Abstract
The group IIA intron Ll.LtrB from Lactococcus lactis and the group IIB intron EcI5 from Escherichia coli have intron-encoded proteins (IEP) with a DNA-binding domain (D) and an endonuclease domain (En). Both have been successfully retargeted to invade target DNAs other than their wild-type target sites. RmInt1, a subclass IIB3/D intron with an IEP lacking D and En domains, is highly active in retrohoming in its host, Sinorhizobium meliloti. We found that RmInt1 was also mobile in E. coli and that retrohoming in this heterologous host depended on temperature, being more efficient at 28°C than at 37°C. Furthermore, we programmed RmInt1 to recognize target sites other than its wild-type site. These retargeted introns efficiently and specifically retrohome into a recipient plasmid target site or a target site present as a single copy in the chromosome, generating a mutation in the targeted gene. Our results extend the range of group II introns available for gene targeting.
Group II introns are catalytic RNAs and mobile retroelements initially identified in the mitochondrial and chloroplast genomes of lower eukaryotes and plants and later found in bacteria and archaea (22). They have recently been identified in the mitochondrial genomes of the annelid Nephtys sp. (39) and basal placozoan Trichoplax adherens (11). It is thought that both nuclear spliceosomal introns and non-long terminal repeat retrotransposons evolved from mobile group II introns (3, 4, 12, 31, 47).
A typical group II intron consists of a highly structured RNA that folds into a conserved three-dimensional structure consisting of six distinct double-helical domains, DI to DVI (22). Most bacterial group II introns have a multifunctional intron-encoded protein (IEP) open reading frame (ORF) within DIV (20). Group II IEPs have an N-terminal reverse transcriptase (RT) domain homologous to retroviral RT sequences, followed by a putative RNA-binding domain with RNA splicing or maturase activity (domain X), and a C-terminal DNA-binding (D)/DNA endonuclease (En) region (23, 30). Mobile group II intron RNAs have coevolved with their IEPs (35) and three main classes (IIA, IIB, and IIC) of group II introns have been described on the basis of conserved intron RNA structures, whereas phylogenetic analysis of RT and X domains has resulted in the classification of the ORFs into several groups (A, B, C, D, E, F, CL1 [chloroplast-like 1], CL2 [chloroplast-like 2], and ML [mitochondrion-like]. The A, C, D, E, and F introns encode proteins lacking the En domain (32, 36, 38, 46).
Group II introns move through a process catalyzed by a ribonucleoprotein (RNP) complex consisting of the IEP and the spliced intron lariat RNA. RNPs initiate mobility by recognizing DNA target sites, through both the IEP and the base-pairing of the intron RNA (exon-binding sites [EBS]) to the target DNA (intron-binding sequences [IBS]). EBS-IBS interactions are particularly important for target sequence recognition. Mobile group II introns can therefore be programmed to insert efficiently into any putative desired target DNA simply by modifying EBS in the intron RNA (15, 18, 24).
The major features of group II intron mobility mechanisms were elucidated by studies of three closely related group IIA/ML introns: the Saccharomyces cerevisiae mtDNA aI1 and aI2 introns and the Lactococcus lactis Ll.LtrB intron (40, 47, 48). These introns insert into a compatible target (intronless alleles) through retrohoming, by a target DNA-primed reverse transcription mechanism, in which the intron RNA reverse splices into a target sequence in a DNA strand, while the IEP uses the C-terminal En domain to cleave the opposite strand. The IEP then uses the cleaved 3′ end as a primer for the synthesis of an intron cDNA, which is integrated into the recipient DNA by host cell DNA recombination or repair mechanisms (10, 13, 14, 34). The variable D region is involved in the recognition of a subset of key nucleotides in the distal 5′exon region via major groove interactions bolstered by phosphate-backbone contacts (33). These interactions probably trigger local DNA unwinding, thereby placing the intron RNA in a position to base pair with the target site for reverse splicing. Group II introns also transpose, with low frequency, to noncognate (ectopic) sites resembling the homing site, primarily by a DNA-target pathway (for a review, see reference 38) using En-independent or En-dependent retrohoming mechanisms, a process influenced by the host (7).
The Ll.LtrB intron from L. lactis has been used to develop gene targeting applications in a number of different Gram-negative (Escherichia coli, Shigella flexneri, Salmonella enterica serovar Typhimurium, Francisella tularensis, Pseudomonas aeruginosa, and Agrobacterium tumefaciens) (18, 41) and Gram-positive bacteria (L. lactis, Clostridium perfringens, Staphylococcus aureus, and Paenibacillus alvei) (5, 42, 43). A new class IIB1/CL1 intron, EcI5, located in an E. coli virulence plasmid, has also recently been used for gene targeting. This intron displays active retrohoming in E. coli and has been retargeted to the chromosomal lacZ gene in this species (45). The IEP encoded by the Ll.LtrB and EcI5 introns has both a DNA-binding domain (D) and a DNA endonuclease (En) domain (44, 45).
Remarkably, the RmInt1 intron of Sinorhizobium meliloti, a IIB3/D group II intron (37) with an IEP with no recognizable D or En domain displays mobility, retrohoming, with a high frequency and efficiency. RmInt1 mobility principally involves reverse splicing of the intron RNA into single-stranded DNA at a replication fork, using the nascent lagging DNA strand as the primer for reverse transcription (21). Nevertheless, the RmInt1 intron can retrohome to both DNA strands (21, 27).
We show here that RmInt1 can be retargeted to invade genes located on plasmids or present as a single copy in the chromosome very efficiently and at high frequency. Our results extend the possibilities for the reprogramming of group II introns for insertion into any desired target sequence regardless of the characteristics of the IEP and the mechanism of mobility.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
S. meliloti strain RMO17 (RmInt1 intronless strain) was grown at 28°C on TY or defined minimal medium (MM) (28). The E. coli DH5α strain was used for the cloning and maintenance of plasmid constructs. The E. coli HMS174(DE3) strain carrying the T7 RNA polymerase gene was used in mobility assays with the pACE system. E. coli strains were routinely grown at 37°C or, when specified, at 28°C, on Luria-Bertani (LB) medium (29). Antibiotics were added to the medium, when required, at the following concentrations: kanamycin at 200 μg/ml for S. meliloti and at 50 μg/ml for E. coli, ampicillin at 200 μg/ml, and tetracycline at 10 μg/ml.
