Abstract
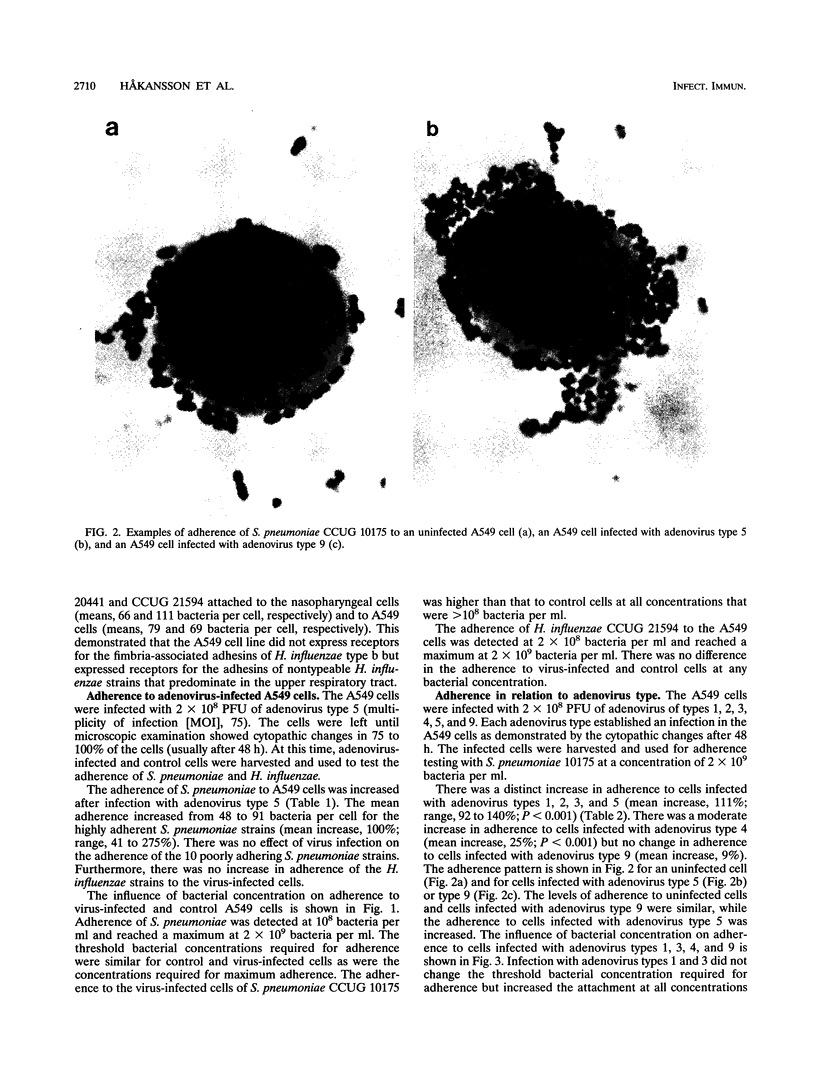
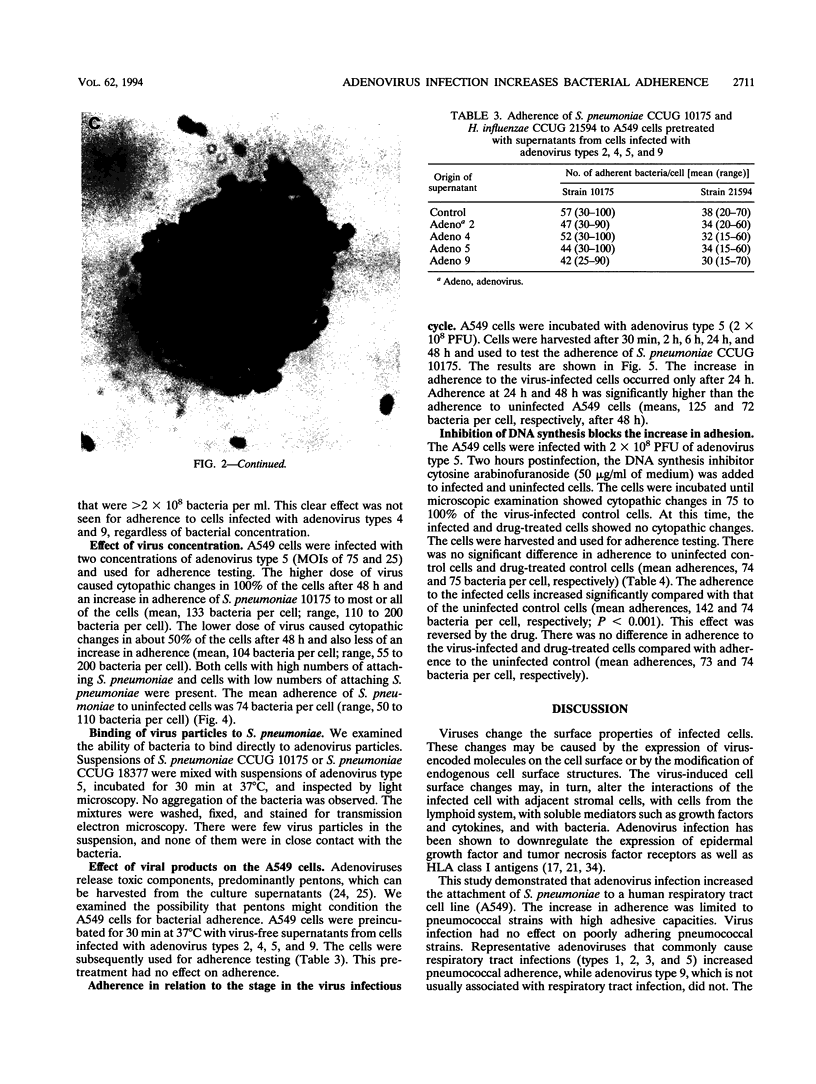
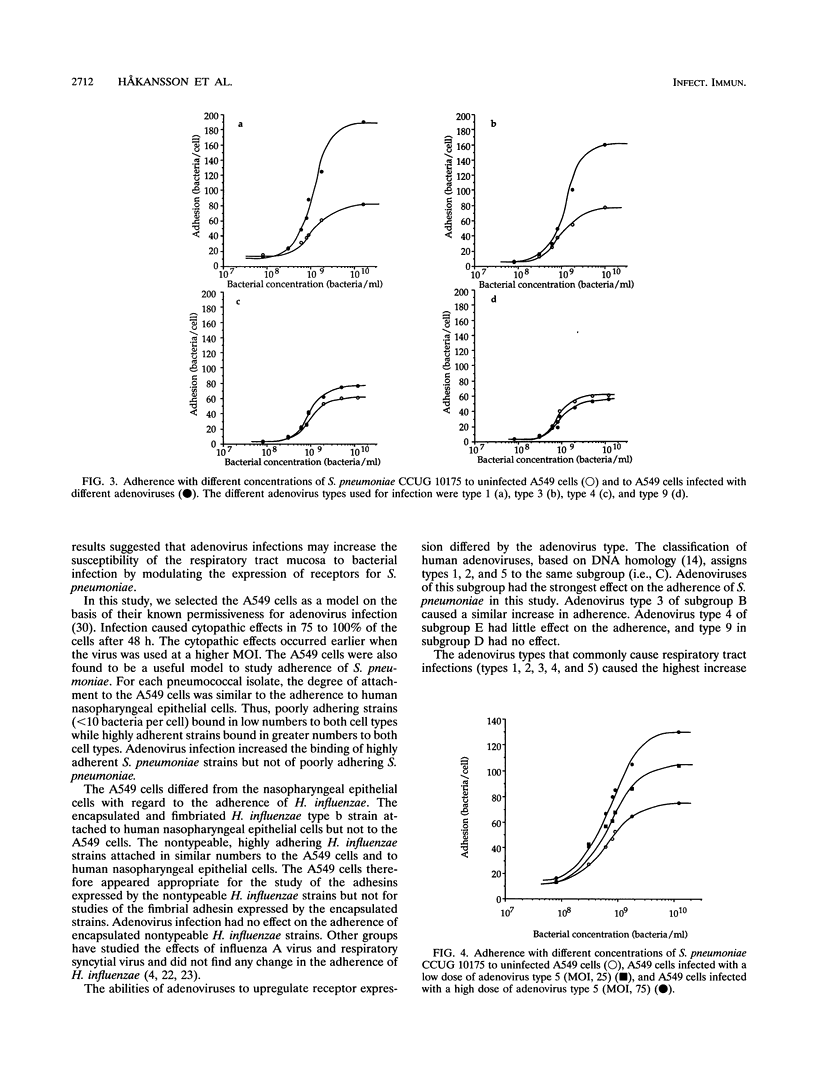
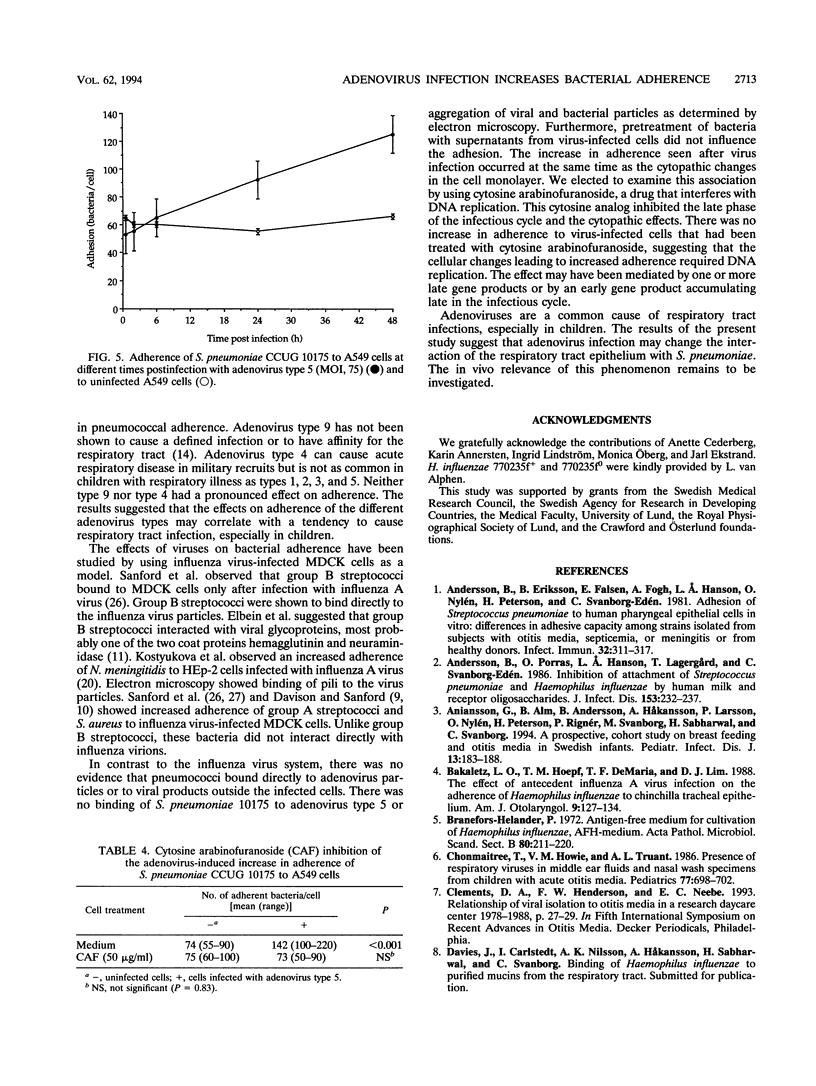
Viruses are thought to facilitate bacterial infections of the respiratory tract, but the mechanisms are poorly understood. The present study analyzed the effect of adenovirus on bacterial adherence to human respiratory tract epithelial cells. The human lung carcinoma cell line A549 was infected with adenovirus of types 1, 2, 3, 4, 5, and 9. At a multiplicity of infection of 75 particles per cell, cytopathic effects occurred in 75 to 100% of the cells within 48 h. The virus-infected cells were harvested at various times after infection and analyzed for the ability to bind strains of Haemophilus influenzae and Streptococcus pneumoniae. Adenovirus (types 1, 2, 3, and 5) commonly causing respiratory tract infections increased the binding of adherent S. pneumoniae strains to the cells. This effect was not seen for other adenovirus types. Adenovirus infection did not change the adherence of cells of poorly adhering strains of S. pneumoniae or H. influenzae. The increase in adherence of S. pneumoniae could be inhibited by the DNA synthesis inhibitor cytosine arabinofuranoside, which is known to block the late phase of the adenovirus infection. When electron microscopy was used, there was no evidence that virus particles bound directly to bacteria. Adherence was not affected by pretreatment of the cells with virus particles or viral proteins. This suggested that adenovirus infection upregulated receptors for S. pneumoniae. The increased attachment may be one mechanism by which viruses precondition the respiratory mucosa for bacterial infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Porras O., Hanson L. A., Lagergård T., Svanborg-Edén C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986 Feb;153(2):232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- Aniansson