chelungpuianus and Methanobacterium palustre FG694aF (JCM 15160; BCRC AR10031), from the Chelungpu fault.
MATERIALS AND METHODS
Sample core handling.
Coring was performed with a diamond-coated hollow bit with an inner diameter of 8.3 cm (57). Drilling fluid consisted of water pumped from an adjacent river, and bentonite and barite (BaSO4) were used to reduce heat, remove rock fragments generated during pulverization, and prevent groundwater intrusion. Rhodamine dye was added to the drilling fluid at a concentration of 50 ppm (wt/vol) to monitor contamination introduced during the coring (57). Each core section, with a maximum length of around 3 meters, was brought to the surface using a wireless core catcher. Intact cores without lithological interclasts or structural features were chosen and sectioned into a length of 20 to 30 cm for geomicrobiological study and prepared by Pei-Ling Wang at the Institute of Oceanography, National Taiwan University. The sectioned core was anaerobically handled and immediately transported to the laboratory in an anaerobic glove chamber within 3 h (57). Anaerobic samples from the central part of the cores at depths ranging from 545 mbls to 1,675 mbls were trimmed and prepared inside a Coy anaerobic chamber by Pei-Ling Wang; these samples were given to us for further enrichment, isolation, and characterization.
Media and culture techniques.
The modified anaerobic technique of Hungate was utilized (1, 50). Sterilized media were prepared in an oxygen-free N2-CO2 (4:1) atmosphere. The MB medium (pH 7.0) consisted of deionized water with MgCl2·6H2O (1.0 g/liter), KCl (0.5 g/liter), NaCl (5 g/liter), CaCl2·2H2O (0.1 g/liter), K2HPO4 (0.4 g/liter), NH4Cl (1.0 g/liter), cysteine-HCl (0.25 g/liter), NaHCO3 (4.0 g/liter), yeast extract (2 g/liter), tryptone (2 g/liter), and resazurin (0.001 g/liter). Vitamin and trace element solutions without tungstate were added to a final concentration of 1% (vol/vol) (32). An MB/W medium was prepared by prepared by adding a 1% (vol/vol) trace element solution containing tungstate (Na2WO4, 0.3 mg/liter) to MB medium (32). MM medium was prepared using MB medium without the addition of yeast extract and tryptone. All of the constituents except sulfide were dissolved in water, boiled, and cooled under an oxygen-free atmosphere of N2-CO2 (4:1). The medium was distributed to serum bottles (Wheaton Scientific, Millville, NJ) or Hungate tubes (Belleco Glass, Inc., Vineland, NJ) under the same atmosphere. The anaerobic tubes were then sealed and autoclaved at 121°C for 20 min. Sodium sulfide from a sterilized anoxic stock solution was added to a final concentration of 1 mM before inoculation. For solid roll tube medium, 20 g/liter of agar was added. To measure the effect of pH on growth, the ratio of N2 to CO2 in the gas phase and the concentration of NaHCO3 in the medium were modified to obtain pH values between 5.6 and 8.3.
Enrichment and isolation.
Enrichment was started immediately after the sample was brought to our laboratory. In an anaerobic chamber, the powdered core samples were added to 160-ml serum bottles that contained 45 ml of MB/W or MM/W medium with sodium formate (50 mM) plus acetate (20 mM), acetate (50 mM), or methanol (50 mM) as the catabolic substrate. These enrichment cultures were incubated at 37 and 45°C for 1 month (see Table S1 in the supplemental material). Methane production was determined by gas chromatography with a flame ionization detector (29). Trace amounts of methane were detected in most of the enrichment cultures after subtransfers with a 1:1 ratio and incubation at 37°C for an additional month. Among them, enrichments of sample St545, from sandstone at a depth of 545 m, with methanol as a substrate, and sample FG694, from a fault gouge at a depth of 694 m, with formate plus acetate as a substrate, accumulated the largest amounts of methane. These two cultures were further subtransferred (1:20) into MB/W medium in Hungate tubes with the same substrate and vancomycin (an antibiotic to inhibit bacteria). This procedure was repeated for four successive transfers. The culture was then diluted and transferred into molten MB agar, and the roll tube technique was performed. Colonies grew on the inner wall of the glass tube after 2 to 3 weeks. Colonies were picked with disposable, sterilized inoculation needles in a Coy anaerobic chamber and transferred to anaerobic tubes containing 5 ml of MB/W medium. Cultures from a single colony were further incubated at 37°C for 1 to 2 weeks. Two methane-producing cultures, St545Mb and FG694aF, derived from two morphologically distinct colonies were further purified with repeated serial dilutions with vancomycin until they were free of contamination with nonmethanogenic bacteria. The axenic nature of the culture was confirmed by microscopic examination, the presence of single colony type in roll tubes, and the absence of growth in anaerobically prepared Bacto thioglycolate (TGC) medium.
Determination of catabolic substrates.
The catabolic substrates tested under N2-CO2 (4:1) were sodium formate (100 mM), sodium acetate (50 mM), trimethylamine (40 mM), dimethylamine (111 mM), methanol (50 mM), ethanol (48 mM), 2-propanol (48 mM), isobutanol (48 mM), 2-butanol (48 mM), methylamine (50 mM), and methyl sulfide (50 mM). H2 was tested by pressurizing the culture tubes with H2 (100%, 200 kPa). Utilization of these substrates in MB/W media was determined by monitoring methane production. Methane production was determined by gas chromatography with flame ionization detection (29).
Antibiotic susceptibility.
Sensitivity to ampicillin, penicillin, spectinomycin, kanamycin, tetracycline, and chloramphenicol (100 μg/ml of each) was tested in MB/W medium with sodium formate (100 mM) at 37°C. Growth was determined by measuring methane production.
Determination of growth rates.
Specific growth rates were calculated from methane production, which was analyzed by linear regression of the logarithm of the total amount of the methane that accumulated with time (29, 46). Inocula were grown under conditions similar to the experimental conditions.
Microscopy.
An Olympus BH-2 microscope was used for phase-contrast microscopy. Preparations for negative staining were performed as described previously (31). Electron micrographs were obtained using models JEM-1200EXII and 200cx (Joel, Ltd.).
Whole-cell protein profile.