Construction of recipient target-site plasmids.
Standard molecular biology techniques were used to generate the various plasmid constructs. pACE and the vectors derived from it are based on the pJB3 plasmid skeleton (2). Briefly, primers TcF (5′-CATATGAAATCTAACAATGCGCTC3′) with an NdeI engineered site and TcR (5′-AGATCTTCAGGTCGAGGTGGCCCGGCT-3′) with a BglII engineered site were used to amplify the tetracycline resistance (Tetr) gene from plasmid pBBR1MCS. This gene was cloned in pET3a digested with NdeI and BamHI to generate plasmid pETc. By using the primers RBSF2 (5′-GGTACCTAGAAATAATTTTGTTTAACT-3′) and RBSR2 (5′-GAATTCGGATATAGTTCCTCCT-3′) with KpnI and EcoRI, respectively, engineered sites, and pETc as a template, a 1.3-kb fragment carrying the ribosome binding site (RBS) and the RNA polymerase T7 transcription terminator (Tφ) was amplified from pET3a, together with the Tetr gene, from plasmid pBBR1MCS. This fragment was cloned in pGEM-T Easy vector system to generate plasmid pGERBS. By KpnI and EcoRI digestion of this plasmid, the 1.3-kb RBS fragment was cloned in pJB3 digested with the same enzymes to give rise to plasmid pJBRBS. The rrnB T1 and (3×) T2 transcription terminators were inserted into the plasmid described above by PCR and insertion of the fragments generated with the primers T1F (5′-GCATGCCGTAGCGCCGATGGTAGTGTG-3′) and T1R (5′-CTGCAGCCGTTGCTTCGCAACGTTCA-3′) for terminators T1 and T2F (5′-GGATCCGGAGGGTGGCGGGCAGGACGC-3′) and T2R (5′-GGTACCAGATCTTGGGGGGATGGCTTGTAGATATGA-3′) for terminator T2. Three consecutive T2 terminators were inserted into plasmid pGEM-T by several rounds of amplification and cloning to produce plasmid pGET2-3. Independently, T1 was also cloned in pGEM-T to give rise to plasmid pGET1. Afterward, T1 was excised from this plasmid by digestion with SphI and PstI and cloned into plasmid pIC20H digested with the same enzymes to produce plasmid pICT1. This plasmid, digested with BamHI and KpnI, was used to introduce the fragment with the three units of terminator T2 from plasmid pGET2-3 to generate plasmid pICT12. A fragment with these terminators in tandem plus a multicloning site between them was excised from pICT12 by digestion with HindIII and KpnI, and it was cloned upstream of the RBS fragment in pJBRBS digested with the same enzymes to generate plasmid pACELEAD. Afterward, the ACE cassette was amplified by the use of the primers T1Eco (5′-GAATTCGCCGTAGCGCCGATGGTAGTGTG-3′) and TφHind (5′-AAGCTTCGGATATAGTACCTCCTTTCAGC-3′) with EcoRI and HindIII engineered sites, respectively, and cloned in pGEM-T to give rise to pGEACE. From this plasmid, by EcoRI and HindIII digestion, the ACE fragment was obtained and cloned into pJB3 digested with the same enzymes in order to generate plasmid pACELAG. In the target-recipient plasmids target site is cloned in either the same (pACELAG) or in the opposite (pACELEAD) orientation, depending on whether the nascent lagging or leading DNA strands could be used as a primer for reverse transcription of the inserted intron RNA. The ACE fragments of both plasmids were sequenced to check for possible changes or modifications due to PCR and cloning processes. A 575-bp RmInt1 target-containing fragment (positions −148 to +427 with respect to the intron insertion site) obtained from pGEM0.6 (21) by PCR amplification with the XbaT600 (5′-ACTCTAGAATGTGGCGCAGTTCGTCA-3′) and SalT600 (5′-ACATGTCGACCTTCGTGCACGAAGA-3′) primers was inserted into both acceptor plasmids to generate pACE0.6LAG and pACE0.6LEAD.
The pACETrxBLAG and pACEOmpTLAG plasmids were generated by inserting the trxB and ompT genes, as PCR fragments amplified from E. coli genomic DNA with the TrxBF-TrxBR and OmpTF-OmpTR primer pairs, into pACELAG.
Construction of intron-donor plasmids.
The pKGEMA4T7 is a similar construct to pKGEMA4 (27), but with a phage T7 promoter inserted into domain IV. For its construction a BamHI-ΔORF-BamHI cassette from pKGΔORF-IEP (a construct carrying RmInt1 deleted of intron 611 to 1,759 nucleotides flanked by exon sequences −50/+146 inserted into a BamHI site just upstream of the IEP coding sequence) was inserted into the pET3a vector to generate pETP1. A fragment consisting of two annealed primers carrying the T7 RNA polymerase promoter (T7-F, 5′-TCGACTCGAGTAATACGACTCACTATAGGG-3′; T7-R, 5′-TCGACCCTATAGTGAGTCGTATTACTCGAG-3′) containing an upstream XhoI site was inserted into the XhoI site engineered in the site of deletion in domain IV of the ΔORF intron (9) cloned in pETP1. The BamHI-ΔORF/pT7-BamHI cassette was then inserted into pKGIEP, a construct carrying the IEP expressed under the kanamycin promoter (26) to generate pKGΔORF/pT7-IEP. The ΔORF/pT7 intron flanked by exon sequences −20/+5 was then generated by PCR amplification of the BamHI ΔORF/pT7 fragment, using pKGΔORF/pT7-IEP as a template and the appropriate primers to yield a DNA product that was inserted as a SacI fragment into pKGIEP downstream the IEP. This DNA fragment also carries PmlI and BlnI sites internal to SacI. The new construct, called pKGEMA4T7, was used as a template for site-specific mutagenesis by two-step PCR in which the first step used primers that contain the specific mutations in the EBS1, IBS2, and IBS1 elements of the intron and flanking 5′ exon region to generate two partially overlapping PCR products. The two PCR products were mixed and amplified with external primers. Thus, the primer sequences for retargeting purposes were as follows: TI-5′exon (5′-CACGTGTCGTTATCCACCACGTCGGTGTGCTGCAGAGGCA-3′), TI-EBS2 (5′-CTGGTTTCGACGACACTTACGATCCAAACGACGCGTCCTC-3′), and TI-EBS1 (5′-GTAAGTGTCGTCGAAACCAGGACCGCGACGTGATCCTGGG-3′) for trxB1; TII-5′exon (5′-CACGTGTCGTTTACTGCGTTTGGTGTGTGCTGCAGAGGCA-3′), TII-EBS2 (5′-CTGGTTTCGACGACACTTACGTACTGAACGACGCGTCCTC-3′), and TII-EBS1 (5′-GTAAGTGTCGTCGAAACCAGGACCGATTAGACATCCTGGG-3′) for trxB2; OI-5′exon (5′-CACGTGTCGTTTACTTCCATCCGTGTGTGCTGCAGAGGCA-3′), OI-EBS2 (5′-CTGGTTTCGACGACACTTACGTACTTAACGACGCGTCCTC-3′), and OI-EBS1 (5′-GTAAGTGTCGTCGAAACCAGGACCGATGGGTGATCCTGGG-3′) for ompT1; and OII-5′exon (5′-CACGTGTCGTTTCCAGCCAGCAGCGTGTGCTGCAGAGGCA-3′), OII-EBS2 (5′-CTGGTTTCGACGACACTTACGTCCAGAACGACGCGTCCTC-3′), and OII-EBS1 (5′-GTAAGTGTCGTCGAAACCAGGACCGGTTGCTGATCCTGGG-3′) for ompT2. A common primer annealing in the 3′ exon region, 3′-SacI (5′-CTGAGCTCAGGGGTGAGTAGGCCGGAGGAG-3′), was used to amplify the final site-specific mutated fragment. Once generated, amplicons were digested with PmlI/XhoI and inserted into pKGEMA4T7, which had been digested in the same way, to generate the reprogrammed introns.
