Abstract
Extracellular polysaccharide (EPS) is produced by diverse bacterial pathogens and fulfills assorted roles, including providing a structural matrix for biofilm formation and more specific functions in virulence, such as protection against immune defenses. We report here the first investigation of some of the genes important for biofilm formation in Photorhabdus luminescens and demonstrate the key role of the phosphomannose isomerase gene, manA, in the structure of functional EPS. Phenotypic analyses of a manA-deficient mutant showed the importance of EPS in motility, insect virulence, and biofilm formation on abiotic surfaces as well as the requirement of this gene for the use of mannose as the sole carbon source. Conversely, this defect had no apparent impact on symbiosis with the heterorhabditid nematode vector. A more detailed analysis of biofilm formation revealed that the manA mutant was able to attach to surfaces with the same efficiency as that of the wild-type strain but could not develop the more extended biofilm matrix structures. A compositional analysis of P. luminescens EPS reveals how the manA mutation has a major effect on the formation of a complete, branched EPS.
Photorhabdus luminescens is a Gram-negative, entomopathogenic gammaproteobacterium belonging to the family Enterobacteriaceae. Photorhabdus has an unusual life-style, displaying a switch from a symbiotic relationship with a heterorhabditid nematode to a virulent infection of an insect host (51). The bacteria colonize the gut of a free-living, host-seeking, infective juvenile (IJ) Heterorhabditis bacteriophora nematode (19). The IJ invades an insect larva, regurgitating P. luminescens into the hemolymph, wherein the bacteria rapidly multiply and kill the insect by using a plethora of virulence factors, including proteases, lipases, and toxin complexes (18, 51). The bacteria proliferate in insect tissues, providing a food source for the symbiotic nematode, which then replicates. After several nematode generations, the IJs reacquire P. luminescens and leave the host in search of new insects to continue the cycle.
Biofilms are likely to be important in Photorhabdus biology, as they allow the establishment of surface-associated populations in order to colonize habitats such as the gut of the nematode vector (13) and the midgut epithelium in the insect host (42). Biofilm formation involves the attachment of bacterial cells to a solid surface, utilizing an extensive matrix of polymers, predominantly extracellular polysaccharides (EPSs), to develop more mature community structures (50). EPSs are secreted by most bacterial species, both Gram positive and Gram negative, as capsular polysaccharides (CPSs) closely associated with cells and/or polysaccharides, which are more diffuse and readily released into the surrounding environment. The variability of EPS types produced by organisms such as Xanthomonas, Pseudomonas, and Escherichia demonstrates that there is no common EPS structure within the Gammaproteobacteria. EPSs from Xanthomonas and Pseudomonas have been particularly well studied due to their widespread commercial uses, especially as gelling agents in foodstuffs (48). Various pathovars and mutants of the plant-pathogenic organism Xanthomonas campestris produce xanthan, with a common general structure of d-mannose and d-glucuronic acid decorating a cellulose-like, glucan backbone but with various levels of complexity of the branching side groups (48). The specific nature of these side chains is essential for defining the properties of the EPS. In contrast, alginates produced by Pseudomonas spp. are linear polysaccharides comprised of β-d-mannuronic acid (M residue) and α-l-guluronic acid (G residue) arranged in blocks or randomly (20).
The roles of EPS produced by pathogens are wide and varied, ranging from the adhesion of pathogenic bacteria to host tissues during the early stages of invasion to the shielding of a pathogen against host immune factors and abiotic stresses (21, 33, 40) and the formation of a biofilm matrix in more persistent infections. In Yersinia pestis, the lack of EPS disrupts biofilm formation, which is essential for bacterial transmission from fleas to mammals (7). Variation in the CPS structure also contributes to the genesis of new Vibrio cholerae epidemic strains (37). During human infections, Pseudomonas aeruginosa secretes large quantities of alginate, causing a blockage of the lower respiratory tract in cystic fibrosis patients (16). Furthermore, biofilm formation by a number of bacterial species on the abiotic surfaces of prostheses (e.g., catheter implants) can be a persistent source of infection (14).
Besides the contribution of EPS to bacterial biofilm formation and persistence, EPS has been implicated in virulence mechanisms that deal with host defenses (4, 11). The role of EPS in the interaction of Photorhabdus with either the innate immune system of the insect host or its nematode partner has not been investigated. This study describes the identification of some of the genes important for biofilm formation in P. luminescens TT01 and presents an investigation into the role of the phosphomannose isomerase (PMI) gene, manA, in the life cycle of the bacterium. Furthermore, we present a compositional analysis of EPSs from wild-type and mutant P. luminescens and use this to elucidate the central role of the enzyme encoded by manA in EPS structure and subsequent biofilm formation.
MATERIALS AND METHODS
Culture conditions for strains.
All strains and plasmids are summarized in Table 1. Strains were grown in Luria-Bertani (LB) (6) broth (Scientific Laboratory Supplies) supplemented with 1.5% (wt/vol) agar (Scientific Laboratory Supplies) for plates. Kanamycin, ampicillin, gentamicin, and rifampin (Fluka Biochemika) were added at 50 μg/ml, and tetracycline was added at 5 μg/ml when required. Photorhabdus luminescens strains were grown at 28°C, and Escherichia coli strains were grown at 37°C. Growth curves of P. luminescens were measured from cultures grown in 100 ml LB broth with an initial optical density at 600 nm (OD600) of 0.002, inoculated from cultures grown overnight. M63 minimal medium (34) was supplemented with 0.2% (vol/vol) glucose or mannose, and 1.5% (wt/vol) agar was added for plates. Grace's and Schneider's insect media (Sigma-Aldrich) and filtered hemolymph from Manduca sexta were used in vitro to mimic growth conditions in the insect.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| TT01 rif | Photorhabdus luminescens subsp. laumondii strain TT01; spontaneous Rifr | 28 |
| manA mutant | TT01 rif with Tn10 insertion in manA gene, isolated from the mutant library; Kmr | This study |
| manA+ | manA mutant complemented with manA gene on vector pBBR1MCS-5 | This study |
| α-Select | Electrocompetent Escherichia coli F− deoR endA1 recA1 relA1 gyrA96 hsdR17 (rK− mK+) supE44 thi-1 phoA Δ(lacZYA argF)U169 φ80lacZΔM15 λ− | Bioline |
| DH5α | Escherichia coli F−recA ΔlacU169 (φ80 lacZΔM15) endA hsdR gyrA | 22 |
| S17-1λpir | Escherichia coli Tpr SmrrecA thi hsdR M+ RP4::2-Tc::Mu::Km::Tn7 λpir lysogen | 43 |
| Plasmids | ||
| pBBR1MCS-5 | Broad-host-range cloning vector; Gmr | 32 |
| pBBR1MCS-5manA | pBBR1MCS-5 vector containing cloned manA with its promoter | This study |
| pHC60 | Broad-host-range vector constitutively expressing GFP; Tetr | 12 |
| pLOF | Delivery plasmid (Apr) with Tn10 (Kmr) | 23 |
| pUC19 | High-copy-number cloning vector; Apr | 54 |
Biofilm and motility assays.
The formation of a biofilm or pellicle at the air-liquid interface was tested, and its cohesiveness/integrity was analyzed according to a modified assay described previously by Spiers et al. (46). Universal test tubes with 10 ml LB broth were inoculated with 1-μl cultures grown overnight (ca. 16 h) and incubated under static conditions at 28°C for 6 to 7 days. Mutants with a phenotype different from that of wild-type strain TT01 rif were streaked out, and the pellicle assay was repeated in triplicate for each of them. The quantification of biofilm strength was assessed by calculating the maximum deformation mass (MDM) (47). Glass beads of ca. 1 mm in diameter (Sigma-Aldrich) were weighed before being placed onto 7-day-old pellicles. When these broke, the total weight (mg) of the beads added was calculated to give an MDM value.