The mid-log-phase cells of Methanolobus chelungpuianus strain St545MbT, Methanolobus psychrophilus strain R15, Methanolobus vulcani, and Methanohalophilus portucalensis strain FDF1T were harvested and resuspended in loading buffer containing 4% sodium dodecyl sulfate at a ratio of 1 ml buffer per optical density (OD) unit. An OD unit was the amount of cells found in 1 ml of culture with an absorbance of 1.0. SDS-PAGE was performed as described by Laemmli (28). Gels were stained with Coomassie blue R-250.
Phylogenetic analysis.
Chromosomal DNA preparations, PCR amplification of 16S rRNA genes, and sequencing were performed as described by Wu et al. (62). PCR amplification primers specific for methanogen were forward primer coccus 1 (5′-CGACTAAGCCATGCGAGTC-3′) and reverse primer reverse 3 (5′-GTGACGGGCGGTGTGTGCAAG-3′). The sequences corresponded to positions 2 to 20 and 1309 to 1329, respectively, in the 16S rRNA nucleotide sequence of Methanofollis formosanus strain ML15T (AY186542). A single PCR product of the expected size was detected from both cultures. The estimated 1,300-bp amplified fragments were further purified, cloned into the pGEM-T Easy vector, and sequenced. The 1,309-bp rRNA gene sequence from strain St545Mb (positions 44 to 1348 in the 16S rRNA nucleotide sequence of Methanolobus psychrophilus strain R15) and the 1,264-bp rRNA gene sequence from strain FG694aF (positions 87 to 1349 in the 16S rRNA nucleotide sequence of Methanobacterium palustre strain F) were obtained and compared with those of related methanogens. Gene sequences of the archaea used were obtained from the Ribosomal Database Project (RDP) Seqmatch (http://rdp.cme.msu.edu/seqmatch/seqmatch_intro.jsp) (10) and NCBI GenBank (http://blast.ncbi.nlm.nih.gov/GenBank). Multiple sequence alignments were analyzed using ClustalW of MEGA4 (http://www.megasoftware.net/) (53), and phylogenetic trees were created using the neighbor-joining method of MEGA4 with bootstrap test of phylogeny with 500 replicates.
DNA-DNA hybridization and DNA G+C content.
Cells of strain St545Mb, Methanolobus psychrophilus R15, Methanolobus vulcani DSM3029T, and Methanohalophilus portucalensis FDF1T were harvested during the late exponential phase and used for DNA isolation as described above. DNA-DNA hybridization experiments were performed by using the dot blot technique as described previously (32) with a DIG DNA labeling and detection kit (Roche Applied Science, Indianapolis, IN). Four target DNA samples were quantified by A260, were diluted to 25, 50, 75, and 100 ng/μl, and were denatured by boiling at 100°C for 10 min. Two microliters of each denatured DNA sample (50, 100, 150, and 200 ng/μl) was blotted on a Biodyne nylon membrane (Pall Corporation, Port Washington, NY). Two membranes containing the same DNA samples were reassociated with the labeled DNA probes from strain St545Mb and strain R15 at 45°C overnight. Hybridization signals were detected and analyzed by TINA software (version 2.09e; Raytest Isotopenmeßgeräte). Triplicate tests were performed for each assay, and self-hybridization of the probe with homologous target DNA was set to 100%.
The DNA G+C content was determined by high-performance liquid chromatography after enzymatic hydrolysis (34) by the Bioresource Collection and Research Center (BCRC) in the Food Industry Research and Development Institute (FIRDI) at Taiwan.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of strain FG694aF and strain St545MbT determined in this study have been deposited in the GenBank database under accession numbers EU293795 and EU293796, respectively.
RESULTS AND DISCUSSION
Methanogen enrichment from core samples of the Chelungpu fault.
The Taiwan Chelungpu Drilling Project (TCDP) near Dai-Keng penetrated through the Pliocene-Pleistocene sedimentary rocks to a depth of 2,000 mbls and encountered the Chelungpu and San-Yi fault zones (57). Anaerobic samples from the central part of the cores at depths ranging from 545 mbls to 1,675 mbls were trimmed and prepared in a Coy anaerobic chamber. Portions of powdered cores were added to serum bottles with methanogen basal medium (MB/W) or MM/W medium with sodium formate plus acetate, acetate, and methanol as the catabolic substrates.
After 1 month of incubation at 37 or 45°C, trace amounts of methane (below 20 μmol per bottle) were detected in most of the enrichment cultures (see Table S1 in the supplemental material). Among these cultures, enrichments of sample St545M, from sandstone at a depth of 545 m using methanol as a substrate, sample FG694F, from a fault gouge at a depth of 694 m using formate as a substrate, and sample SS1033F, from siltstone at a depth of 1,033 m using formate plus acetate as a substrate, accumulated the largest amounts of methane (2,152, 1,246, and 113 μmol, respectively). These three cultures were further transferred (1:20) to MB/W medium in Hungate tubes with the respective substrate and vancomycin (100 μg/ml) to inhibit bacterial growth. Methane was not detected upon transfer of culture SS1033F.
After successive transfers, serial dilution, and the roll tube technique (30), a small transparent hemispherical colony (<2 mm) was selected from the enrichment sample, collected from sandstone at a depth of 545 m, with methanol as a substrate. Upon inoculation into thioglycolate (TGC) medium, no growth was observed, indicating that the culture was free of anaerobic heterotrophic bacteria (30). This methanoarchaeal isolate (strain St545Mb) was deposited into DSMZ as DSM 19953, JCM as JCM 15159, and the BCRC (Taiwan) as AR10030. Another methane-producing isolate (strain FG694aF) was isolated from the enrichment of the sample, collected from a fault gouge at a depth of 694 m, with formate as a substrate. After further purification using the roll tube technique, a white opaque colony was selected and cultivated. A TGC medium growth test showed no trace of microbial growth. Strain FG694aF was deposited into JCM as JCM 15160 and BCRC as AR10031.
Methanolobus chelungpuianus St545MbT.