Southern hybridization.
Southern blotting of genomic DNA was performed on total DNA isolated with a Real-Pure genomic DNA extraction kit (Real-Durviz, SLU), according to the manufacturer's protocols. DNA (2 μg) was digested with EcoRI, subjected to electrophoresis in a 1% TAE agarose gel, and vacuum blotted onto nylon filters (Pall Corp.) according to the manufacturer's instructions. A DNA probe for RmInt1 was obtained by PCR amplification in the presence of digoxigenin-11-dUTP (DIG; Boehringer Mannheim), using pKGEMA4 as the template and the Epsilon (5′-GTGAGCGTCGGATGAAAC-3′) and 18R0 (5′-ACGTTTCTCAATTCGAAACG-3′) primers.
The mobility of RmInt1 was revealed by a two-plasmid assay and further Southern hybridizations. Plasmid DNA isolated by alkaline extraction (1) from S. meliloti RMO17 transconjugants were analyzed by SalI digestion, agarose gel electrophoresis, and Southern blotting with an ISRm2011-2 probe (575-bp target-containing fragment). The homing efficiency was calculated as the ratio of homing product (H) to noninvaded recipient plasmid (R), expressed as a percentage, according to the following formula: (H/H+R) × 100.
Hybridizations were carried out at high stringency; the membranes were washed, and the hybridization signals were detected according to the manufacturer's instructions (Boehringer Mannheim).
Curing of the intron donor plasmid.
Cells were cured of the donor plasmid by introducing an incompatible plasmid expressing an ampicillin-resistant gene, followed by incubation overnight in the presence of ampicillin.
Detection of intron insertion by colony PCR.
Intron mobility and insertion were detected by colony PCR (16), with primers binding to the target gene and a primer specific for the intron. The following primers were used: for ompT, OmpTF (5′-GCGAACTGCAGATGCGGGCGAAACTTCTGGG-3′) and OmpTR (5′-ACTAGTCTAGATTAAAATGTGTACTTAAGAC-3′); for trxB, TrxBF (5′-ACTAGTCTAGATTATTTTGCGTCAGCTAAAC-3′) and TrxBR (5′-GCGAACTGCAGATGGGCACGACCAAACACAG-3′); and for the intron, Pi (5′-TCAGGAGCCTGGTTCCTGCAGTTC-3′). The insertion-specific sets of primers used for PCR amplification were Pi/OmpTR for the ompT gene and Pi/TrxBR for the trxB gene. The gene-specific sets of primers used were OmpTF/OmpTR for the ompT gene and TrxBF/TrxBR for the trxB gene.
Colorimetric assays.
HMS174(DE3) and the ompT mutant derivative were grown overnight in LB medium. Cells were harvested and washed with M9 medium. Washed cells were used to inoculate 3 ml of M9 medium (OD600 = 0.03) plus chromogenic substrate S-2251 (0.45 mM; Chromogenix). These cultures were grown at 37°C for 24 h, the cells were then pelleted, and the supernatant measured at 405 nm. The OD600 reached by the cultures were 1.9 to 2.3 for HMS174(DE3) and 1.7 to 2.3 for HMS174(DE3) ompT mutant.
RESULTS
Design and validation of a two-plasmid retrohoming assay for RmInt1 mobility.
For mobility assays, a system based on intron donor and recipient target site vectors similar to that previously described for Ll.LtrB (15, 18) was designed (Fig. 1 A). The intron-donor plasmid, pKGEMA4T7 contains a RmInt1-ΔORF intron and short flanking exons −20/+5, with a phage T7 promoter inserted into domain IV and the IEP expressed from a position upstream from the 5′ exon (E1). The kanamycin promoter is used to express the IEP, flanking exons and intron ΔORF. The recipient plasmids, pACE0.6LAG or pACE0.6LEAD, are compatible ampicillin resistance (Ampr) plasmids containing a 575-bp target-containing fragment (positions −148 to +427 with respect to the intron insertion site) upstream from a promoterless Tetr gene. An E. coli rrnB T1 transcription terminator was inserted upstream from the target site, and three E. coli rrnB T2 transcription terminators were placed between the target site and the Tetr gene. A phage T7 TΦ terminator was inserted downstream from the Tetr gene to terminate T7 RNA polymerase transcription.