The visualization of P. luminescens surface-associated biofilms was done by inoculating a chambered microscopy slide (μ-slide, 8 well; Ibidi) with 300 μl of P. luminescens TT01 rif and the manA mutant containing green fluorescent protein (GFP)-expressing plasmid pHC60 at an OD600 ∼0.002 and incubating these static cultures for 7 days. z-stack images were obtained by using an inverted confocal microscope (LSM510, 40× oil immersion objective; Zeiss). Analysis of the images was performed by using Imaris (Bitplane) three-dimensional (3D) data analysis software, and 10 lateral sections per sample were measured.
For the swimming motility assays, 1 μl of exponentially grown cultures was inoculated into the center of a petri dish containing semisolid agar (0.25% [wt/vol] agar, 0.1% [wt/vol] LB broth). Each strain was tested in triplicate, and the diameter of the resulting halo was measured 6 days after inoculation.
Construction of a transposon mutant library and sequencing of the isolated mutants.
The P. luminescens TT01 rif transposon library was created by using a pLOF suicide vector containing a kanamycin resistance cassette flanked by insertion sequences from Tn10 (23). The vector was conjugated from E. coli strain S17-1λpir into P. luminescens TT01 rifampin resistant (TT01 rif). The mating mix was plated onto LB agar supplemented with kanamycin and rifampin. More than 3,000 transconjugants were arrayed in 96-well plates to form the mutant library.
To identify the interrupted gene, sequencing of the mutants was done by arbitrary primed PCR (AP-PCR) and plasmid rescue cloning. The AP-PCR was performed in two rounds of reactions, with the following conditions for the first reaction: 94°C for 15 min; 6 cycles of 94°C for 30 s and 42°C for 30 s, at −1°C per cycle; and 72°C for 3 min, repeated 20 times, and then 94°C for 30 s, 65°C for 30 s, and 72°C for 3 min, repeated 25 times. In the first round, a forward primer (kanF1 [5′-GTT TCA TTT GAT GCT CGA TGA G-3′]) and three arbitrary reverse primers were used, diluted to 20 pmol/μl and mixed 1:1:1 {CEKG_2A [5′-GGC CAC CGC TCG ACT AGT AC (AGCT) × 10 AGA G-3′], CEKG_2B [5′-GGC CAC GCG TCG ACT AGT AC (AGCT) × 10 ACG CC-3′], and CEKG_2C [5′-GGC CAC GCG TCG ACT AGT AC (AGCT) × 10 GAT AT-3′]}. One microliter each of kanF1 and the CEKG mix was added to a 25-μl final reaction mixture using Immomix Hot Start Taq (Bioline). One microliter of the product from the first round was used as a template in the second round with a nested forward primer (kanF2 [5′-CCA CCT ACA ACA AAG CTC TC-3′]) and a reverse primer (CEKG_4 [5′-GGC CAC GCG TCG ACT AGT AC-3′]). Conditions for the second reaction were as follows: 94°C for 10 min; 94°C for 30 s, 60°C for 30 s, and 72°C for 3 min, repeated 30 times; and 72°C for 10 min. All primers were obtained from MWG. Cloning of the transposon/chromosome junction was done by digestion of the genomic DNA of the selected mutants with EcoRI, ligation with pUC19, and electroporation into α-Select electrocompetent E. coli cells, selected with kanamycin for transposon-containing inserts. The sequencing of the AP-PCR products and resulting clones was performed by Qiagen.
Cloning of manA for transcomplementation.
Forward and reverse PCR primers containing restriction sites (manAapaI [5′-CGA TGG GCC CCA TTG AGT CTT TTT C-3′] and manAkpnIrev [5′-CGC TAG GGT ACC TAT GCA TTC TCT-3′]) were designed to amplify the manA gene (NCBI accession number NP_929606.1) with its putative promoter region. The PCR was done by using the PicoMaxx high-fidelity PCR system (Stratagene) and genomic DNA from TT01 rif as a template, and the product was cloned into pBBR1MCS-5 using ApaI and KpnI. The clones containing an insert were checked by PCR using primers that amplify across the multiple-cloning site of the vector (pBBR1MCS-5 for [5′-CGT AAT ACG ACT CAC TAT AGG GCG-3′] and pBBR1MCS-5 rev [5′-CGG CTC GTA TGT TGT GTG GAA TTG TG-3′]). The pBBR1MCS-5manA plasmid was transformed into S17-1λpir E. coli and transferred to the manA mutant strain by mating.
SPR measurement of attachment.
The attachment of P. luminescens strains to a solid surface was measured by surface plasmon resonance (SPR), a technique which has been previously used to measure bacterial attachment (27). Cultures grown overnight were diluted to an OD600 of 1, and 50 μl was applied to channel 1 of an Autolab Esprit instrument (Eco Chemie, BV, Netherlands) and compared with 50 μl bacterium-free medium control. The difference between the angles (reflectivity angle) is indicative of the change produced in the refractive index by bacterial attachment. After stabilization of the baseline, the reflectivity angle was recorded every 2 s for 1,000 s or until stabilization. Washed cells resuspended in phosphate-buffered saline (PBS; Oxoid) and filtered supernatant were also measured.
Nematode transmission assays, insect virulence, and resistance to antimicrobials.
The ability of the manA mutant and TT01 rif to be transmitted to IJs was determined by homogenizing 100 surface-sterilized IJs and plating the equivalent of 1 and 0.1 IJs expected to yield ca. 1,000 and 100 CFU, respectively. Strains were GFP labeled, allowing the maternal transmission of bacterial symbionts to IJs to be directly observed by fluorescence microscopy (DM5000; Leica). A value for the transmission efficiency was determined from the proportion of IJs out of at least 200 examined containing GFP-labeled P. luminescens. The presence of a persistent biofilm in the maternal nematode intestine was determined by pulse-chase experiments as was described previously (13).
Virulence data were obtained by using Galleria mellonella (Livefood, United Kingdom) and Manduca sexta larvae. Cultures of P. luminescens grown overnight were diluted to an OD600 of 0.1 in PBS, and E. coli DH5α and PBS were used as controls. The M. sexta colony, maintained at the University of Bath, was reared under controlled conditions (25°C in 80% humidity) on an artificial diet (39). For the test, fifth-instar larvae were injected with ca. 1,000 cells of each strain. For G. mellonella assays, 20 larvae were injected per strain with 10 μl of diluted cultures corresponding to ca. 100 cells. All insects were incubated at 25°C and scored for mortality daily up to 1 week.
For the assay of sensitivity to defense-related antimicrobial compounds, 5 ml of TT01 rif and manA mutant cultures grown overnight (adjusted to 108 CFU/ml) were treated with H2O2 (Fluka Biochemika) and cecropin A (Sigma-Aldrich) at final concentrations of 0.03% and 10 μM, respectively, and H2O as a control. The samples were incubated at 28°C for 1 h before serial dilution and plating. Survival was expressed as a percentage of the H2O-treated controls.
EPS extraction and analysis.