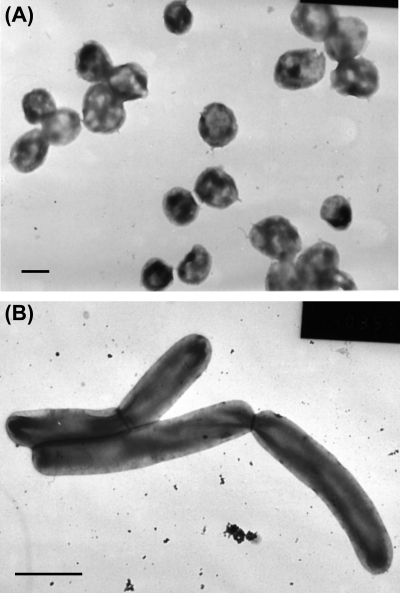
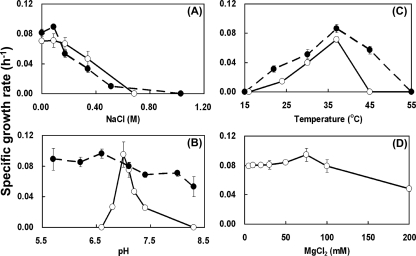
Strain St545MbT was isolated from the enrichment of a sandstone sample collected at a depth of 545 m with methanol as a substrate. Cells of strain St545MbT were irregular cocci with diameters of 0.5 to 1.0 μm (Fig. 1A). The cells stained Gram negative and lysed upon addition of 0.01% (wt/vol) SDS, indicating that a proteinaceous cell wall is present (4). The DNA G+C content of strain St545MbT is 48.3 mol%. Strain St545MbT can use methanol and trimethylamine as catabolic substrates but not formate, acetate, dimethylamine, ethanol, 2-propanol, isobutanol, 2-butanol, methylamine, or methyl sulfide. Cells of strain St545MbT grew over the NaCl range of 0 to 0.68 M (Fig. 2A), a pH range of 6.8 to 7.4 (Fig. 2B), a temperature range of 24 to 45°C (Fig. 2C), and a Mg2+ range of 5 to 200 mM (Fig. 2D). The optimal growth conditions for strain St545MbT were 37°C, pH 7.0, 0.00 to 0.08 M NaCl, and 75 mM MgCl2. The specific growth rates of strain St545MbT in MM/W and MB/W with methanol (50 mM) as a substrate were 0.041 h−1 and 0.091 h−1, respectively. Thus, strain St545MbT is a mesophilic, neutrophilic, methylotrophic methanoarchaea.
FIG. 1.
Electron micrographs of negatively stained cells of strain St545MbT (A) (bar, 0.5 μm) and strain FG694aF (B) (bar, 1.0 μm).
FIG. 2.
Influence of NaCl (A), pH (B), temperature (C), and MgCl2 (D) on the growth of strains St545MbT (○) and FG694aF (•) in MB/W medium with methanol or formate as the catabolic substrate, as indicated by specific growth rates. Specific growth rates were calculated from rates of methane production and are the means of triplicate cultures.
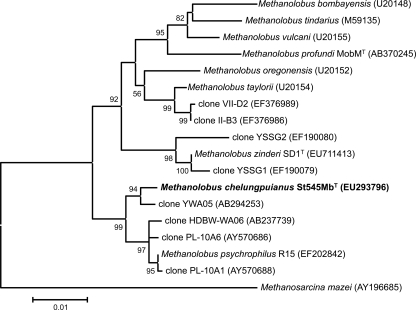
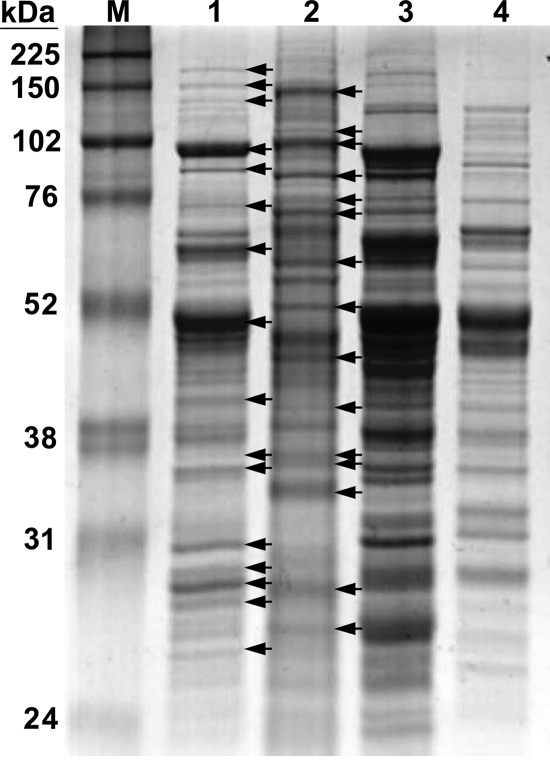
The 16S rRNA gene sequence (1,309 bp, EU293796) of strain St545MbT was determined, and a phylogenetic tree was constructed using a selection of sequences from related methanogens and the environmental DNA library from the GenBank (Fig. 3). The 16S rRNA gene sequence analysis placed strain St545MbT in the family Methanosarcinaceae and close to Methanolobus psychrophilus (sequence similarity, 99.0%), Methanolobus taylorii (97.5%), Methanolobus oregonensis (97.1%), Methanolobus zinderi SD1T (97.3%), M. vulcani (97.1%), Methanolobus tindarius (96.4%), Methanolobus profundi strain MobMT (96.3%), and Methanolobus bombayensis (95.9%). A similarity of less than 98% in a 16S rRNA sequence is evidence for separate species (23). The 16S rRNA gene sequence similarity between strain St545MbT and Methanolobus psychrophilus was higher than 98%. The psychrophilic methanogen Methanolobus psychrophilus (isolated from the Zoige wetland of the Tibetan plateau) can grow over a temperature range of 0 to 25°C, with the optimal growth temperature at 18°C, and utilizes methyl sulfide as a catabolic substrate (64); by comparison, strain St545MbT, isolated from subsurface sandstone at 30°C from the Taiwan Chelungpu fault, grows over the temperature range of 24 to 45°C, with an optimal growth temperature of 37°C (Table 1; Fig. 2C), and cannot utilize methyl sulfide (Table 1). The whole-cell protein profile analysis of strain St545MbT, M. psychrophilus, M. vulcani, and M. portucalensis FDF1T revealed that the protein profile of St545MbT was obviously distinct from the those of the other three strains (Fig. 4). Additionally, in DNA-DNA hybridization experiments, the DNA of strain St545MbT exhibited 14 to 20% hybridization with the DNA of M. psychrophilus strain R15. Therefore, based on the differential characteristics listed in Table 1, combined with the whole-cell protein analysis and DNA-DNA hybridization data, we propose that strain St545MbT represents a novel Methanolobus species and that it be named Methanolobus chelungpuianus sp. nov.
FIG. 3.