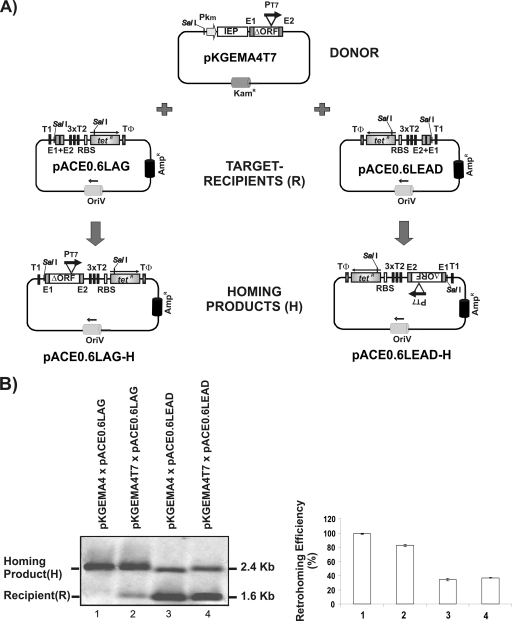
FIG. 1.
Plasmid-based genetic system for invasion event selection. (A) The donor plasmid pKGEMA4T7 uses the km promoter to express a cassette containing the IEP-ΔORF, with the T7 promoter (PT7) inserted into DIV, and short flanking 5′ and 3′ exons (−20/+5). The recipient plasmids pACE0.6LAG and pACE0.6LEAD harbor the wild-type target site for RmInt1 (E1+E2) inserted upstream from a promoterless Tetr gene. T1, T2, and TΦ are E. coli rrnB T1 and T2 and phage T7 TΦ transcription terminators. RBS, ribosome binding site. The arrow above Tetr indicates the transcription orientation of the Tetr gene. The homing products shown result from intron insertion when pKGEMA4T7 is used as a donor, and the expression of Tetr gene is under the control of the T7 promoter. (B) Southern blot analyses of the invasion of pACE0.6LAG and pACE0.6LEAD by introns expressed from pKGEMA4T7 and pKGEMA4 (a similar construct to pKGEMA4T7, but without the T7 promoter). A target-site DNA probe (575-bp target-containing fragment of ISRm2011-2) was used. The homing products (H) and target-recipient plasmids (R) were quantified with the QuantityOne software package (Bio-Rad Laboratories), and the retrohoming efficiency was calculated as (H/H+R) × 100 and is shown in the histogram. The data represent mean determinations for at least four independent assays, with the standard deviations (SD) indicated by thin lines.
For mobility assays, plasmids were transferred into S. meliloti RMO17 by triparental mating. Figure 1B shows a comparison of the intron mobility of pKGEMA4T7 with that of pKGEMA4 (a similar construct, but without the T7 promoter), using the recipient plasmids pACE0.6LAG or pACE0.6LEAD. Integration of the intron into the target site was detected by Southern hybridization. Both intron donor plasmids displayed closed mobilization efficiencies, when using pACE0.6LEAD (31.4 and 30%, respectively) or pACE0.6LAG (82 and 98%, respectively). The difference in homing efficiency observed between recipient plasmids pACE0.6LEAD and pACE0.6LAG is due to the fact that RmInt1 uses most efficiently the nascent lagging strand as a primer for reverse transcription (21).
We then evaluated (Table 1) the capacity of this two-plasmid assay to generate Tetr colonies. In the case of a homing event, the promoterless Tetr gene would be under the control of the T7 promoter, resulting in a Tetr colony. Since S. meliloti lacks T7 RNA polymerase, plasmid DNA was isolated from RMO17 transconjugant cells harboring donor and recipient plasmids and used for the electroporation of E. coli strain HMS174(DE3), which harbors an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible phage T7 RNA polymerase. Mobility events were selected by plating on LB agar supplemented with tetracycline and ampicillin but without IPTG. The addition of IPTG (at a concentration of 1 or 0.1 mM) to the plates decreased cell viability and slowed colony growth. The low levels of T7 RNA polymerase expression occurring in the absence of IPTG were nonetheless sufficient for transcription of the Tetr gene under the control of the T7 promoter. Retrohoming efficiency was calculated as follows: [(Tetr + Ampr)/Ampr] × 100. The RmInt1-ΔORF intron harboring the T7 promoter had a mobilization efficiency of ∼63 and 27% when using pACE0.6LAG and pACE0.6LEAD as recipient plasmids. In controls, the same intron without the T7 promoter (pKGEMA4) or with the recipient plasmid alone gave less than 0.0001% tetracycline- and ampicillin-resistant colonies, demonstrating that Tetr gene expression was under the control of the T7 promoter rather than any other putative promoter present in the intron or the recipient plasmid.
TABLE 1.
Retrohoming efficiency
| Plasmid(s) | Mean retrohoming efficiencya (%) ± SD |
|---|---|
| pKGEMA4T7 × pACE0.6LAG | 63.30 ± 16.10 |
| pKGEMA4 × pACE0.6LAG | ≤0.0001 |
| pACE0.6LAG | ≤0.0001 |
| pKGEMA4T7 × pACE0.6LEAD | 27.70 ± 3.5 |
| pKGEMA4 × pACE0.6LEAD | ≤0.0001 |
| pACE0.6LEAD | ≤0.0001 |
Retrohoming efficiency is expressed as the percentage of Tetr colonies calculated as follows: [(number of Tetr colonies + number of Ampr colonies)/number of Ampr colonies] × 100.
RmInt1 is mobile in E. coli.
The Ll.LtrB-ΔORF intron expressed under the control of the T7 promoter in the absence of IPTG, at 37°C, was active in E. coli HMS174 (DE3), with a mobilization efficiency of 24% (45). We investigated whether RmInt1 was mobile in E. coli and determined the efficiency of retrohoming events, by electroporating E. coli HMS174 (DE3) with the recipient pACE0.6LAG and the intron donor pKGEMA4T7 plasmids. The electroporated cells were diluted 1/400 in LB medium supplemented with kanamycin and ampicillin and grown at one of two different temperatures, 37 and 28°C, overnight. The cultures were then serially diluted and plated on LB agar supplemented with tetracycline and ampicillin. Mobilization efficiencies were then calculated as indicated above. RmInt1 had a mobilization efficiency of 10.4% at 28°C, 1,000 times higher than that at 37°C (0.01%). The insertion of the intron at the correct site was confirmed by sequencing. In controls, pKGEMA4XT7 (pKGEMA4T7 derivative, which has a frameshift within the IEP coding sequence generated by eliminating the internal EcoRI, producing a truncated IEP), gave 0.0035% and 0.001% Tetr + Ampr colonies at 28 and 37°C, respectively. Thus, the RmInt1 intron is active in E. coli but is considerably more efficient at 28°C, the optimal temperature for its natural host S. meliloti.
Retargeting of RmInt1 for insertion into sites within the E. coli trxB and ompT genes.