About 50 140-mm petri dishes (Sterilin) per strain of cultures grown overnight were washed with 5 to 10 ml of 0.9% NaCl. The EPS was released by mixing for 15 to 20 s in a blender and separated by centrifugation at 18,000 × g for 10 min (Avanti J-25 centrifuge; Beckman-Coulter). The supernatant was frozen at −80°C for 4 to 6 h and concentrated to ca. 30 to 40 ml by freeze-drying (Edwards Modulyo freeze dryer). The sample was thawed, and 3 volumes of chilled acetone were added, mixed, and chilled at −20°C overnight before centrifugation at 3,000 × g for 20 min. The pellet was freeze-dried for 4 to 6 h and then dissolved in a minimal volume of incubation buffer (10 mM Tris-HCl, 5 mM MgCl2·7H2O, 0.5 mM CaCl2·2H2O, 15 mM KCl) and treated with DNase and RNase (100 μg/ml; Sigma-Aldrich) for 4 h at 37°C and pronase (250 μg/ml; Sigma-Aldrich) for a further 24 h at 37°C. Residual proteins were removed by heating at 80°C for 30 min before ultracentrifugation (L-80 ultracentrifuge; Beckman-Coulter) at 100,000 × g for 4 h. The supernatant was dialyzed for 24 h (dialysis membrane molecular cutoff of 12 to 14 kDa; Medicell International Ltd.), with at least two changes of water, and freeze-dried until it was powder. The EPS was dissolved in endotoxin-free water (0.5 mg/ml; Cambio), and Triton X-114 (Sigma-Aldrich) was added to a final concentration of 1% (vol/vol) to remove contaminating lipopolysaccharide (1, 8). The sample was chilled on ice for 1 h and stirred at 4°C for 20 h before incubation at 37°C until phase separation occurred. The phases were recovered by centrifugation for 30 min at 1,000 × g at 30°C. The lower phase was discarded, and Triton X-114 was added to the upper aqueous phase to a final concentration of 5%. The detergent wash was repeated. The EPS-containing aqueous phase was extracted three times with 3 volumes of chloroform-methanol (2:1) to remove detergent before freeze-drying.
Composition and linkage analysis of the EPS samples was performed at the Complex Carbohydrate Research Center (CCRC), University of Georgia. For composition analysis the samples were first subjected to acidic methanolysis to produce monosaccharide methyl glycosides. Combined gas chromatography-mass spectroscopy (GC-MS) was then performed on the per-O-methylsilyl derivates of these groups using an Alltech EC-1 fused silica capillary column. Linkage analysis was performed by first producing partially methylated alditol acetates (PMAAs) from the samples and analyzing these by GC-MS on a Supelco 2330 silica capillary column.
Statistical methods.
Statistical analyses of SPR data were undertaken by using the statistical application included with the Igor Pro 6.0 data analysis software (WaveMetrics). The Kruskal-Wallis test was used to avoid assuming a normal distribution of the population. The maximum angle variation of each strain was compared with that of TT01 rif (n = 3), with the significance being set at 95%. For the other assays, significant differences were tested by one-way analysis of variance (ANOVA) and a Tukey-Kramer HSD (honestly significant difference) test to confirm differences between treatments.
RESULTS
Screening of a random transposon insertion library of P. luminescens TT01 for biofilm defective mutants.
We took advantage of the inherent link between biofilm and EPS to screen for EPS mutants by observing differences in macroscopic biofilm structure. More than 3,000 transposon-insertion mutants were screened, and of those, 29 mutants (approximately 1% of the library) were identified as forming an aberrant or atypical biofilm (pellicle) at the air-liquid interface, as described in Table 2. The pellicle morphology showed significant variation between mutants, including differences in the thickness of the pellicle, changes in porosity of the matrix surface, and various degrees of pigmentation. The most striking mutants did not exhibit complete pellicle formation. In these cases the development of the structure halted at an early stage following the adhesion of the first subpopulations of cells to the glass of the tube at the air-liquid interface. It was common to observe a severely decreased or nonexistent development of the pellicle across the surface of the liquid in these mutants. Identification of the genes disrupted by the transposon was attempted by sequencing either from the AP-PCR product (12 strains) or from the plasmid after rescue cloning (17 strains). However, we failed to identify the transposon insertion site for 6 mutants.
TABLE 2.
Characteristics of the biofilm mutants identifieda
| Gene hit | Strain | Phenotype |
||
|---|---|---|---|---|
| Pellicle morphology (thickness score/porosity score/ pigmentation)b | Attachment (mDeg)c | Motility (% of wild type)d | ||
| — | TT01 rif | 5/5/orange | 116 | 100 |
| plu2360 manA | P56 | 1/0/none | 96 | 16 |
| plu4799 wblD | P73 | 4/1/orange | 106 | 89 |
| plu0934 suhB | P36 | 0/0/none | 90 | ND |
| plu4004 diaA | P27 | 0/0/none | 46 | 13 |
| plu2728 entB | P32 | 0/0/none | 103 | ND |
| plu2728 entB | P40 | 0/0/none | 85 | ND |
| plu3085 nuoF | P72 | 4/2/pale | 105 | 80 |
| plu3085 nuoF | P38 | 3/2/pale | 93 | ND |
| plu1849 motA | P79 | 0/0/none | 95 | 0 |
| plu1850 motB | P69 | 0/0/none | 140 | 0 |
| plu1919 flgF | P30 | 0/0/none | 83 | ND |
| plu1920 flgG | P84 | 0/0/none | 100 | 0 |
| plu1851 cheA | P71 | 0/0/none | 67 | 0 |
| plu4890 | P74 | 4/4/white | 133 | 86 |
| plu2203 hcaR | P82 | 3/2/orange | 84 | 105 |
| plu2209 hcaD | P78 | 2/3/white | 79 | 107 |
| plu3559 | P31 | 0/0/none | 93 | ND |
| plu0499 | P75 | 2/0/orange | 88 | 84 |
| plu2244 | P86 | 2/1/orange | 81 | 98 |
| plu2548 | P77 | 2/1/dark orange | 68 | 97 |
| plu1730 | P29 | 0/0/none | 91 | ND |
| plu2412 | P76 | 3/2/red | 116 | 93 |
| plu4230 | P33 | 1/0/orange | ND | ND |
| ND | P80 | 3/3/dark orange | 78 | 100 |
| ND | P85 | 5/5/white | 59 | 110 |
| ND | P89 | 0/0/none | 108 | 15 |
| ND | P88 | 2/2/orange | 108 | 65 |
| ND | P81 | 0/0/none | 123 | 0 |
| ND | P83 | 2/3/dark orange | 96 | 95 |
Gene disrupted by transposon, pellicle morphology, attachment ability, and swimming motility. ND, not determined.
Scores for pellicle assay to estimate thickness/porosity/pigmentation (arbitrary scale from 0 [none] to 5 [wild-type level]).
Surface plasmon resonance measurements are maximum angle variation (mDeg) after 1,000 s.
Diameter (percentage of TT01 rif) of swimming halo after 6 days.
Characterization of biofilm mutants.
The initial attachment of the mutant strains to an abiotic surface was quantified by using surface plasmon resonance (SPR), a technique previously used to measure cellular and molecular binding to an abiotic gold surface (28). The majority of mutants showed surface attachment similar to that of parental strain TT01 rif (confirmed by Kruskal-Wallis statistical analysis). The only mutant that displayed a significant difference (less than 50% compared to the wild type) was P27, in which plu4004 was interrupted, encoding a protein which contains a phosphoheptose isomerase domain, shared with proteins involved in the biosynthesis of the lipopolysaccharide core region (Table 2).
Flagella are involved in motility and have been shown to be important for biofilm formation by other pathogens (31). Therefore, we assessed the motility of the majority of identified mutants using standard flagellum-mediated swimming agar assays (Table 2). Among the tested mutants, 8 showed a decrease in motility greater than 80%. As expected, mutants with defects in genes involved in motility, such as the flagellum (flgG), motor proteins (motA and motB), and the chemotaxis two-component system (cheA), did not swim at all. Other nonswimmers for which sequences could be determined included P56, a putative EPS mutant.
Further analyses were carried out to test the symbiotic and pathogenic phenotypes of the biofilm mutants. In vitro gut colonization assays with Heterorhabditis bacteriophora IJs in plates fed with each individual mutant showed no significant defect in the colonization of the symbiotic nematode, recovering hundreds of symbiotic bacteria per IJ nematode (data not shown). Initial infection bioassays were carried out by using the larvae of the greater wax moth Galleria mellonella, which, due to its small size and commercial availability, allowed the testing of a large number of bacterial strains. The insects injected with each of the mutants showed no significant attenuation (greater than 20%, as occurred in controls) of larval mortality after 48 h in this insect model (data not shown).