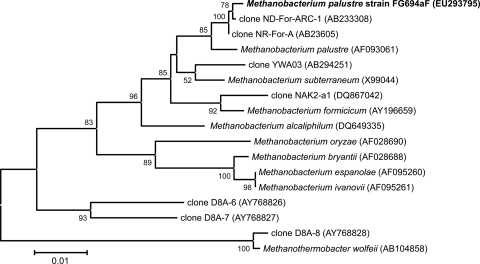
Phylogenetic analysis of 16S rRNA genes showed the relationships between strain St545MbT and related methanogen isolates and environmental clones. Bootstrap values at nodes are the percentages of 500 replicates. The accession number for each reference species is shown in parentheses. The bar indicates evolutionary distance.
TABLE 1.
Characteristics of Methanolobus species and strain St545MbT
| Characteristic | Result forc: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain St545MbT (this study) | M. bombayensis (22) | M. oregonensis (5) | M. taylorii (41) | M. tindarius (26) | M. vulcani (21) | M. psychrophilus (64) | M. profundi (36) | M. zinderi (11) | |
| Cell size (μm) | 0.5-0.7 | 1.0-1.5 | 1.0-1.5 | 0.5-1.0 | ND | 1.0-1.25 | 0.7-1.2 | 0.9-1.2 | 0.5-1.0 |
| Substratea | M, T | M, T, Ds, Ms | M, T, Ds, Ms | M, Ma, Ms | M, Ma | M, Ma | M, T, Ms | M, Ma, D, T | M, Ma, D, T |
| Temp range (°C) (optb) | 24-45 (37) | 13-46 (37) | 7-44 (34) | 11-40 (39) | 7-50 (28) | 13-45 (40) | 0-25 (18) | 9-37 (30) | 25-50 (40-50) |
| pH range (opt) | 6.8-7.4 (7.0) | 6.5-8.0 (7.2) | 7.4-9.5 (8.6) | 7.0-8.6 (8.0) | 5.5-8.0 (6.5) | 6.0-7.5 (7.2) | 6.0-8.0 (ND) | 6.1-7.8 (6.5) | 6.0-9.0 (7.0-8.0) |
| Na+ concn range (M) (opt) | 0.00-0.68 (0.00-0.17) | 0.3-2.0 (0.50) | 0.1-1.6 (0.50) | 0.2-1.2 (0.60) | 0.06-1.3 (0.49) | 0.1-1.2 (0.50) | 0.01-0.8 (ND) | 0.1-1.0 (0.35) | 0.2-0.6 (0.05-1.8) |
| Mg2+ concn range (mM) (opt) | 5-200 (75) | 30-80 (33) | 13-220 (40) | 13-40 (40) | ND (ND) | 0.5-85 (13) | ND (ND) | 10-400 (15-25) | 10-≥200 (100-≥200) |
| G+C (mol%) | 48.3 | 39 | 41 | 41 | 46 | 39 | 44.9 | 42.4 | 41-43 |
| Habitat | Chelungpu fault (545 m) | Arabian Sea sediments | Anoxic aquifer 3 m deep near Alkali Lake | Estuarine sediments from San Francisco Bay | Sediments | Sea sediments near Vulcano Island, Italy | Zoige wetland of the Tibetan plateau | Deep subsurface sediments in natural gas field (350 m) | Deep subsurface coal seam (926 m) |
M, methanol; Ma, methylamine; D, dimethylamine; T, trimethylamine; Ds, dimethyl sulfide; Ms, methylsulfides.
opt, optimum.
The source or reference is in parentheses. ND, not determined.
FIG. 4.
Whole-cell protein profile of Methanolobus and Methanohalophilus species determined by SDS-PAGE. Lane 1, Methanolobus chelungpuianus strain St545MbT; lane 2, Methanolobus psychrophilus strain R15; lane 3, Methanolobus vulcani; lane 4, Methanohalophilus portucalensis strain FDF1T; lane M, protein marker. Arrows indicate polypeptide bands with significant differences between strain St545MbT and strain R15.
The genus Methanolobus belongs to the order Methanosarcinales and the family Methanosarcinaceae. The genus Methanolobus (26) includes M. bombayensis (22), M. oregonensis (5), M. taylorii (41), M. tindarius (26), M. vulcani (21), M. psychrophilus (64), M. profundi strain MobMT (36), and M. zinderi SD1T (11). Most of these organisms are mesophilic, highly irregular cocci, use methanol for growth and methanogenesis, and require sodium chloride (0.06 to 1.20 M) and magnesium (0.5 to 400 mM) (Table 1). All previously described isolates are from coastal sediments; however, the recently published strains of M. psychrophilus, M. profundi, and M. zinderi were isolated from cold wetlands, a deep subsurface gas field, and a coal seam, respectively. The new Methanolobus chelungpuianus strain St545MbT was isolated from the subtropical terrestrial deep underground fault environment and could grow well with no additional NaCl. Interestingly, surveys of 16S rRNA genes in environmental DNA have found sequences with 98 to 99% sequence similarity (Fig. 3) in clone YWA05, from a coal seam aquifer located 843 to 907 mbls in northern Japan (48), clone HDBW-WA06, from a fault-bordered aquifer located 374 mbls in the Miocene formation of northern Japan (49), and clones PL-10A1 and 10A6, from production waters of a low-temperature biodegraded oil reservoir in Canada (14). The phylotypes with high sequence similarity within the clade of Methanolobus chelungpuianus and M. psychrophilus may be widespread in terrestrial sediment and the subsurface.
Methanobacterium palustre FG694aF.
In addition to the methylotrophic Methanolobus chelungpuianus strain St545MbT, the hydrogenotrophic methanogen Methanobacterium palustre strain FG694aF was isolated from the Chelungpu fault. Strain FG694aF came from the enrichment sample, collected from a fault gouge at a depth of 694 m, with formate as a substrate. Cells of strain FG694aF are filamentous, thin rods with diameters of 0.4 to 0.6 μm by 1.7 to 3.4 μm (Fig. 1B). Aggregations were observed under a microscope. The cells stained Gram negative and could not lyse upon the addition of 0.01% (wt/vol) SDS, indicating that a pseudomurein cell wall was present as in other Methanobacterium species. Inside the roll tube, white opaque colonies were observed.