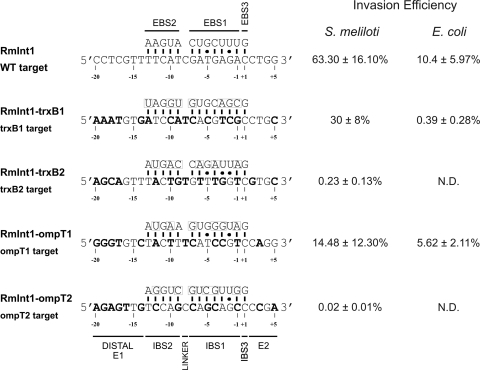
We investigated whether RmInt1 could be retargeted for insertion into different DNA target sequences within any gene, using two E. coli genes: trxB, which encodes the thioredoxin reductase monomer, and ompT, which encodes an outer membrane protease. These genes are of biotechnological significance and were chosen for the present study because their mutation facilitates the production and expression of recombinant proteins. Each gene (full-length, coordinate positions for ompT 583906 to 584856 and for trxB 930308 to 931272) was inserted into the recipient construct, pACELAG. The cloned target sites are orientated in the target recipient plasmids so that the lagging strand will be the same strand that in the chromosomal location, and it could be used as a primer for reverse transcription of the inserted intron RNA at a replication fork. Sixteen potential target sites for ompT and 12 for trxB were identified on the basis of target sequence requirements for RmInt1 recognition (17). These target sites contain the T-N14-C-N2-G sequence, with the positions T-15 in the 5′exon and the IBS3 (C+1) and G+4 in the 3′ exon fixed. Only two target sites for each gene were chosen, and the selected target sites were those requiring the smallest number of modifications to the intron EBS. Four intron-donor pKGEMA4T7 derivatives were constructed, each carrying retargeted RmInt1 for insertion into these sites by modification of the EBS1 and EBS2 sequences to form Watson-Crick base pairs at the positions recognized during base pairing of the intron RNA (IBS2, −13 to −9; and IBS1, −7 to −1). IBS2 and IBS1, in the 5′ exon of the donor plasmid, were also modified to make them complementary to the retargeted EBS2 and EBS1 sequences for efficient splicing. The pKGEMA4T7trxB1 and pKGEMA4T7trxB2 constructs are retargeted to insert at nucleotide positions 9 and 857 in the antisense strand of trxB, whereas pKGEMA4T7ompT1 and pKGEMA4T7ompT2 are designed to insert at positions 261 and 345 in the antisense strand of the ompT gene, respectively (Fig. 2). Donor and recipient plasmids were transferred into S. meliloti RMO17 by triparental mating, and plasmid DNA was isolated from transconjugants and used to electroporate E. coli HMS174(DE3) cells, which were then plated on LB agar supplemented with ampicillin and tetracycline. Integration of the intron was confirmed by colony-PCR and sequencing. Figure 2 shows the retrohoming efficiencies obtained with the various intron-donor constructs, ranging from 0.02% for the least efficiently targeted site (ompT2) to 30% for the most efficiently targeted site (trxB1). These findings indicate that RmInt1 can be efficiently retargeted using a minimal set of DNA target site positions.
FIG. 2.
Sequences and base-pairing interactions with retargeted introns of the selected targets, trxB and ompT. The wild-type RmInt1 target site and base-pairing interactions are shown at the top. The nucleotide residues of target sites differing from the wild-type sequence are indicated in boldface. Nucleotides in the EBSs modified to optimize base pairing with the target sites are boxed. The invasion efficiencies of the various retargeted introns are expressed as the percentage of Tetr colonies in S. meliloti and E. coli (experiments performed at 28°C). The data are means ± the SD of at least three independent experiments. N.D., not determined.
Gene targeting in E. coli.
For gene targeting in E. coli, RmInt1 retargeted to the most efficient target sites, trxB1 and ompT1, were used. The plasmids used to disrupt the trxB and ompT target sites were the donor plasmids, pKGEMA4T7trxB1 and pKGEMA4T7ompT1, and the recipient plasmids, pACEtrxBLAG and pACEompTLAG, respectively. All plasmids were introduced into E. coli HMS174(DE3) by electroporation. Electroporated cells were diluted 1/400 in LB medium supplemented with kanamycin and ampicillin, grown overnight at 28°C, and serially diluted on LB agar plates supplemented with ampicillin and tetracycline, as described above. As shown in Fig. 2, the RmInt1-ΔORF had a mobilization efficiency of 5.62% for the ompT1 target site and 0.39% for the trxB1 target site. Integration of the intron at the correct site was confirmed by colony-PCR and sequencing. The targeting frequency in E. coli was lower than that in its natural host, S. meliloti, but ompT1 was unexpectedly found to be the most efficient target, suggesting that targeting efficiency may depend on the host.
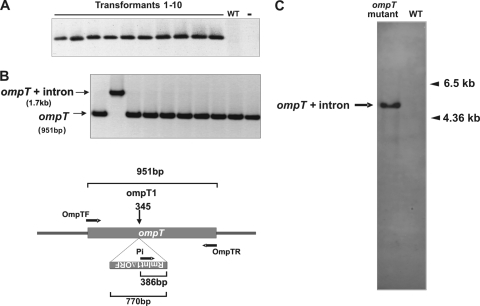
The reprogrammed intron with the highest mobilization efficiency was subsequently used to generate the corresponding chromosomal ompT gene disruption. E. coli HMS174(DE3) was electroporated with the donor plasmid pKGEMA4T7ompT1. The electroporated cells were incubated for 1 h at 28°C and plated on LB agar plus kanamycin to select for the donor plasmid. Note that selection of the chromosomal intron insertion event cannot be detected by antibiotics addition; hence, PCR screening was used. Ten colonies were randomly selected from the plates and screened, with intron insertion-specific primers (Pi/OmpTR), to confirm the presence of the intron insertion into ompT. All 10 colonies tested displayed intron insertion at the target site (Fig. 3 A). However, when the same colonies were analyzed with a gene-specific set of primers (OmpTF/OmpTR), they gave only a PCR product corresponding to the wild-type ompT gene (not shown). These findings suggest that, although all colonies have cells harboring ompT with intron insertions, there are still a large number of cells carrying the wild-type copy of the ompT gene. Nonetheless, a pure population of the ompT mutant, generated by intron insertion into the ompT1 site, was obtained by growing cells from one of the above colonies in liquid medium overnight and plating on LB agar. PCR of 10 of the resulting colonies with a gene-specific primer pair (Fig. 3B) revealed full disruption of the ompT gene in one of them, which was confirmed by DNA sequencing. Furthermore, the ompT intron insertion mutant was cured of the donor plasmid and, as analyzed by Southern hybridization with an intron-specific probe, the mutant gave only one band of the size expected for insertion at the desired site in the ompT gene (Fig. 3C). To check the absence of the OmpT activity in the mutant, we analyzed its capacity to breakdown the chromogenic substrate S-2251 (19). Hydrolyzes of the substrate S-2251 was 3-fold higher in the wild-type cells that in the ompT mutant (data not shown). These findings demonstrate that the RmInt1 intron can be reprogrammed to insert efficiently and specifically into chromosomal DNA targets.
