Abstract
This study demonstrates a functional twin-arginine (Tat) translocation pathway present in the tsetse fly symbiont Sodalis glossinidius and its potential to export active heterologous proteins to the periplasm. Functionality was demonstrated using green fluorescent protein (GFP) fused to the Tat signal peptide of Escherichia coli trimethylamine N-oxide reductase (TorA).
Expression of proteins that interfere with pathogen development by genetically modified symbiotic bacteria of arthropod disease vectors may serve as a powerful approach to control disease transmission (paratransgenesis). The use of bacterial symbionts expressing foreign proteins in disease-carrying arthropods has also an intriguing potential for studying insect-pathogen interactions (2). Sodalis glossinidius, a maternally inherited Gram-negative symbiont of the tsetse fly, is one of the few insect symbiotic bacteria that can be cultured and genetically modified in vitro (2, 6, 19) and is currently considered a promising drug delivery tool to block trypanosome development in the tsetse fly (1). Active protein secretion is crucial to target the effector molecules to where the trypanosome parasites reside. To date, there have been no studies regarding the export of heterologous proteins to the periplasmatic and/or outer environment of S. glossinidius.
In prokaryotes, most secreted proteins are translocated across the cytoplasmic membrane in an unfolded conformation by the Sec pathway (for a comprehensive review, see reference 10). A recently described Sec-independent pathway mediates the export of proteins in a folded conformation. This alternative pathway has been designated the twin-arginine translocation (Tat) system because of the characteristic twin-arginine motif (S/T-R-R-x-F-L-K) present in the signal peptide of proteins translocated via this system (3, 11, 13, 18, 20; for a recent comprehensive review, see reference 9). In Escherichia coli, a functional Tat pathway requires a minimum set of three gene products: TatA, TatB, and TatC (4). In the present study, we report on the presence of a functional Tat secretory pathway in S. glossinidius and its potential to be exploited for export of heterologous proteins in an active manner to the periplasm.
Analysis of the completed S. glossinidius genome (GenBank accession no. NC_007712) (16) revealed the presence of a tat locus, composed of tatA, tatB, and tatC (GenBank accession no., respectively, SG0112, SG0113, and SG0114), on the circular chromosome with a high level of homology to the corresponding E. coli genes and with identities ranging from 47 to 77% on the amino acid level. A putative promoter was identified (BPROM Server algorithm) 421 bp upstream of tatA, while the close proximity of the individual tat genes (4 bp between tatA and tatB and 4 bp between tatB and tatC) is suggestive for an arrangement as a single operon. Topology prediction (TMpred; http://www.expasy.org/) suggested similar membrane insertion of the S. glossinidius TatABC proteins compared to the corresponding E. coli counterparts. The extent to which this secretory pathway is used in prokaryotes strongly varies according to bacterial species and is not yet well characterized (8). A genome survey to identify putative Tat substrates in the predicted proteome of Sodalis glossinidius using “pattern search” in the Pedant database (http://pedant.gsf.de) resulted in the identification of six substrates (Table 1). The search pattern used was x-RR-x-[VFLIMA]-x. The results from the pattern search were analyzed using the TatP program (http://www.cbs.dtu.dk/services/TatP). To reduce the number of false positives, we excluded all proteins that had negative results for two or more parameters measured by the program. The majority of these predicted Tat substrates require cofactor binding and appear primarily involved in redox regulation, which are recurrent features of Tat substrates in other prokaryotes.
TABLE 1.
List of predicted Tat-dependent proteins of Sodalis glossinidius with their predicted subcellular localizations and biological functions
| Sodalis glossindius protein | Accession no. | Localization | Biological function |
|---|---|---|---|
| N-Acetylmuramoyl-l-alanine amidase (AmiC) | YP_455641.1 | Periplasm | Cell envelope biogenesis |
| Proline aminopeptidase P II (PepP) | YP_455685.1 | Cytoplasm | Amino acid transport and metabolism |
| Membrane protein TonB (TonB) | YP_455061.1 | Outer membrane | Iron ion transmembrane transporter activity |
| Cell division inhibitor (MinD) | YP_455015.1 | Cytoplasm | Cell cycle |
| Cell division protein (FtsN) | YP_455847.1 | Inner membrane | Cell cycle |
| 3-Oxoacyl-(acyl-carrier-protein) reductase | YP_454740.1 | Cytoplasm | Oxidoreductase |
For investigation of Tat functionality in S. glossinidius, the RR-signal peptide of TMAO reductase (TorA), a molybdopterin-containing protein that is known to be exported by the Tat pathway in E. coli (11, 13), was used. The TorA signal sequence was demonstrated to be highly Tat specific and has been efficiently employed to target heterologous proteins to the periplasm of various prokaryotes (5, 7, 17). The pBADtorA-gfp plasmid (15), encoding a green fluorescent protein (GFP) reporter fused to the E. coli TorA signal peptide, was introduced into S. glossinidius via a heat shock procedure (2). Cells were grown to mid-exponential growth phase (optical density at 600 nm [OD600] = 0.2) before TorA-GFP expression was induced by arabinose (0.15%) for 5 h. Next, the location of the expressed GFP was monitored by immunoblotting of periplasmic and cytoplasmic cell fractions. Cells were fractionated according to the method of Skerra and Plückthun (14). Samples were heat denatured at 95°C in the presence of SDS-PAGE loading buffer and analyzed on a 12% (wt/vol) polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane (Whatman) and blocked overnight in 5% skim milk to be assayed by Western blot analysis using an anti-GFP detection antibody (1:3,000; Sigma). Figure 1 shows that two forms of GFP could be detected in S. glossinidius cells harboring the pBADtorA-gfp plasmid: precursor GFP (30 kDa) and a mature-sized protein with a molecular mass corresponding to that of GFP devoid of the TorA leader sequence (27 kDa). Cytoplasmic extracts contained both mature and precursor forms, while only the mature-sized GFP could be detected in the periplasm, indicating the predicted cleavage of the TorA leader sequence during periplasmic transport.