G., Alm B., Andersson B., Håkansson A., Larsson P., Nylén O., Peterson H., Rignér P., Svanborg M., Sabharwal H. A prospective cohort study on breast-feeding and otitis media in Swedish infants. Pediatr Infect Dis J. 1994 Mar;13(3):183–188. doi: 10.1097/00006454-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Bakaletz L. O., Hoepf T. M., DeMaria T. F., Lim D. J. The effect of antecedent influenza A virus infection on the adherence of Hemophilus influenzae to chinchilla tracheal epithelium. Am J Otolaryngol. 1988 May-Jun;9(3):127–134. doi: 10.1016/s0196-0709(88)80018-8. [DOI] [PubMed] [Google Scholar]
- Branefors-Helander P. Antigen-free medium for cultivation of Haemophilus influenzae, AFH-medium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(2):211–220. doi: 10.1111/j.1699-0463.1972.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Howie V. M., Truant A. L. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986 May;77(5):698–702. [PubMed] [Google Scholar]
- Davison V. E., Sanford B. A. Adherence of staphylococcus aureus to influenza A virus-infected Madin-Darby canine kidney cell cultures. Infect Immun. 1981 Apr;32(1):118–126. doi: 10.1128/iai.32.1.118-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison V. E., Sanford B. A. Factors influencing adherence of Staphylococcus aureus to influenza A virus-infected cell cultures. Infect Immun. 1982 Sep;37(3):946–955. doi: 10.1128/iai.37.3.946-955.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Sanford B. A., Ramsay M. A., Pan Y. T. Effect of inhibitors on glycoprotein biosynthesis and bacterial adhesion. Ciba Found Symp. 1981;80:270–287. doi: 10.1002/9780470720639.ch17. [DOI] [PubMed] [Google Scholar]