Notably, from this screen only a single mutant was identified in a putative EPS biosynthesis gene, P56, in which the manA phosphomannose isomerase (PMI) gene was interrupted. ManA catalyzes the reversible interconversion of mannose-6-phosphate to fructose-6-phosphate. Given that the manA mutant was clearly deficient in biofilm formation and motility and that ManA has a role in EPS biosynthesis in other bacteria (15), we selected this mutant strain as a model to further investigate the role of EPS production in the Photorhabdus life cycle.
Phenotypic characterization and complementation of the manA mutant.
Analysis of the genomic organization around the manA gene showed that it appears to be monocistronic, with no similar sugar metabolism or EPS production/export genes in the vicinity. Furthermore, there were no obvious homologues of regulators tightly linked to the manA gene. Directly upstream of manA is fumC, a gene involved in the reversible conversion of fumarate to l-malate in the tricarboxylic acid (TCA) cycle. However, this gene is encoded on the opposite strand of manA and is unlikely to have an effect on its regulation or function.
Photorhabdus luminescens TT01 harbors a unique copy of manA in its genome. The closest homologue of the plu2360 manA gene in other bacteria occurs in the close relative Photorhabdus asymbiotica (pau02157), which shows 87% sequence identity by BLAST-P analysis (2, 3, 41). Other than Photorhabdus spp. the closest homologue of manA is found in Providencia rettgeri (NCBI accession number ZP_03639012), showing 66% sequence identity. This gene is also conserved in other members of the Gammaproteobacteria, including Escherichia spp., Yersinia spp., and Salmonella spp.
In order to rule out the possibility that the manA mutant's inability to form a biofilm pellicle was the result of a general growth defect, we compared growth curves with the parental strain. The manA mutant was able to grow as efficiently as the parental strain in rich medium (LB broth), with early stationary phase attained at ca. 16 to 18 h and the doubling times of TT01 rif and the manA mutant strain calculated as 74 and 77 min, respectively. We also compared the growths of TT01 rif and the manA mutant strain in Grace's and Schneider's insect defined media and in clarified hemolymph from larvae of the insect model Manduca sexta in order to mimic the growth conditions encountered inside the insect. There was no growth difference between the manA mutant and TT01 rif under the conditions tested (data not shown).
The transcomplementation of the manA mutant with a functional copy of the gene and its putative promoter region confirmed that this was the mutation responsible for the biofilm and motility defects. The pellicle assay (Fig. 1 A) demonstrated that 6 days after inoculation, TT01 rif developed a thick, structured pellicle with complete coverage of the liquid surface and an orange pigmentation, presumably due to anthraquinone pigment production (9). In contrast to this, the manA mutant did not produce orange pigmentation and formed a ring of cells which adhered to the glass at the air-liquid interface with little spread across the surface of the liquid, resulting in a very thin pellicle. Conversely, the transcomplemented manA+ strain showed a complete recovery of phenotype, with the pellicle structure, pigmentation, and development time consistent with those of parental strain TT01 rif.
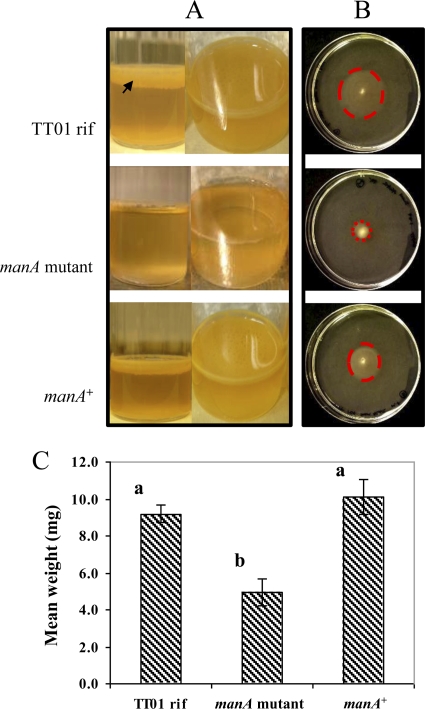
FIG. 1.
Complementation of pellicle formation, motility, and maximum deformation mass (MDM) assays. (A and B) TT01 rif, the manA mutant, and the transcomplemented strain (manA+) were tested with the pellicle (A) and motility (B) assays for 6 days. (A) Typical pellicle formation resulted in the formation of a thick matrix of EPS spread across the surface of the liquid (arrow). The manA mutant strain showed attachment to the glass but a much reduced spread across the surface, whereas with the manA+ strain, the phenotype was rescued to TT01 rif levels. (B) The defect in motility shown by the manA mutant strain was rescued to nearly wild-type levels in the complemented manA+ strain (halos marked with dotted circles). (C) The MDM values for 7-day-old pellicles of TT01 rif and the manA mutant and manA+ strains were estimated by dropping preweighed glass beads onto the surface of the biofilms until the matrix broke to allow the bead through (error bars calculated by standard error). One-way ANOVA revealed a significant difference (P = 0.0057), and a comparison of means by the Tukey-Kramer honestly significant difference (HSD) test (P = 0.05) revealed strain-specific differences shown by letters above the columns.
Differences in the strengths of these floating biofilms were quantified by adding preweighed glass beads to the surface of manA and parental strain pellicles and calculating the maximum deformation mass (MDM) (47). TT01 rif and the transcomplemented manA+ strain displayed similar MDMs, ca. 9 to 10 mg, whereas the manA mutant MDM was only half that value, ca. 5 mg (Fig. 1C).
The manA+ strain was also rescued for motility (Fig. 1B). The TT01 rif cells swam forming a halo with a mean diameter of 35 mm (standard error [SE], ±2 mm), in contrast to that of the manA mutant strain, which only extended to a mean of 8 mm (SE, ±0). The halo of the manA+ strain extended three times further than that of the manA mutant strain (24-mm mean diameter; SE, ±0), but the complementation was not to the level of TT01 rif.
The manA mutant is unable to utilize mannose as a sole carbon source.
ManA (PMI) was previously reported to be involved in the metabolism of mannose in E. coli (52). In addition to its role in biofilm production, we tested the role of ManA in the central metabolism of P. luminescens. The manA strain and strain TT01 rif were streaked from rich LB medium onto minimal medium agar containing either glucose, fructose, or mannose as the sole carbon source (Fig. 2 A). TT01 rif grew well on all three sugars, whereas the growth of the manA mutant was severely and specifically impaired on the mannose agar. However, we noted the presence of a few colonies after 2 to 3 days. We isolated four of these colonies that appeared on the mannose plates, which did not contain kanamycin, to select for the maintenance of the transposon. PCR and sequencing of the manA gene showed an absence of the transposon coinciding with the ability to grow on mannose and restoration of the pellicle phenotype (data not shown), demonstrating that these colonies had reverted to the wild type.
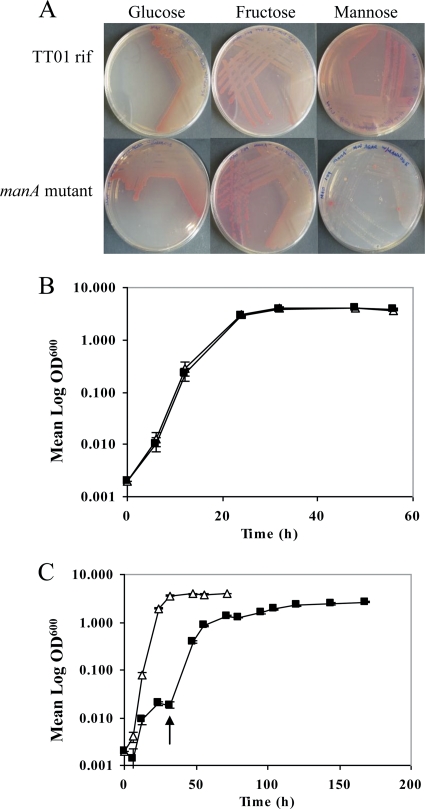
FIG. 2.