Strain FG694aF can use only formate and H2 plus CO2 to produce methane (Table 2). Under a N2-CO2 atmosphere, it could not produce methane from acetate, methanol, trimethylamine, ethanol, 2-propanol, isobutanol, or 2-butanol. Strain FG694aF was resistant to ampicillin, penicillin, kanamycin, tetracycline, spectinomycin, and vancomycin but was sensitive to chloramphenicol. Cells of strain FG694aF tolerated a NaCl range of 0 to 0.54 M (Fig. 2A), a pH range of 5.7 to 8.3 (Fig. 2B), and a temperature range of 20 to 45°C (Fig. 2C). The optimal growth conditions were 37°C, pH 5.7 to 6.8, and 0.08 M NaCl. Strain FG694aF, isolated from a subsurface fault gouge at 40°C from the Taiwan Chelungpu fault, grows over a temperature range of 20 to 45°C, with an optimal growth temperature of 37°C (Table 2; Fig. 2C). The specific growth rates of strain FG694aF in MM/W and in MB/W with formate as the catabolic substrate were similar (0.081 h−1 and 0.080 h−1, respectively). Acetate was not required for cell growth. Thus, strain FG694aF is an autotrophic, hydrogenotrophic methanogen. Interestingly, the addition of tungsten significantly increased growth. The specific growth rate of strain FG694aF grown in MB medium without the addition of tungsten was 0.043 h−1. The addition of tungsten increased the rate to 0.081 h−1. The thermophilic methanogenic archaea Methanobacterium wolfei and Methanobacterium thermoautotrophicum require tungsten or molybdenum for growth, as has been discussed previously (15, 24). Schmitz et al. (47) showed that tungsten does not support synthesis of active formylmethanofuran dehydrogenase in Methanosarcina barkeri and concluded that mesophilic methanogens appear to use only molybdenum. However, mesophilic methanogens Methanofollis aquaemaris (29), Methanofollis formosanus (62), Methanocalculus taiwanensis (32), Methanocalculus chunghsingensis (30), and Methanobacterium palustre FG694aF (this study), which we isolated from estuarine, marine, and deep subsurface fault environments, all demonstrated that trace amounts of tungsten not only promoted growth but also extended the temperature, pH, and salt ranges of growth conditions.
TABLE 2.
Characteristics of Methanobacterium species and strain FG694aF
| Characteristic | Result forb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Strain FG694aF (this study) | M. formicicum (3) | M. subterraneum (27) | M. palustre (63) | M. alcaliphilum (61) | M. bryantii (1, 3) | M. espanolae (43) | M. ivanovii (18) | |
| Morphology | Thin filament rod, 0.5-0.7 μm by 1.7-2.4 μm | Rod, 0.4-0.8 μm by 2-15 μm, filaments, clumps | Short rod, 0.1-0.15 μm by 0.6-1.2 μm, aggregates | Rod, 0.5 μm by 3-5 μm, filaments | Rod, 0.5-0.6 μm by 2-5 μm, filaments | Rod, 0.5-1.0 μm by 1.5 μm, filaments, clumps | Rod, 0.8 μm by 3-22 μm, filaments | Rod, 0.5-0.8 μm by 1.2 μm, filaments |
| Substratea | H, F | H, F, P, I | H, F | H, F, P, I | H | H, P, I | H | H |
| Stimulatory factor | Tungsten | Acetate, cysteine | None | Not reported | Trypticase peptone, yeast extract, peptone | Yeast extract, acetate, cysteine B, cysteine, Fe, W, Ni | Vitamins | Acetate, cysteine |
| Temp range (°C) (optimum) | 20-45 (37) | 25-50 (37-45) | 3.6-45 (20-40) | 20-45 (33-37) | 25-45 (37) | ND (37-39) | 15-50 (35) | 15-55 (45) |
| pH range (optimum) | 5.7-8.3 (5.7-6.8) | ND (6.6-6.8) | 6.5-9.2 (7.8-8.8) | 7.0 (ND) | 7.0-9.9 (8.1-9.1) | ND (6.9-7.2) | 4.6-7.0 (5.6-6.2) | 6.5-8.5 (7.0-7.4) |
| Optimum salinity (M) | 0.086 | 0.25 | 0.2-1.25 | 0.2 | 0.012 | 0.26 | ND | 0.19 |
| G+C (mol%) | ND | 38.0-48.0 | 54.5 | 34.0 | 57.0 | 31.0-38.0 | 34.0 | 36.6 |
H, H2 plus CO2; F, formate; P, 2-propanol plus CO2; I, isobutanol plus CO2.
The source or reference(s) is in parentheses. ND, not determined.
The 16S rRNA gene sequence (1,240 bp; EU293795) of strain FG694aF was determined, and phylogenetic trees were constructed using a selection of sequences from related methanogens and the environmental DNA library from GenBank (Fig. 5). The sequence analysis placed strain FG694aF within the genus Methanobacterium (95.8 to 99.3%) and showed that it is closely related to M. palustre (99.3%). The genus Methanobacterium belongs to the order Methanobacteriales and family Methanobacteriaceae, which include hydrogen-utilizing rods. The hydrogenotrophic, mesophilic Methanobacterium species have been isolated from anaerobic waste digesters (7), peat bogs (63), marshy soil (25), rice fields, and various freshwater lake sediments (2). The catabolic substrates Methanobacterium uses can be divided into four groups: (i) H2 plus CO2 only, (ii) H2 plus CO2 and formate, (iii) H2 plus CO2, 2-propanol plus CO2, and isobutanol plus CO2, and (iv) H2 plus CO2, formate, 2-propanol plus CO2, and isobutanol plus CO2 (Table 2). These organisms can tolerate only low-salt environments, with an optimal salinity for growth of less than 0.26 M NaCl. However, the pH tolerance is diverse. Some species are moderately acidic, such as Methanobacterium espanolae, which has a pH range of 4.6 to 7.0 and an optimal pH of 5.6 to 6.2 (43), and some are alkalophiles, such as Methanobacterium alcaliphilum, which has a pH range of 7.0 to 9.9 and an optimal pH of 8.1 to 9.1 (61) (Table 2). An exception is Methanobacterium subterraneum, the first methanogen isolated from the deep granitic groundwater at depths of 68, 409, and 420 m (27). It is eurythermic (3.6 to 45°C) and grows optimally at 20 to 40°C, pH 7.8 to 8.8, and 0.2 to 1.2 M NaCl.
FIG. 5.
Phylogenetic analysis of 16S rRNA genes showed the relationships between strain FG694aF and related methanogen isolates and environmental clones. Bootstrap values at nodes are the percentages of 500 replicates. The accession number for each reference species is shown in parentheses. The bar indicates evolutionary distance.