- Fiala M. A study of the combined role of viruses, mycoplasmas and bacteria in adult pneumonia. Am J Med Sci. 1969 Jan;257(1):44–51. doi: 10.1097/00000441-196901000-00005. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Berzins I. K., Marker S. C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980 Nov;30(2):445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Ruuskanen O., Waris M., Ziegler T., Arola M., Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991 Apr;145(4):445–448. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Sanyal M. A., Watkins J. M., Fairclough D. L., Clyde W. A., Jr, Denny F. W. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982 Jun 10;306(23):1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Burgert H. G. E3/19K from adenovirus 2 is an immunosubversive protein that binds to a structural motif regulating the intracellular transport of major histocompatibility complex class I proteins. J Exp Med. 1990 Dec 1;172(6):1653–1664. doi: 10.1084/jem.172.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Dollete F. R., Yolken R. H. The role of respiratory syncytial virus and other viral pathogens in acute otitis media. J Pediatr. 1982 Jul;101(1):16–20. doi: 10.1016/s0022-3476(82)80172-8. [DOI] [PubMed] [Google Scholar]
- Kuivinen E., Hoffman B. L., Hoffman P. A., Carlin C. R. Structurally related class I and class II receptor protein tyrosine kinases are down-regulated by the same E3 protein coded for by human group C adenoviruses. J Cell Biol. 1993 Mar;120(5):1271–1279. doi: 10.1083/jcb.120.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Connor E., Penney D. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988 Feb;56(2):484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA H. G. A protein factor responsible for the early cytopathic effect of adenoviruses. Virology. 1958 Dec;6(3):601–611. doi: 10.1016/0042-6822(58)90109-0. [DOI] [PubMed] [Google Scholar]