FIG. 1.

Expression and export of TorA-GFP by the Tat pathway in Sodalis glossinidius cells. Expression was induced with arabinose. The location of the expressed GFP was analyzed by immunoblotting of periplasmic (P) and cytoplasmic (C) fractions using an anti-GFP antibody (1:3,000; Sigma) for detection. Presented data are representative for two independent experiments.
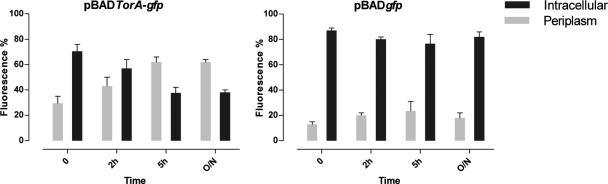
Kinetics and activity of the periplasmically transported GFP were assessed by fluorimetry on prepared cell fractions (Fig. 2). S. glossinidius cells expressing TorA-GFP were induced at 26°C for 2 h using 0.15% arabinose. To prevent saturation of the Tat pathway, cells were washed to remove the arabinose from the culture medium and subsequently 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to repress the pBAD promoter. Samples were taken immediately (t = 0), 2 h and 5 h after removal of arabinose. The relative fluorescence levels present in the different cellular fractions were quantified using a PerkinElmer Victor3 1420 multilabel counter (485-nm excitation/535-nm emission; measurement time, 0.1 s). At t = 0 and t = 2 h after arabinose removal, the majority of GFP was still located in the cytoplasm. However, 5 h after arabinose induction, a clear shift in the presence of fluorescence was observed from the cytoplasm toward the periplasm. We calculated that a maximal export efficiency of around 65% could be achieved within 5 h after induction, which is consistent with previous studies in E. coli (11). In S. glossinidius harboring the pBADgfp control plasmid devoid of the TorA leader sequence, GFP clearly remained accumulated in the cytoplasm whereas only a small and constant amount of GFP could be detected in the periplasmic extracts.
FIG. 2.
Quantitative export of active TorA-GFP by the Tat-pathway in Sodalis glossinidius cells. Cells were grown in the presence of arabinose, and samples were taken immediately (0), 2 h, 5 h, and 24 h (O/N) after removal of arabinose and IPTG repression. Values are given for the fluorescence of periplasmic and cytoplasmic fractions of cells harboring the pBADtorA-gfp and pBADgfp plasmid. As a control to determine the background fluorescence, cell fractions from noninduced S. glossinidius containing the respective plasmids were used. Presented data are representative for three independent experiments; data in the graph are means ± standard deviations.
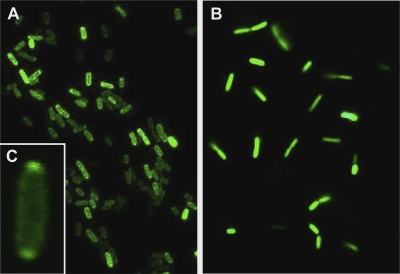
The in vivo cellular localization of GFP in TorA-GFP-expressing cells was assessed more directly by fluorescence microscopy (Fig. 3). In concordance with the fluorimetry experiment, cells were induced by arabinose for 2 h and samples were taken immediately, 2 h, and 5 h after washing and repression with IPTG. Consistent with the fluorimetry data, immediately and 2 h after arabinose removal, GFP remained uniformly distributed in TorA-GFP-expressing cells. However, 3 h later (t = 5 h), cells showed prominent halos of periplasmic GFP. Occasionally, GFP was observed to concentrate at the poles in the periplasm. This polar compartmentalization has been shown to be reversible and is suggestive of free movement of proteins within the periplasm in response to environmental changes (12). In S. glossinidius cells harboring the pBADgfp control plasmid, GFP remained uniformly distributed, with no signs of enrichment of the signal in the periplasm. Collectively, these data confirm that active GFP specifically accumulates in the periplasm, which results from the recognition of the TorA signal peptide that is cleaved off upon transport.