Growth of TT01 rif and the manA mutant strain on different carbon sources. (A) Minimal agar containing glucose, fructose, or mannose as a sole carbon source. The manA mutant strain did not grow on mannose plates; however, a few revertant colonies can be observed. (B and C) Strain TT01 rif (triangles) and the manA mutant strain (squares) were also grown in liquid cultures containing glucose (B) or mannose (C). In mannose cultures, spontaneous revertants (arrow) appeared after 2 days, which resumed normal growth into the exponential and stationary phases.
We also examined the effect of the manA mutation on planktonic growth in shaking cultures of minimal medium containing either glucose (Fig. 2B) or mannose (Fig. 2C) as a sole carbon source. Consistent with the results with solid media, the manA mutant strain and the TT01 rif parent grew identically in minimal medium supplemented with glucose. However, with mannose as the sole carbon source, the manA mutant strain displayed severely attenuated growth. TT01 rif reached early stationary phase after ca. 40 h, whereas by this time the manA mutant strain was still in the very early exponential phase. The lag phase in the manA mutant strain lasted until ca. 32 h, after which the culture began to grow in a way similar to that of TT01 rif, presumably due to the generation of spontaneous revertants, as seen with the plate assays (Fig. 2C, arrow). Taken together, these observations indicate that ManA plays a role in mannose utilization in the central carbon metabolism of P. luminescens TT01.
The manA gene influences biofilm maturation but not initial attachment.
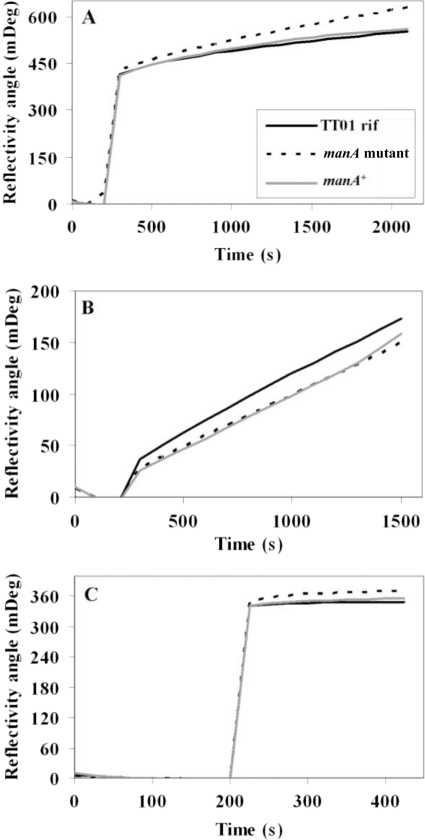
Biofilm formation is preceded by the initial attachment of cells onto a solid surface, in this case the glass surface at the air-liquid interface, followed by the second stage of further development and spread of the pellicle across the surface of the liquid. We quantified this initial attachment stage of biofilm formation using SPR (Fig. 3). Cells and supernatants from broth cultures grown overnight were measured independently of each other, allowing us to differentiate between cell surface features and secreted bacterial factors. The SPR analysis showed that there were no significant differences between TT01 rif, the manA mutant strain, and the complemented mutant manA+ strain in the binding to the abiotic gold surface of whole cultures (Fig. 3A), washed cells resuspended in PBS (Fig. 3B), or supernatants alone (Fig. 3C) from the three strains. These observations support those seen with the biofilm assay, that the manA mutant strain has no deficiency in initial attachment.
FIG. 3.
SPR measurements of TT01 rif (solid line), the manA mutant strain (broken line), and the manA+ strain (gray line). The measurements per strain were made in triplicate. (A) Cultures grown overnight were diluted to an OD600 of 1 in LB broth and measured. (B and C) Cells from the same dilution were washed and resuspended in PBS (B), and the supernatants from the diluted cultures were also measured (C).
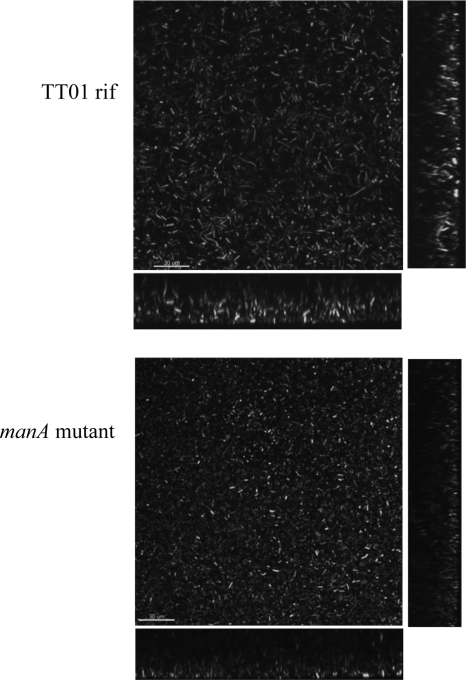
To elucidate the impact of the manA disruption on the architecture of the mature biofilm on an abiotic surface, we used confocal microscopy (Fig. 4) to compare 7-day-old biofilms grown on a microscope chamber slide. The distributions of cells on the surface appeared comparable between the mutant and parental strain biofilms, and the lateral sections showed that they developed similar undulating structures of peaks and troughs. However, the average height of the biofilms produced by the manA mutant was markedly reduced, with a mean of 15 μm (SE, ±2 μm), compared to 35 μm (SE, ±3 μm) for the parental strain. This suggested that while ManA is not involved in the initial colonization of a surface, it does have a role in the maturation of the normal biofilm structure. This is consistent with the SPR data and the observation that the manA mutant strain can attach to the glass of the tube at the air-liquid interface but cannot form a completely developed pellicle.
FIG. 4.
Confocal microscopy images of GFP-labeled TT01 rif and manA mutant biofilms. Transversal views show the heights of the biofilms formed by the manA mutant strain (15 ± 2 μm) and TT01 rif (35 ± 3 μm). Measurements are the means of 10 transects ± SE. Scale bar, 30 μm.
Role of manA in the life cycle of P. luminescens TT01.
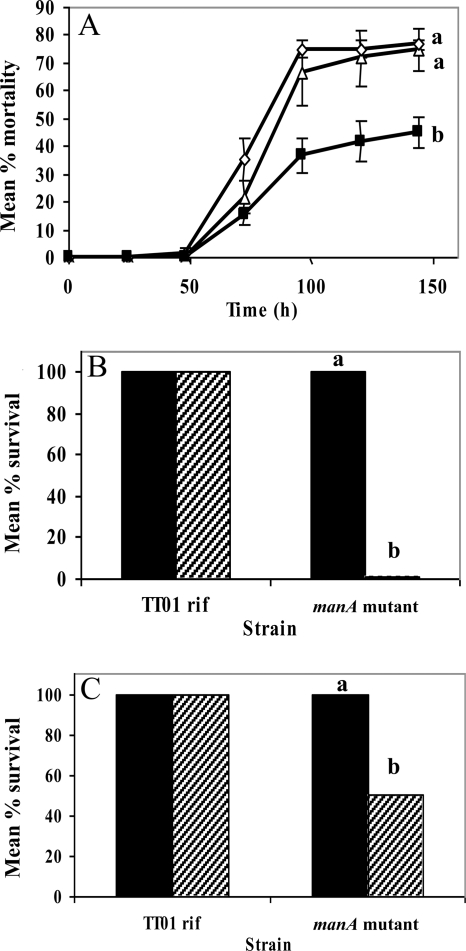
The influence of manA on insect pathogenicity was assessed by using a well-known insect model: the tobacco hornworm Manduca sexta (29). Approximately 1,000 cells of the manA mutant strain and strain TT01 rif were each injected into 20 insect larvae. The manA mutant strain displayed attenuated virulence 30% lower than that of TT01 rif (Fig. 5 A). Significantly, virulence in the transcomplemented manA+ strain was restored to the level of the parental strain. These results suggest that EPS has a significant role in the virulence of P. luminescens in an insect host.
FIG. 5.