Distribution of Methanobacterium spp. throughout the subsurface.
Methanobacterium palustre strain FT was isolated from a peat bog (63). Additional isolates and environmental clones have also been obtained from anaerobic digesters and rice paddy fields (Fig. 5). Strain FG694aF is the first strain of Methanobacterium palustre isolated from the deep subsurface. Compared with Methanobacterium palustre strain FT, strain FG694aF is more tolerant of acids and salts, and the addition of tungsten doubles its growth rate. However, strain FG694aF cannot utilize 2-propanol plus CO2 and isobutanol plus CO2 (Table 2). Most of the environmental methanoarchaeal clones previously characterized from the terrestrial deep subsurface are related to Methanobacterium and Methanothermobacter, although a few are related to Methanosaeta (36, 37). Moser et al. (37) studied the microbial community of the fault at the Drietontein Consolidated Mine at Johannesburg, South Africa, by PCR amplification of 16S rRNA gene sequences. Their work indicated that Methanobacterium spp. dominate at 4 to 5 kilometers deep. We reanalyzed these clones and found that clone D8A-8, which is closely related to Methanothermobacter wolfeii as well as to clones D8A-6 and D8A-7, forms its own clade, which is separate from known Methanobacterium species. Furthermore, these phylotypes may belong to a new genus (Fig. 5). In addition, the dominant environmental NAK2-a1 clones, obtained from a depth of 703 to 805 m in a natural gas well in Japan (36), were related to Methanobacterium formicicum. Clone YWA03, from a coal seam aquifer located 843 to 907 m below ground level in northern Japan, was formerly reported to be related to M. formicicum (48); however, our phylogenetic analysis suggests that it is related to M. subterraneum (Fig. 5). In this report, we isolated and purified Methanobacterium palustre strain FG694aF from the Chelungpu fault at 694 m below the surface. Both cultivation and molecular studies suggested that Methanobacterium species are widespread in subsurface environments.
The methane-producing archaea have long been believed to be one of the most abundant organisms in the deep subsurface. Although many environmental 16S rRNA gene surveys revealed the diversity of methanoarchaea, only a few methanogens have been isolated from this habitat. Here, we demonstrated the occurrence of methanoarchaea in the Chelungpu fault, Taiwan, and enriched, purified, and characterized two different types of methanogens, the methylotrophic Methanolobus chelungpuianus St545MbT and hydrogenotrophic Methanobacterium palustre FG694aF, obtained from fault samples at depths of 545 m and 694 m, respectively. Scientific studies often focus solely on hydrogenotrophic methanogens within the subsurface biosphere and neglect the methylotrophic methanogens. Bacterial rRNA gene sequencing efforts for the Antrim Shale have shown that a variety of fermentative, syntrophic, and homoacetogenic bacteria inhabit the shale (13). These organisms are believed to convert bioavailable components of shale organic matter into substrates for methanogenesis, such as H2, CO2, acetate, and methylamines (55). The rock formations of the TCDP coring site, where Methanolobus chelungpuianus St545MbT was isolated, are mainly composed of sandstone and shale in different proportions (see Table S1 in the supplemental material) (56, 57). It is likely that the catabolic substrates for methylotrophic methanogens may also be produced by the biodegradation of organic matter in shale. Although we do not know exactly how methylotrophic methanogens obtain their catabolic substrates within the deep subsurface, our isolate Methanolobus chelungpuianus St545MbT and environmental DNA surveys clearly indicate that Methanolobus and Methanosaeta live within the deep subsurface. The results of this investigation expand our understanding of the microbial life deep underground.
Description of Methanolobus chelungpuianus sp. nov.
Methanolobus chelungpuianus sp. nov. (che.lung.pu.i.a′nus. N.L. adj. chelungpuianus, Chelungpu fault, the place of isolation).
Cells are irregular cocci (diameter, 0.5 to 1.0 μm) and are strictly anaerobic and Gram negative. The cell wall has an SDS-sensitive surface layer protein. Methane is produced from methanol and trimethylamine, but not formate, acetate, dimethylamine, ethanol, 2-propanol, isobutanol, 2-butanol, methylamine, or methyl sulfide. Cells are mesophilic and grow at 24 to 45°C, with optimal growth at 37°C. Cells grow at pH 6.8 to 7.4, with optimal growth at pH 7.0. Cells grow well in 0.00 to 0.68 M NaCl and 5 to 200 mM Mg2+, with optimal growth at 0.00 to 0.08 M NaCl and 75 mM MgCl2. No organic compounds are required for growth; however, cells grow better in medium with yeast extract and tryptone. Growth is completely inhibited by chloramphenicol and partly inhibited by tetracycline, but not by ampicillin, penicillin, kanamycin, spectinomycin, or vancomycin. The DNA G+C content of strain St545MbT (DSM 19953T; JCM 15159T, BCRC AR10030T) is 48.3 mol%. This strain was isolated from the deep subsurface at a depth of 545 mbls in sandstone in Chelungpu fault near Dai-Keng, Taiwan.
Supplementary Material
Acknowledgments
We thank Pei-Ling Wang at the Institute of Oceanography, National Taiwan University, for sampling preparation and geological background and sampling information. We thank Xiuzhu Dong at Chinese Academy of Sciences, Beijing, for supplying Methanolobus psychrophilus R15 and Methanolobus vulcani DSM3029T.
This project is supported by the National Science Council of Taiwan, Republic of China (NSC 94-2621-B005-001).
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Balch, W. E., G. E. Fox, L. J. Margum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbial. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyvae, S. S., A. Y. Obraztcova, K. S. Laurinavichus, and L. V. Bezrukova. 1986. Characterization of rod-like methanogens isolated from oil field. Microbiology 55:1014-1020. [Google Scholar]
- 3.Boone, D. R. 1987. Replacement of the type strain of Methanobacterium formicicum and reinstatement of Methanobacterium bryantii sp. nov. nom. rev. (ex Balch and Wolfe, 1981) with M.o.H. (DSM 863) as the type strain. Request for an opinion. Int. J. Syst. Bacteriol. 37:172-173. [Google Scholar]
- 4.Boone, D. R., and W. B. Whitman. 1988. Proposal of minimal standards for describing new taxa of methanogenic bacteria. Int. J. Syst. Bacteriol. 38:212-219. [Google Scholar]
- 5.Boone, D. R., et al. 1993. Isolation and characterization of Methanohalophilus portucalensis sp. nov. and DNA reassociation study of the genus Methanohalophilus. Int. J. Syst. Bacteriol. 43:430-437. [Google Scholar]
- 6.Boone, D. R., W. B. Whitman, and P. Rouvière. 1993. Diversity and taxonomy of methanogens, p. 35-80. In J. G. Ferry (ed.), Methanogenesis. Chapman & Hall, New York, NY.