FIG. 3.
Localization of GFP in cells harboring the pBADtorA-gfp (A) and pBADgfp (B) plasmid by fluorescence microscopy. Samples were immobilized on poly-l-lysine-coated slides and analyzed on an Olympus BX-41 UV microscope equipped with a filter set for fluorescein isothiocyanate and a 60× (U Plan FLN 1.25) oil immersion objective lens. Occasionally, GFP was found to accumulate at the poles of pBADtorA-gfp-containing cells (C). Presented data are representative for at least three independent observations.
So far, the functionality of the bacterial Tat system has been analyzed mainly in free-living organisms. In this study we demonstrated that the Tat pathway is biologically active in Sodalis glossinidius, a bacterial symbiont of the tsetse fly, and identified a number of predicted natural endogenous substrates that contain the characteristic twin-arginine motif. Moreover, we illustrated that the Tat system has the potential to be exploited for exporting heterologous proteins in an active manner to the periplasm, which will be further explored for paratransgenesis approaches to control trypanosome infection in the tsetse fly vector.
Acknowledgments
We thank Mauricio Pontes for his valuable comments on the manuscript. We are grateful to James P. Barnett for providing the plasmid pJDT1 and to Maria Soledad Saldias Morales for the pBAD24gfp plasmid.
This research was supported by the Belgian Co-operation (DGOS), SOFI-B grant, InterUniversity Attraction Pole program (IAP), and FWO (1.5.147.09), which funded part of the equipment. This work is also performed in the framework of a FAO/IAEA Coordinated Research Project, “Improving SIT for tsetse flies through research on their symbionts and pathogens.”
Footnotes
Published ahead of print on 10 December 2010.
REFERENCES
- 1.Aksoy, S., I. Maudlin, C. Dale, A. S. Robinson, and S. L. O'Neill. 2001. Prospects for control of African trypanosomiasis by tsetse vector manipulation. Trends Parasitol. 17:29-35. [DOI] [PubMed] [Google Scholar]
- 2.Beard, C. B., et al. 1993. Genetic transformation and phylogeny of bacterial symbionts from tsetse. Insect Mol. Biol. 1:123-131. [DOI] [PubMed] [Google Scholar]
- 3.Berks, B. C. 1996. A common export pathway for proteins binding complex redox factors? Mol. Microbiol. 22:393-404. [DOI] [PubMed] [Google Scholar]
- 4.Bogsch, E. G., et al. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003-18006. [DOI] [PubMed] [Google Scholar]
- 5.Brüser, T. 2007. The twin-arginine translocation system and its capability for protein secretion in biotechnological protein production. Appl. Microbiol. Biotechnol. 76:35-45. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, Q., and S. Aksoy. 1999. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 8:125-132. [DOI] [PubMed] [Google Scholar]
- 7.Cristobal, S., J. W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilks, K., R. W. Rose, E. Hartmann, and M. Pohlschröder. 2003. Prokaryotic utilization of the twin-arginine translocation pathway: a genomic survey. J. Bacteriol. 185:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, P. A., D. Tullman-Ercek, and G. Georgiou. 2006. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 60:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manting, E. H., and A. J. Driessen. 2000. Escherichia coli translocase: the unravelling of a molecular machine. Mol. Microbiol. 37:226-238. [DOI] [PubMed] [Google Scholar]
- 11.Santini, C. L., et al. 1998. A novel Sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santini, C. L., et al. 2001. Translocation of jellyfish green fluorescent protein via the Tat-system of Escherichia coli and change of its periplasmic localization in response to osmotic up-shock. J. Biol. Chem. 276:8159-8164. [DOI] [PubMed] [Google Scholar]
- 13.Sargent, F., et al. 1998. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 17:3640-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skerra, A., and A. Plückthun. 1988. Assembly of a functional immunoglobulin-FV fragment in Escherichia coli. Science 240:1038-1041. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47-52. [DOI] [PubMed] [Google Scholar]
- 16.Toh, H., et al. 2006. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 16:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tullman-Ercek, D., et al. 2007. Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J. Biol. Chem. 282:8309-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner, J. H., et al. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 19.Welburn, S. C., I. Maudlin, and D. S. Ellis. 1987. In vitro cultivation of rickettsia-like-organisms from Glossina spp. Ann. Trop. Med. Parasitol. 81:331-335. [DOI] [PubMed] [Google Scholar]
- 20.Yates, M. G., E. M. De Souza, and J. H. Kahindi. 1997. Oxygen, hydrogen and nitrogen fixation in Azotobacter. Soil Biol. Biochem. 29:863-869. [Google Scholar]