Virulence assay and resistance to antimicrobial compounds. (A) Cohorts of 20 Manduca sexta larvae per treatment were injected with 1,000 cells each of P. luminescens strain TT01 rif (triangles), the manA mutant strain (squares), and the manA+ strain (diamonds). The experiment was repeated three times. The insects were scored for mortality each day for 6 days (144 h), and those injected with the manA mutant showed decreased mortality. One-way ANOVA revealed a significant difference (P < 0.0001; df = 3), with specific effects of strain (P = 0.0007; df = 2) and time (P < 0.0001; df = 1). Significantly different mean values for the three strains were revealed by a Tukey-Kramer HSD test (P = 0.05), as shown by letters beside each line. (B) Cultures of TT01 rif and the manA mutant strain were treated with 0.03% H2O2 (hatched bars) and water as a control (solid bars) for 1 h before dilution and plating to determine the percentage of cells that could survive exposure. (C) The strains were also treated with 10 μM cecropin A (hatched bars) and an equivalent volume of water as a control (solid bars). Significant differences were revealed by one-way ANOVA (B, P < 0.0001; df = 3; C, P < 0.0179. df = 1) and by a Tukey-Kramer HSD test (P = 0.05 for both B and C), as shown by letters above the columns.
To address if the attenuation results from a failure to form a biofilm or through some other virulence-related function of the EPS, we examined the role of EPS in bacterial protection from known immune factors in vitro. The manA mutant exhibited a greater sensitivity than the parental strain both to reactive oxygen species (ROS), in the form of H2O2, (Fig. 5B) and to cecropin A, an insect-derived antimicrobial peptide (AMP) (Fig. 5C). TT01 rif showed 100% recovery when treated with both H2O2 and water as a control (Fig. 5B); however, the manA mutant recovery was negligible when subjected to the H2O2 treatment. TT01 rif showed 100% recovery when treated with 10 μM cecropin A and water (Fig. 5C), whereas manA mutant recovery after cecropin A treatment was only 50%. The differential survival of the strains when exposed to these compounds indicates that a fully functional EPS is required to protect the bacterium against antimicrobial agents.
As described above, none of the mutants identified in the screening showed a defect in the colonization of the IJ nematode gut. Further investigations were done to establish if EPS production by P. luminescens is important for the maternal transmission of the bacterial symbionts to new IJs developing in the maternal body cavity. The transmission of the symbiotic bacteria takes place by several stages, the first of which is the attachment and formation of a microcolony on the maternal posterior intestine (13). For this assay the manA mutant strain and strain TT01 rif were GFP labeled to be detected by fluorescence microscopy in IJs and during maternal transmission. TT01 rif gave a mean value of 0.95 (SE, ±0.02), and the manA mutant strain gave a mean value of 0.96 (SE, ±0.03), showing that the transmission efficiency of the manA mutant was not compromised (a transmission efficiency of 1.00 means that bacteria are transmitted by all nematodes). No defects were observed in other steps of transmission, including symbiont biofilm formation on the posterior intestinal cells of the maternal nematode (data not shown).
Structural analysis of EPS from P. luminescens TT01.
An analysis of the composition and linkage of highly purified EPS from the manA mutant strain and TT01 rif was conducted in order to determine the core units and linkage groups of the P. luminescens EPS and to elucidate the role of the ManA enzyme in its formation. TT01 wild-type strain EPS was also analyzed (data not shown), which ruled out any impact of the spontaneous rifampin resistance mutation necessary to make the initial transposon mutant library. Interestingly, although the manA mutant strain does not appear to produce a large mature biofilm, it was nevertheless possible to purify EPS for the analysis. This indicates that the manA mutant biofilm defect is likely to be related to alterations in EPS properties rather than the lack of it.
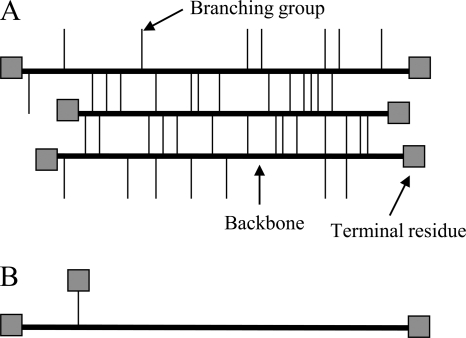
The results of the composition analysis (Table 3) show that the monomeric components of EPS in both strains are nearly identical. The percentages of each of the sugar residues present are also very similar between the two strains, with the main difference being the relative amounts of glucose, present at 7% in TT01 rif and 1.6% in the manA mutant strain. However, the amount of mannose present in the EPS of the manA mutant strain is very similar to that found in TT01 rif. Significantly, the linkage analysis revealed striking differences between the TT01 rif and manA mutant EPS (Table 4). EPS from TT01 rif contained a total of nine different linkage groups, whereas EPS from the manA mutant strain contained only a total of four. Most of the missing groups were residues linked in two and three positions (e.g., 3,4-linked galactopyranose and 3,4,6-linked galactopyranose). From these results we inferred that the missing groups from manA mutant EPS result in a less branched, linear structure (Fig. 6).
TABLE 3.
Composition analysis of purified EPS from TT01 rif and the manA mutant strain
| Residue | Mol% attachment |
|
|---|---|---|
| TT01 rif | manA mutant | |
| Xylose | 8.1 | 7.1 |
| Glucuronic acid | 5.3 | 2.5 |
| Mannose | 9.0 | 10.9 |
| Galactose | 67.3 | 72.2 |
| Glucose | 7.0 | 1.6 |
| N-Acetylglucosamine | 3.4 | 5.6 |
| Total | 100.1 | 99.9 |
TABLE 4.
Linkage analysis of purified EPS from TT01 rif and the manA mutant straina
| Glycosyl residue | % linkage |
|
|---|---|---|
| TT01 rif | manA mutant | |
| Terminal Manp | 6.8 | 8.2 |
| Terminal Galp | 4.5 | 4.4 |
| Terminal Glcp | 0 | 3.5 |
| 2,3-linked Manp | 0 | 4.6 |
| 2-linked Manp | 4.3 | 0 |
| 3-linked Galp | 41.0 | 45.7 |
| 4-linked Galp | 7.0 | 3.2 |
| 4-linked Glcp | 14.5 | 30.4 |
| 3,4-linked Galp | 5.9 | 0 |
| 3,4-linked Glcp | 3.6 | 0 |
| 4,6-linked Galp | 1.7 | 0 |
| 3,6-linked Galp | 5.0 | 0 |
| 3,4,6-linked Galp | 5.8 | 0 |
| Total | 100.1 | 100.0 |
Manp, mannopyranose; Galp, galactopyranose; Glcp, glucopyranose.
FIG. 6.
Low-resolution model of the EPS branching of P. luminescens TT01. Data from the linkage analysis allowed us to present this simple model illustrating that the EPS of TT01 rif (A) has a more branched and cross-linked organization than that of the manA mutant strain, which has a more linear structure (B).
DISCUSSION
The initial screening of the Photorhabdus luminescens TT01 rif transposon library for EPS-deficient mutants utilized the ability of these bacteria to form extensive floating biofilm structures in static rich medium, a method previously used for other bacteria (46, 47). The phenotypes observed included subtle macroscopic alterations to biofilm structure, porosity, and pigmentation and more obvious differences of developmental arrest of the structure before the matrix could be fully matured. A determination of the genes interrupted in the selected biofilm-deficient mutants revealed homologues of genes implicated in biofilm formation in other species (31).