- 7.Bryant, M. P., E. A. Wolin, M. J. Wolin, and R. S. Wolfe. 1967. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 59:20-31. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, L., et al. 2007. Methermicoccus shengliensis gen. nov., sp. nov., a thermophilic, methylotrophic methanogen isolated from oil-production water, and proposal of Methermicoccaceae fam. nov. Int. J. Syst. Evol. Microbiol. 57:2964-2969. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, L., et al. 2008. Isolation and characterization of Methanoculleus receptaculi sp. nov. from Shengli oil field, China. FEMS Microbiol. Lett. 285:65-71. [DOI] [PubMed] [Google Scholar]
- 10.Cole, J. R., et al. 2009. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37:D141-D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doerfert, S. N., M. Reichlen, P. Lyer, M. Wang, and J. G. Ferry. 2009. Methanolobus zinderi sp. nov., a methylotrophic methanogen isolated from a deep subsurface coal seam. Int. J. Syst. Evol. Microbiol. 59:1064-1069. [DOI] [PubMed] [Google Scholar]
- 12.Ferry, J. G. 1997. Methane: small molecules, big impact. Science 278:1413-1414. [DOI] [PubMed] [Google Scholar]
- 13.Formolo, M. J., J. M. Salacup, S. T. Petsch, A. M. Martini, and K. Nüsslein. 2008. A new model linking atmospheric methane sources to Pleistocene glaciation via methanogenesis in sedimentary basins. Geology 36:139-142. [Google Scholar]
- 14.Grabowski, A., O. Nercessian, F. Fayolle, D. Blanchet, and C. Jeanthon. 2005. Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol. Ecol. 54:427-443. [DOI] [PubMed] [Google Scholar]
- 15.Hochheimer, A., R. Hedderich, and R. K. Thauer. 1998. The formylmethanofuran dehydrogenase isoenzymes in Methanobacterium wolfei and Methanobacterium thermoautotrophicum: induction of the molybdenum isoenzyme by molybdate and constitutive synthesis of the tungsten isoenzyme. Arch. Microbiol. 170:389-393. [DOI] [PubMed] [Google Scholar]
- 16.Huber, H., M. Thomm, H. König, G. Thies, and K. O. Stetter. 1982. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch. Microbiol. 132:47-50. [Google Scholar]
- 17.Inagaki, F., et al. 2006. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl. Acad. Sci. U. S. A. 103:2815-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain, M. K., Thompson, E. Conway De Macario, and J. G. Zelkus. 1987. Speciation of Methanobacterium strain Ivanov as Methanobacterium ivanovii, sp. nov. Syst. Appl. Microbiol. 9:77-82. [Google Scholar]
- 19.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from submarine hydrothermal vent. Arch. Microbiol. 136:254-261. [Google Scholar]
- 20.Jones, W. J., J. D. Nagle, Jr., and W. B. Whitman. 1987. Methanogens and the diversity of Archaebacteria. Microbiol. Rev. 51:135-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadam, P. C., and D. R. Boone. 1995. Physiological characterization and emended description of Methanolobus vulcani. Int. J. Syst. Bacteriol. 45:400-402. [Google Scholar]
- 22.Kadam, P. C., D. R. Ranade, L. Mandelco, and D. R. Boone. 1994. Isolation and characterization of Methanolobus bombayensis sp. nov., a methylotrophic methanogen that requires high concentrations of divalent cations. Int. J. Syst. Bacteriol. 44:603-607. [Google Scholar]
- 23.Keswani, J., and W. B. Whitman. 2001. Relationship of 16S rRNA sequence similarity to DNA hybridization in prokaryotes. Int. J. Syst. Evol. Microbiol. 51:667-678. [DOI] [PubMed] [Google Scholar]
- 24.Kletzin, A., and M. W. W. Adams. 1996. Tungsten in biological systems. FEMS Microbiol. Rev. 18:5-63. [DOI] [PubMed] [Google Scholar]
- 25.König, H. 1984. Isolation and characterization of Methanobacterium uliginosum sp. nov. from a marshy soil. Can. J. Microbiol. 30:1477-1481. [Google Scholar]
- 26.König, H., and K. O. Stetter. 1982. Isolation and characterization of Methanolobus tindarius, sp. nov., a coccoid methanogen growing only on methanol and methylamines. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 3:478-490. [Google Scholar]
- 27.Kotelnikova, A., A. J. L. Macario, and K. Pedersen. 1998. Methanobacterium subterraneum sp. nov., a new alkaliphilic, eurythemic and halotolerant methanogen isolated from deep granitic groundwater. Int. J. Syst. Evol. Microbiol. 48:357-367. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lai, M. C., and S. C. Chen. 2001. Methanofollis aquaemaris sp. nov., a methanogen isolated from an aquaculture fish pond. Int. J. Syst. Evol. Microbiol. 51:1873-1880. [DOI] [PubMed] [Google Scholar]
- 30.Lai, M. C., C. C. Lin, P. H. Yu, Y. F. Huang, and S. C. Chen. 2004. Methanocalculus chungshingensis sp. nov., isolated from an estuary and a marine fishpond in Taiwan. Int. J. Syst. Evol. Microbiol. 54:83-189. [DOI] [PubMed] [Google Scholar]
- 31.Lai, M. C., et al. 2000. Methanosarcina mazei strain O1M9704, methanogen with novel tubule isolated from estuarine environment. Curr. Microbiol. 41:15-20. [DOI] [PubMed] [Google Scholar]
- 32.Lai, M. C., et al. 2002. Methanocalculus taiwanensis sp. nov., isolated from an estuarine environment. Int. J. Syst. Evol. Microbiol. 52:1799-1806. [DOI] [PubMed] [Google Scholar]
- 33.Lanoil, B. D., et al. 2005. Archaeal diversity in ODP legacy borehole 892b and associated seawater and sediments of the Cascadia Margin. FEMS Microbiol. Ecol. 54:167-177. [DOI] [PubMed] [Google Scholar]
- 34.Mesbah, M., U. Premachandran, and W. B. Whitman. 1989. Precise measurement of the guanine-plus-cytosine content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Bacteriol. 39:159-167. [Google Scholar]