FIG. 3.
Disruption of the chromosomal ompT gene. (A) Colony-PCR analysis of a primary transformant of E. coli (pKGEMA4T7ompT1), using an insertion-specific set of primers (Pi-OmpTR). WT, untransformed wild type; -, control PCR with the same set of primers and 100 ng of donor plasmid (pKGEMA4T7ompT1) and recipient plasmid (pACEompTLAG) as a template. (B) Colony-PCR analysis of isolates obtained after one of the primary transformants was cultured overnight in liquid medium and plating the cells on LB agar. PCR of the resulting colonies was performed with a gene-specific set of primers (OmpTF-OmpTR). Schematic diagram of ompT with or without the inserted retargeted intron and the location of the primers used for PCR is shown. (C) Southern blot of genomic DNA from a cured intron-donor plasmid of the ompT mutant and the wild-type strain, with an intron-specific probe.
DISCUSSION
It has been shown that En+ group IIA (Ll.LtrB) and IIB (EcI5) introns are mobile in E. coli and could be retargeted to insert into different DNA target sequences by modifying the base-pairing sequences of the intron RNA (15, 24, 45). In the present study, we found that the group II intron RmInt1, the IEP of which contains no recognizable D or En domain, displays active retrohoming in E. coli and could be retargeted for the very efficient invasions, at high frequency, of genes present in plasmids or as single copies on the chromosome. In our assays, the intron donor plasmid pKGEMA4T7 uses the km promoter to generate the IEP from a position upstream from the 5′ exon and the RmInt1-ΔORF intron and flanking exons, with a phage T7 promoter inserted in DIV. A similar intron configuration has been shown to increase the homing efficiency of RmInt1 (27), presumably by decreasing the susceptibility of the intron RNA to nucleases.
Ll.LtrB and EcI5 intron derivatives expressed at low levels (in the absence of IPTG) using the T7lac promoter to express the intron-ΔORF and flanking exons, with a phage T7 promoter inserted in DIV and the IEP expressed from a position downstream from the 3′ exon have mobilization efficiencies in E. coli of 24 and 77%, respectively (45). Interestingly, the group II intron RmInt1, a subclass IIB3/D intron isolated from S. meliloti, retrohomes efficiently in E. coli, with a mobilization efficiency of 10.4%, one-sixth that in its natural host, S. meliloti. These findings are even more remarkable if we consider that, for introns Ll.LtrB (44) and EcI5 (45), En domain mutations strongly inhibit retrohoming and truncations that delete both the putative D and En domains abolish mobility with or without IPTG induction. We have recently reported that these IEPs lacking the En domain may have adapted to mobilization via different mechanisms to make use of either leading- or lagging-oriented targets (25). It has been hypothesized that the acquisition of a C-terminal DNA binding and an En domain enabled group II introns to retrohome more efficiently into double-stranded DNA target sites without requiring nascent strands at DNA replication forks for the priming of reverse transcription (44), whereas introns, such as RmInt1, lacking DNA endonuclease activity may have additional features facilitating mobility through reverse splicing into single-stranded DNA at a replication fork, followed by the use of a nascent lagging strand as a primer.
Like other mobile group II introns, RmInt1 recognizes DNA target sequences through both the IEP and base pairing of the intron RNA. For class IIB introns, the base-pairing interactions involve EBS1 and EBS2, which base pair to the 5′ exon, and EBS3, which base pairs to the 3′ exon (8, 17). Group II IEPs appear to recognize sequences in both the distal 5′ and 3′ exons, but apparently with no critical nucleotide residues in common. Thus, for EcI5, the most strongly conserved distal 5′ exon nucleotide residues are C−18, C−17, A−15, and A−14, the most critical position being A−14. For Ll.LtrB, the most critical nucleotide residues in this region are T−23, G−21, A−20, T−19, and G−15. In the 3′ exon, for both introns, the most critical residue is T+5, which is required for second-strand cleavage by the En domain. RmInt1 has less stringent requirements for recognition of the distal 5′ and 3′ exon regions, with T−15 and G+4 the only critical nucleotide residues (17), but is sufficiently long to confer high specificity. The RmInt1 IEP may also interact with nucleotide residues −20 to −16 in the distal 5′ exon, because the elimination of these residues decreases retrohoming efficiency. Nevertheless, for RmInt1 retargeting for insertion into sites in the E. coli trxB and ompT genes, we selected potential targets retaining only critical nucleotide residues T−15, G+4, and a wild-type nucleotide residue at the IBS3 (C+1) position. The targeting frequencies of the retargeted introns were from 0.02% (ompT2) to 30% (trxB1), whereas that for the wild-type target in the natural host, S. meliloti, was 63.30%. For EBS/IBS base pairing in the most efficient target (trxB1), we replaced 7 of the 12 nucleotides in the intron RNA exon-binding sequences (EBS). Furthermore, this target has only the wild-type nucleotide residue at position −16 (G) in the distal 5′ exon. Thus, these results indicate that the RmInt1 intron has flexible enough EBS sequences and IEP specificity for gene targeting.
The introns retargeted to the novel target sites had different retrohoming efficiencies. These findings may be accounted for by a decrease in the spliceability of the retargeted introns due to nucleotide residue changes that might affect the structure of the ribozyme, and the sequence requirements of RmInt1, which remain incompletely defined. Thus, positions other than T−15, C+1, and G+4 within the exons or in the EBS-IBS interactions may be required. Differences in insertion rates for different targets have also been reported for the Ll.LtrB and EcI5 introns, for which algorithms for predicting better targets have already been developed. However, for these introns, some targets with high scores predicting higher invasion rates are no more frequently invaded than targets with lower scores (45).