The manA mutant was the only gene directly implicated in EPS production as opposed to other biofilm formation defects. For this reason we used this strain as a model to investigate the role of EPS production per se, which may have functions independent of its role in biofilm matrix composition. ManA (PMI) is responsible for the reversible catalysis of mannose-6-phosphate and fructose-6-phosphate and has a dual function in the early metabolism stages of mannose (52) and also as part of the alginate production machinery in P. aeruginosa (15). As such, we infer the function of P. luminescens TT01 ManA from evidence from other organisms. It should be noted that the phenotype of this mutant in the biofilm assay was extremely similar to that of the flagellum mutants: a ring of cells developed on the glass at the air-liquid interface, and further spread across the surface of the liquid was severely limited. In addition, like the flagellum mutants, the manA mutant strain was also attenuated in the ability to swim through semisolid medium, revealing an interesting link between EPS production and flagellum-mediated motility in Photorhabdus. A deficiency in EPS production and swimming motility was previously reported for Stenotrophomonas maltophilia (26). Other reports have linked swimming motility and exopolysaccharide production through the action of regulators common to both mechanisms (5, 25, 53), but in the case of this manA mutant, this is an unlikely explanation, as manA is not known to function as a regulator, and also, there are no other regulators in the immediate vicinity of the manA loci which could be affected. Moreover, transmission electron microscopy (TEM) revealed that flagella were present in both TT01 rif and the manA mutant strain (data not shown). It is possible that the manA mutation is causing a more general pleiotropic effect through its role in central carbon metabolism. Further insights into the role of manA in the biology of P. luminescens TT01 were gained by growing the manA mutant strain in media containing glucose, mannose, or fructose as a sole carbon source. In contrast with the use of other sugars, the inability of the manA mutant to use mannose as the sole carbon source and the appearance of spontaneous revertants indicate that manA is essential not only for EPS and biofilm formation but also for the metabolism of mannose.
Biofilm formation in bacteria has been divided into five general stages (35). The first stage is reversible attachment to a surface, often mediated by electrostatic forces, which soon develops into the second stage of irreversible adhesion and the early production of EPS material. As the bacteria develop into larger microcolonies on the surface, they become embedded in a more extensive matrix of EPS (stage 3), which forms the base of the more mature “mushroom” structures containing both dead and live subpopulations of cells (stage 4). The final stage is the release of living planktonic cells from these “mushrooms,” which colonize new surfaces, which restarts the process. Importantly, Photorhabdus has never been isolated away from its symbiont or insect host, and as such, it exists in an entirely biotic landscape (51); therefore, the classic abiotic surface biofilm structures previously reported for other Gram-negative bacteria may not be as relevant. Screen-identified mutants were deficient in the initial attachment and/or later biofilm maturation. SPR revealed that the efficiency of the initial attachments of TT01 rif and the manA mutant strain to the abiotic surface were identical. This suggests that a normal EPS structure is not crucial for cell attachment by Photorhabdus. Confocal microscopy indicated that under these conditions, P. luminescens does not form the typical “mushroom” formations observed for other bacterial species such as P. aeruginosa (31), although the bacterial mat did form undulating surface structures. The general aspects of the parental and manA mutant biofilms on a glass surface were similar, with the exception of the thicknesses of the biofilms, suggesting that an intact EPS is required for the full growth of the biofilm.
The mechanisms of the biphasic life-style of P. luminescens, switching from a symbiont to a pathogen, are still poorly understood. It is not known whether, as in other pathogenic bacteria (24), EPS production and its involvement in biofilm formation is an important factor in the establishment of associations with the vector. This seems unlikely, as the transmission efficiency of the manA mutant strain in the host nematode was found to be close to 100% and therefore very similar to that of TT01 rif, suggesting that EPS-mediated biofilm formation on abiotic surfaces is not a requirement for successful symbiosis. Conversely, the mad fimbria locus, involved in adhesion, and genes implicated in lipopolysaccharide (LPS) biosynthesis were previously shown to be essential for an efficient symbiosis of P. luminescens with the nematode vector (17, 44). The colonization of IJs by P. luminescens is described as a biofilm. However, the conditions under which that particular biofilm is formed and its genetic requirements are very different from those of the biofilm structures observed in the present study. It was shown previously that four out of the six mutants defective for nematode colonization formed biofilms in vitro that were similar to those of wild type (17). Similarly, the symbiosis-deficient madA fimbria mutant was able to produce biofilm in vitro (44), indicating that the formation of a biofilm in the nematode vector has different characteristics than those of biofilms formed in other niches.
When introduced to the insect host M. sexta, the manA mutant strain displays a significant reduction in virulence. The decrease in virulence is unlikely to be due to a growth defect, since the in vitro growth of the manA strain in M. sexta clarified hemolymph was identical to that of the wild type. The decrease in virulence was not observed when G. mellonella larvae were used as a model. This could be due to differences in the sensitivities of the hosts and suggests that a manA mutation may affect P. luminescens toxicity only in certain hosts. Considering the armory of insecticidal toxins and delivery systems that Photorhabdus possesses (10, 18), the finding that a reduction in EPS levels can have such an effect on the pathogenic behavior of the cells is informative and highlights its importance in infection. EPS is known to be involved in limiting access by antimicrobial compounds (11), masking immune recognition (36), suppressing defense signaling by chelating calcium ions (4), and neutralizing virulence effectors in some pathogens (38). Also, we speculate that a structurally aberrant or underproduced EPS, such as that of the manA mutant strain, may not serve to effectively shield bacterial defense-eliciting microbe-associated molecular patterns (MAMPs), leaving it more susceptible to triggering immune recognition and responses. As reported here, and previously for Xanthomonas (30), the presence of a mature EPS provides the bacterial cell with protection from reactive oxygen species in the form of H2O2 and from cecropin A, an antimicrobial peptide (AMP). AMP production is one of the mainstays of insect innate immunity, so one role of EPS in immune evasion seems clear. Overall, it is evident that EPS can function to shield the bacterial cells from various components of the host immune system.
Linkage analysis revealed that the EPS of the manA mutant strain contains markedly fewer linkage groups, which in turn leads to an EPS with a minimally branched, almost linear structure. Prima facie, the low-branched EPS produced by the manA mutant strain would not cross-link as readily, leading to the formation of a truncated, less extended matrix structure, which would also be structurally weaker, as suggested by the MDM results. Lower mechanical strength is likely to make it more difficult for the manA mutant strain to form a mature biofilm with the structural stability of that produced by the parental strain. The viscosity of EPS from a becA (PMI homologue) mutant of Burkholderia cepacia was much lower than that of the wild type (45). The authors of that study speculated that the reduced viscosity could be due to a reduction in the cellular concentration of UDP-sugar precursors resulting in a shorter polysaccharide chain, as seen previously for Streptococcus pneumoniae (49). Although the present study shows no evidence of a reduction in EPS chain length in the manA mutant strain, it may be possible that a deficiency in mannose metabolism could lead to a downstream reduction in the availability of UDP-sugars normally utilized in the branching groups, resulting in the more linear structure of manA mutant EPS. The levels of mannose in the EPS from strain TT01 rif and the manA mutant were surprisingly very similar. This suggests that although manA is involved in the shuttling of mannose in the central metabolism, it is not required directly for the incorporation of this residue into the EPS structure.
EPS has been repeatedly demonstrated to have an important role as a virulence factor in a variety of pathogenic bacterial species, and this study shows that the EPS of P. luminescens TT01 is no exception. Here we reveal not only that a mutation in a gene encoding a mannose metabolism enzyme (ManA or PMI) has an effect on the ability of P. luminescens TT01 to proliferate within a host organism but also that the fundamental structure of its major extracellular polysaccharide is severely altered. We have presented evidence that the consequences of this can have an impact on cellular motility and that the survival of the bacteria when exposed to the host immune system is likely to be impaired. These results highlight the complete requirement for fully functional extracellular polysaccharide material in the pathogenicity and life-style success of P. luminescens TT01.
Acknowledgments
This work was funded by grants from the BBSRC (BB/E021328/1) and the EU 7th Framework (EU FP7 EMBEK1) to N.R.W. and from the Department of Energy (DE-FG09-93ER-20097) to the Center for Plant and Microbial Complex Carbohydrates (University of Georgia).
Footnotes
Published ahead of print on 10 December 2010.