- 35.Mikucki, J. A., Y. Liu, M. Delwiche, F. S. Colwell, and D. R. Boone. 2003. Isolation of a methanogen from deep marine sediments that contain methane hydrates, and description of Methanoculleus submarinus sp. nov. Appl. Environ. Microbiol. 69:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochimaru, H., et al. 2009. Methanolobus profundi sp. nov., a methylotrophic methanogen isolated from deep subsurface sediments in a natural gas field. Int. J. Syst. Evol. Microbiol. 59:714-718. [DOI] [PubMed] [Google Scholar]
- 37.Moser, D. P., et al. 2005. Desulfotomaculum and Methanobacterium spp. dominate a 4- to 5-kilometer-deep fault. Appl. Environ. Microbiol. 71:8773-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ollivier, B., P. Caumette, J. L. Garcia, and R. A. Mah. 1994. Anaerobic bacteria from hypersaline environments. Microbiol. Rev. 58:27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ollivier, B., et al. 1997. Methanoplanus petrolearius sp. nov., a novel methanogenic bacterium from an oil-producing well. FEMS Microbiol. Lett. 147:51-56. [DOI] [PubMed] [Google Scholar]
- 40.Ollivier, B., et al. 1998. Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. Int. J. Syst. Bacteriol. 48:821-828. [DOI] [PubMed] [Google Scholar]
- 41.Oremland, R. S., and D. R. Boone. 1994. Methanolobus taylorii sp. nov., a new methylotrophic, estuarine methanogen. Int. J. Syst. Bacteriol. 44:573-575. [Google Scholar]
- 42.Orphan, V. J., L. T. Taylor, D. Hafenbradl, and E. F. Delong. 2000. Culture-dependent and culture-independent characterization of microbial assemblages associated with high temperature petroleum reservoirs. Appl. Environ. Microbiol. 66:700-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel, G. B., G. D. Sprott, and J. E. Fein. 1990. Isolation and characterization of Methanobacterium espanolae sp. nov. a mesophilic, moderately acidiphilic methanogen. Int. J. Syst. Bacteriol. 40:12-18. [Google Scholar]
- 44.Pedersen, K. 1993. The deep subterranean biosphere. Earth Sci. Rev. 34:243-260. [Google Scholar]
- 45.Pedersen, K. 1997. Microbial life in deep granitic rock. FEMS Microbiol. Rev. 20:399-414. [Google Scholar]
- 46.Powell, G. E. 1983. Interpreting the gas kinetics of batch cultures. Biotechnol. Lett. 5:437-440. [Google Scholar]
- 47.Schmitz, R. A., P. A. Bertram, and R. K. Thauer. 1994. Tungstate does not support synthesis of active formylmethanofuran dehydrogenase in Methanosarcina barkeri. Arch. Microbiol. 161:528-530. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu, S., et al. 2007. Molecular characterization of microbial communities in deep coal seam groundwater of northern Japan. Geobiology 5:423-433. [Google Scholar]
- 49.Shimizu, S., et al. 2006. Molecular characterization of microbial communities in fault-bordered aquifers in the Miocene formation of northernmost Japan. Geobiology 4:147-223. [Google Scholar]
- 50.Sowers, K. R., and K. M. Noll. 1995. Techniques for anaerobic growth, p. 15-48. In K. R. Sowers and H. T. Schreier (ed.), Archaea: a laboratory manual, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 51.Stetter, K. O., et al. 1993. Hyperthermophilic Archaea are thriving in deep North Sea and Alaskan reservoirs. Nature 365:743-745. [Google Scholar]
- 52.Takai, K., et al. 2003. Shifts in archaeal communities associated with lithological and geochemical variations in subsurface Cretaceous rock. Environ. Microbiol. 5:309-320. [DOI] [PubMed] [Google Scholar]
- 53.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 54.Teng, L. S., C. T. Lee, Y. B. Tsai, and L. Y. Hsiao. 2000. Slab breakoff as a mechanism for flipping of subduction polarity in Taiwan. Geology 28:155-158. [Google Scholar]
- 55.Waldron, P. J., S. T. Petsch, A. M. Martini, and K. Nüsslein. 2007. Salinity constraints on subsurface archaeal diversity and methanogenesis in sedimentary rock rich in organic matter. Appl. Environ. Microbiol. 73:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, C. Y., C. H. Chang, and H. Y. Yen. 2000. An interpretation of the 1999 Chi-Chi earthquake in Taiwan based on the thin-skinned thrust model. Terr. Atmos. Ocean Sci. 11:609-630. [Google Scholar]
- 57.Wang, P. L., et al. 2007. Cultivated-based characterization of microbial communities associated with deep sedimentary rocks from Taiwan Chelungpu Drilling Project cores. Terr. Atmos. Ocean Sci. 18:395-412. [Google Scholar]
- 58.Watanabe, K., Y. Kodama, and N. Kaku. 2002. Diversity and abundance of bacteria in an underground oil-storage cavity. BMC Microbiol. 2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. U. S. A. 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolfe, R. S. 1996. 1776-1996: Alessandro Volta's combustible air. ASM News 62:529-534. [Google Scholar]
- 61.Worakit, S., D. R. Boone, R. A. Mah, M. E. Abdel-Samie, and M. M. El-Halwagi. 1986. Methanobacterium alcaliphilum sp. nov., an H2-utilizing methanogen that grows at high pH values. Int. J. Syst. Bacteriol. 36:380-382. [Google Scholar]
- 62.Wu, S. Y., M. C. Lai, and S. C. Chen. 2005. Methanofollis formosanus., isolated from a fish pond. Int. J. Syst. Evol. Microbiol. 55:837-842. [DOI] [PubMed] [Google Scholar]
- 63.Zellner, G., et al. 1989. Characterization of new mesophilic, secondary alcohol-utilizing methanogen, Methanobacterium palustre sp. nov. from a peat bog. Arch. Microbiol. 151:1-9. [Google Scholar]
- 64.Zhang, G., N. Jiang, X. Liu, and X. Dong. 2008. Methanogenesis from methanol at low temperatures by a novel psychrophilic methanogen, “Methanolobus psychrophilus” sp. nov., prevalent in Zoige wetland of the Tibetan plateau. Appl. Environ. Microbiol. 74:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.