RmInt1 had lower retrohoming efficiency in E. coli than in its natural host, which is consistent with previous findings indicating that host background influences intron homing (18). In addition, RmInt1 mobility in E. coli is considerably more efficient at 28°C than at 37°C, presumably reflecting adaptation for retromobility in the native host. We have recently reported (6) that RmInt1 excision in E. coli at 37°C was 95% less efficient than excision of the intron from the wild-type construct in S. meliloti, suggesting that host factors may play an important role in the splicing reaction that is essential for the retrohoming process. Nevertheless, in E. coli, ompT1 was more efficiently targeted than trxB1, with retargeting efficiencies of 5.62% and 0.39%, respectively, contrasting with the pattern in S. meliloti, in which the corresponding retargeting efficiencies were 14.48 and 30%, respectively. Thus, targeting efficiency is not solely dependent on target site recognition rules, since host factors may influence the efficiency of retrohoming to specific target sites.
The target sites used in our assays are oriented so that the inserted intron RNA uses the nascent lagging strand as a primer for reverse transcription, since this is the most efficient mobility pathway for RmInt1 (21). Insertion rates of the intron RNA into pACE0.6LEAD, in which intron cannot use the nascent lagging strand as a primer for reverse transcription after insertion into its target site, were nonetheless 27.70%, half those for pACE0.6LAG. This DNA strand targeting bias may limit the use of En− introns such as RmInt1 for gene disruption, but it also has the potential advantage of ensuring the control of specific processes associated with DNA replication, such as cell proliferation and differentiation. In addition, in eukaryotic organisms DNA is much more accessible during replication as the chromatin disorganizes. Furthermore, we have recently found (6) that RmInt1 splicing led to the accumulation of detectable lariat forms but that this process was inefficient, shown by a low detection of ligated exons. This inefficiency of splicing has direct consequences for the use of RmInt1 since the intron could be targeted to both sense and antisense strands causing unconditional disruption of the gene. Finally, the observation that inserting the phage T7 promoter into DIV of the intron RNA does not significantly decrease RmInt1 retrohoming suggests that this intron may be able to transport and insert foreign DNA into any desired target, through the introduction of selectable markers into RmInt1 for the selection of invasion events. Our results extend the range of group II introns available for gene targeting.
Acknowledgments
This study was supported by research grants BIO2008-00740 from the Spanish Ministerio de Ciencia e Innovación, CSD 2009-0006 of Programme Consolider-Ingenio 2010, and CVI-01522 from the Junta de Andalucía, including FEDER (Fondo Europeo de Desarrollo Regional) funds. F.M.G.-R. was supported by project CVI-01522, A.B.-D. by a research grant from the Ministerio de Ciencia e Innovación, and V.D.-P. by the I3P-Tec program of the Consejo Superior de Investigacions Científicas.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Birboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blatny, J. M., T. Brautaset, H. C. Winther-Larsen, K. Haugan, and S. Valla. 1997. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl. Environ. Microbiol. 63:370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalier-Smith, T. 1991. Intron phylogeny: a new hypothesis. Trends Genet. 7:145-148. [PubMed] [Google Scholar]
- 4.Cech, T. R. 1986. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell 44:207-210. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., B. A. McClane, D. J. Fisher, J. I. Rood, and P. Gupta. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ. Microbiol. 71:7542-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chillón, I., F. Martínez-Abarca, and N. Toro. 2010. Splicing of the Sinorhizobium meliloti RmInt1 group II intron provides evidence of retroelement behavior. Nucleic Acids Res. [Epub ahead of print.] doi: 10.1093/nar/gkq847. [DOI] [PMC free article] [PubMed]
- 7.Coros, C. J., et al. 2005. Retrotransposition strategies of the Lactococcus lactis Ll.LtrB group II intron are dictated by host identity and cellular environment. Mol. Microbiol. 56:509-524. [DOI] [PubMed] [Google Scholar]
- 8.Costa, M., F. Michel, and E. Westhof. 2000. A three-dimensional perspective on exon binding by a group II self-splicing intron. EMBO J. 19:5007-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa, M., F. Michel, M. D. Molina-Sánchez, F. Martinez-Abarca, and N. Toro. 2006. An alternative intron-exon pairing scheme implied by unexpected in vitro activities of group II intron RmInt1 from Sinorhizobium meliloti. Bichimie 88:711-717. [DOI] [PubMed] [Google Scholar]
- 10.Cousineau, B., et al. 1998. Retrohoming of a bacterial group II intron mobility via complete reverse splicing, independent of homologous DNA recombination. Cell 94:451-462. [DOI] [PubMed] [Google Scholar]
- 11.Dellaporta, S. L., et al. 2006. Mitochondrial genome of Trichoplax adherens supports placozoa as the basal lower metazoan phylum. Proc. Natl. Acad. Sci. U. S. A. 103:8751-8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eickbush, T. H. 1994. Origins and evolutionary relationships of retroelements, p. 121-157. In S. S. Morse (ed.), The evolutionary biology of viruses. Raven Press, Inc., New York, NY.