REFERENCES
- 1.Adam, O., A. Vercellone, F. Paul, P. F. Monsan, and G. Puzo. 1995. A nondegradative route for the removal of endotoxin from exopolysaccharides. Anal. Biochem. 225:321-327. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam, S. N., et al. 2008. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 18:1078-1083. [DOI] [PubMed] [Google Scholar]
- 5.Bahlawane, C., M. McIntosh, E. Krol, and A. Becker. 2008. Sinorhizobium meliloti regulator MucR couples exopolysaccharide synthesis and motility. Mol. Plant Microbe Interact. 21:1498-1509. [DOI] [PubMed] [Google Scholar]
- 6.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobrov, A. G., O. Kirillina, S. Forman, D. Mack, and R. D. Perry. 2008. Insights into Yersinia pestis biofilm development: topology and co-interaction of Hms inner membrane proteins involved in exopolysaccharide production. Environ. Microbiol. 10:1419-1432. [DOI] [PubMed] [Google Scholar]
- 8.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 9.Brachmann, A. O., et al. 2007. A type II polyketide synthase is responsible for anthraquinone biosynthesis in Photorhabdus luminescens. Chembiochem 8:1721-1728. [DOI] [PubMed] [Google Scholar]
- 10.Brugirard-Ricaud, K., et al. 2005. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 7:363-371. [DOI] [PubMed] [Google Scholar]
- 11.Bylund, J., L. A. Burgess, P. Cescutti, R. K. Ernst, and D. P. Speert. 2006. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 281:2526-2532. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciche, T. A., K. S. Kim, B. Kaufmann-Daszczuk, K. C. Nguyen, and D. H. Hall. 2008. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 74:2275-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 15.Darzins, A., L. L. Nixon, R. I. Vanags, and A. M. Chakrabarty. 1985. Cloning of Escherichia coli and Pseudomonas aeruginosa phosphomannose isomerase genes and their expression in alginate-negative mutants of Pseudomonas aeruginosa. J. Bacteriol. 161:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deretic, V., M. J. Schurr, and H. Yu. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 3:351-356. [DOI] [PubMed] [Google Scholar]
- 17.Easom, C. A., S. A. Joyce, and D. J. Clarke. 2010. Identification of genes involved in the mutualistic colonization of the nematode Heterorhabditis bacteriophora by the bacterium Photorhabdus luminescens. BMC Microbiol. 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ffrench-Constant, R., and D. Bowen. 1999. Photorhabdus toxins: novel biological insecticides. Curr. Opin. Microbiol. 2:284-288. [DOI] [PubMed] [Google Scholar]
- 19.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 20.Gacesa, P., and J. B. Goldberg. 1992. Heterologous expression of an alginate lyase gene in mucoid and non-mucoid strains of Pseudomonas aeruginosa. J. Gen. Microbiol. 138(Pt. 8):1665-1670. [DOI] [PubMed] [Google Scholar]
- 21.Hall-Stoodley, L., and P. Stoodley. 2009. Evolving concepts in biofilm infections. Cell. Microbiol. 11:1034-1043. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 23.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinnebusch, B. J., and D. L. Erickson. 2008. Yersinia pestis biofilm in the flea vector and its role in the transmission of plague. Curr. Top. Microbiol. Immunol. 322:229-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoang, H. H., N. Gurich, and J. E. Gonzalez. 2008. Regulation of motility by the ExpR/Sin quorum-sensing system in Sinorhizobium meliloti. J. Bacteriol. 190:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, T. P., E. B. Somers, and A. C. Wong. 2006. Differential biofilm formation and motility associated with lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes in Stenotrophomonas maltophilia. J. Bacteriol. 188:3116-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins, A. T., R. Ffrench-Constant, A. Buckling, D. J. Clarke, and K. Jarvis. 2004. Study of the attachment of Pseudomonas aeruginosa on gold and modified gold surfaces using surface plasmon resonance. Biotechnol. Prog. 20:1233-1236. [DOI] [PubMed] [Google Scholar]
- 28.Jones, R. T., et al. 2010. Photorhabdus adhesion modification protein (Pam) binds extracellular polysaccharide and alters bacterial attachment. BMC Microbiol. 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanost, M. R., H. Jiang, and X. Q. Yu. 2004. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198:97-105. [DOI] [PubMed] [Google Scholar]
- 30.Kemp, B. P., J. Horne, A. Bryant, and R. M. Cooper. 2004. Xanthomonas axonopodis pv. manihotis gumD gene is essential for EPS production and pathogenicity and enhances epiphytic survival on cassava (Manihot esculenta). Physiol. Mol. Plant Pathol. 64:209-218. [Google Scholar]
- 31.Klausen, M., et al. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 32.Kovach, M. E., et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 33.Little, S. F., and B. E. Ivins. 1999. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1:131-139. [DOI] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 35.Monroe, D. 2007. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 5:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raffatellu, M., et al. 2006. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 74:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramamurthy, T., S. Yamasaki, Y. Takeda, and G. B. Nair. 2003. Vibrio cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect. 5:329-344. [DOI] [PubMed] [Google Scholar]
- 38.Reckseidler-Zenteno, S. L., R. DeVinney, and D. E. Woods. 2005. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect. Immun. 73:1106-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds, S. E., S. F. Nottingham, and A. E. Stephens. 1985. Food and water economy and its relation to growth in 5th-instar larvae of the tobacco hornworm, Manduca sexta. J. Insect Physiol. 31:119-127. [Google Scholar]
- 40.Roberts, I. S. 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50:285-315. [DOI] [PubMed] [Google Scholar]
- 41.Schaffer, A. A., et al. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29:2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva, C. P., et al. 2002. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell. Microbiol. 4:329-339. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 44.Somvanshi, V. S., B. Kaufmann-Daszczuk, K. S. Kim, S. Mallon, and T. A. Ciche. 2010. Photorhabdus phase variants express a novel fimbrial locus, mad, essential for symbiosis. Mol. Microbiol. 77:1021-1038. [DOI] [PubMed] [Google Scholar]
- 45.Sousa, S. A., et al. 2007. The Burkholderia cepacia bceA gene encodes a protein with phosphomannose isomerase and GDP-D-mannose pyrophosphorylase activities. Biochem. Biophys. Res. Commun. 353:200-206. [DOI] [PubMed] [Google Scholar]
- 46.Spiers, A. J., J. Bohannon, S. M. Gehrig, and P. B. Rainey. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50:15-27. [DOI] [PubMed] [Google Scholar]
- 47.Spiers, A. J., and P. B. Rainey. 2005. The Pseudomonas fluorescens SBW25 wrinkly spreader biofilm requires attachment factor, cellulose fibre and LPS interactions to maintain strength and integrity. Microbiology 151:2829-2839. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland, I. W. 1998. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 16:41-46. [DOI] [PubMed] [Google Scholar]
- 49.Ventura, C. L., R. T. Cartee, W. T. Forsee, and J. Yother. 2006. Control of capsular polysaccharide chain length by UDP-sugar substrate concentrations in Streptococcus pneumoniae. Mol. Microbiol. 61:723-733. [DOI] [PubMed] [Google Scholar]
- 50.Vu, B., M. Chen, R. J. Crawford, and E. P. Ivanova. 2009. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterfield, N. R., T. Ciche, and D. Clarke. 2009. Photorhabdus and a host of hosts. Annu. Rev. Microbiol. 63:557-574. [DOI] [PubMed] [Google Scholar]
- 52.Weisser, P., R. Kramer, and G. A. Sprenger. 1996. Expression of the Escherichia coli pmi gene, encoding phosphomannose-isomerase in Zymomonas mobilis, leads to utilization of mannose as a novel growth substrate, which can be used as a selective marker. Appl. Environ. Microbiol. 62:4155-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells, D. H., E. J. Chen, R. F. Fisher, and S. R. Long. 2007. ExoR is genetically coupled to the ExoS-ChvI two-component system and located in the periplasm of Sinorhizobium meliloti. Mol. Microbiol. 64:647-664. [DOI] [PubMed] [Google Scholar]
- 54.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]