- Patel J., Faden H., Sharma S., Ogra P. L. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int J Pediatr Otorhinolaryngol. 1992 Jan;23(1):15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Höglund S. Sructural proteins of adenoviruses. 3. Purification and characterization of the adenovirus type 2 penton antigen. Virology. 1969 Sep;39(1):90–106. doi: 10.1016/0042-6822(69)90351-1. [DOI] [PubMed] [Google Scholar]
- Sajjan U., Reisman J., Doig P., Irvin R. T., Forstner G., Forstner J. Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):657–665. doi: 10.1172/JCI115632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Fibrinogen-mediated adherence of group A Streptococcus to influenza A virus-infected cell cultures. Infect Immun. 1982 Nov;38(2):513–520. doi: 10.1128/iai.38.2.513-520.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Ramsay M. A. Bacterial adherence to the upper respiratory tract of ferrets infected with influenza A virus. Proc Soc Exp Biol Med. 1987 Jun;185(2):120–128. doi: 10.3181/00379727-185-42525. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Shelokov A., Ramsay M. A. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis. 1978 Feb;137(2):176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- Simpson D. A., Ramphal R., Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992 Sep;60(9):3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Craft D. W., Shiromoto R. S., Yan P. O. Alternative cell line for virus isolation. J Clin Microbiol. 1986 Aug;24(2):265–268. doi: 10.1128/jcm.24.2.265-268.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. A., Schaeffer M., Fox J. P., Brandt C. D., Romano M. Standardization and certification of reference antigens and antisera for 30 human adenovirus serotypes. Am J Epidemiol. 1967 Nov;86(3):617–633. doi: 10.1093/oxfordjournals.aje.a120771. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Young L. S., LaForce F. M., Head J. J., Feeley J. C., Bennett J. V. A simultaneous outbreak of meningococcal and influenza infections. N Engl J Med. 1972 Jul 6;287(1):5–9. doi: 10.1056/NEJM197207062870102. [DOI] [PubMed] [Google Scholar]