- 13.Eskes, R., J. Yang, A. M. Lambowitz, and P. S. Perlman. 1997. Mobility of yeast mitochondrial group II introns: engineering a new site specificity and retrohoming via full reverse splicing. Cell 88:865-874. [DOI] [PubMed] [Google Scholar]
- 14.Eskes, R., et al. 2000. Multiple homing pathways used by yeast mitochondrial group II introns. Mol. Cell. Biol. 20:8432-8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, H., et al. 2000. Group II introns designed to insert into therapeutically relevant DNA target sites in human cells. Science 289:452-457. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann, M., and D. Brian. 1991. Sequencing PCR DNA amplified directly from a bacterial colony. Biotechniques 11:30-31. [PubMed] [Google Scholar]
- 17.Jiménez-Zurdo, J. I., F. M. García-Rodríguez, A. Barrientos-Durán, and N. Toro. 2003. DNA target site requirements for homing in vivo of a bacterial group II intron encoding a protein lacking the DNA endonuclease domain. J. Mol. Biol. 326:413-423. [DOI] [PubMed] [Google Scholar]
- 18.Karberg, M., et al. 2001. Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat. Biotechnol. 19:1162-1167. [DOI] [PubMed] [Google Scholar]
- 19.Kukkonen, M., et al. 2001. Protein regions important for plasminogen activation and inactivation of α2-antiplasmin in the surface protease Pla of Yersinia pestis. Mol. Microbiol. 40:1097-1111. [DOI] [PubMed] [Google Scholar]
- 20.Lambowitz, A. M., and S. Zimmerly. 2004. Mobile group II introns. Annu. Rev. Genet. 38:1-35. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Abarca, F., A. Barrientos-Durán, M. Fernández-López, and N. Toro. 2004. The RmInt1 group II intron has two different retrohoming pathways for mobility using predominantly the nascent lagging strand at DNA replication forks for priming. Nucleic Acids Res. 32:2880-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel, F., K. Umesomo, and H. Ozeki. 1989. Comparative and functional anatomy of group II catalytic introns: a review. Gene 82:5-30. [DOI] [PubMed] [Google Scholar]
- 23.Mohr, G., P. S. Perlman, and A. M. Lambowitz. 1993. Evolutionary relationships among group II intron-encoded proteins and identification of a conserved domain that may be related to maturase function. Nucleic Acids Res. 21:4991-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohr, G., D. Smith, M. Belfort, and A. M. Lambowitz. 2000. Rules for DNA target-site recognition by a lactotococcal group II intron enable retargeting of the intron to specific DNA sequences. Genes Dev. 14:559-573. [PMC free article] [PubMed] [Google Scholar]
- 25.Molina-Sánchez, M. D., F. Martínez-Abarca, and N. Toro. 2010. Structural features in the C-terminal region of the Sinorhizobium meliloti RmInt1 group II intron-encoded protein contribute to its maturase and intron DNA-insertion function. FEBS J. 277:244-254. [DOI] [PubMed] [Google Scholar]
- 26.Munóz-Adelantado, E., et al. 2003. Mobility of the Sinorhizobium meliloti group II intron RmInt1 occurs by reverse splicing into DNA, but requires and unknown reverse transcriptase priming mechanism. J. Mol. Biol. 327:931-943. [DOI] [PubMed] [Google Scholar]
- 27.Nisa-Martínez, R., J. I. Jiménez-Zurdo, F. Martínez-Abarca, E. Muñoz-Adelantado, and N. Toro. 2007. Dispersion of the RmInt1 group II intron in the Sinorhizobium meliloti genome upon acquisition by conjugative transfer. Nucleic Acids Res. 35:214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertsen, B. K., P. Aiman, A. G. Darvill, M. McNeil, and P. Alberstein. 1981. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol. 67:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.San Filippo, J., and A. M. Lambowitz. 2002. Characterization of the C-terminal DNA-binding/DNA endonuclease region of a group II intron-encoded protein. J. Mol. Biol. 324:933-951. [DOI] [PubMed] [Google Scholar]
- 31.Sharp, P. A. 1985. On the origin of RNA splicing and introns. Cell 42:397-400. [DOI] [PubMed] [Google Scholar]
- 32.Simon, D. M., et al. 2008. Group II introns in eubacteria and archaea: ORF-less introns and new varieties. RNA 14:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, N. N., and A. M. Lambowitz. 2001. Interaction of a group II intron ribonucleoprotein endonuclease with its DNA target site investigated by DNA footprinting and modification interference. J. Mol. Biol. 309:361-386. [DOI] [PubMed] [Google Scholar]
- 34.Smith, D., J. Zhong, M. Matsuura, A. M. Lambowitz, and M. Belfort. 2005. Recruitment of host functions suggests a repair pathway for late steps in group II intron retrohoming. Genes Dev. 19:2477-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toor, N., G. Hausner, and S. Zimmerly. 2001. Coevolution of group II intron RNA structures with their intron-encoded reverse transcriptases. RNA 7:1142-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toro, N., M. D. Molina-Sánchez, and M. Fernández-López. 2002. Identification and characterization of bacterial class E group II introns. Gene 299:245-250. [DOI] [PubMed] [Google Scholar]
- 37.Toro, N. 2003. Bacteria and archaea group II introns: additional mobile elements in the environment. Environ. Microbiol. 5:143-151. [DOI] [PubMed] [Google Scholar]
- 38.Toro, N., J. I. Jiménez-Zurdo, and F. M. García-Rodríguez. 2007. Bacterial group II introns: not just splicing. FEMS Microbiol. Rev. 31:342-358. [DOI] [PubMed] [Google Scholar]
- 39.Vallès, Y., K. M. Halanych, and J. L. Boore. 2008. Group II introns break new boundaries: presence in a bilaterian's genome. PLoS One 3:e1488. doi: 10.1371/journal.pone.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, J., S. Zimmerly, P. S. Perlman, and A. M. Lambowitz. 1996. Efficient integration of an intron RNA into double-stranded DNA by reverse splicing. Nature 381:332-335. [DOI] [PubMed] [Google Scholar]
- 41.Yao, J., and A. M. Lambowitz. 2007. Gene targeting in Gram-negative bacteria by use of a mobile group II intron (“targetron”) expressed from a broad-host-range vector. Appl. Environ. Microbiol. 73:2735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao, J., et al. 2006. Use of targetrons to disrupt essential and nonessential genes in Staphylococcus aureus reveals temperature sensitivity of Ll.LtrB group II intron splicing. RNA 12:1271-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarschler, K., B. Janesch, S. Zayni, C. Schäffer, and P. Messner. 2009. Construction of a gene knockout system for application in Paenibacillus alvei CCM 2051T exemplified with the initiation enzyme WsfP of S-layer glycan biosynthesis. Appl. Environ. Microbiol. 75:3077-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong, J., and A. M. Lambowitz. 2003. Group II intron mobility using nascent strands at DNA replication forks to prime reverse transcription. EMBO J. 22:4555-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang, F., M. Karberg, J. Perutka, and A. M. Lambowitz. 2009. EcI5, a group IIB intron with high retrohoming frequency: DNA target site recognition and use in gene targeting. RNA 15:432-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerly, S., G. Hausner, and X. Wu. 2001. Phylogenetic relationships among group II intron ORFs. Nucleic Acids Res. 29:1238-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerly, S., H. Guo, P. S. Perlman, and A. M. Lambowitz. 1995. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell 82:545-554. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerly, S., et al. 1995. A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell 83:529-538. [DOI] [PubMed] [Google Scholar]