Abstract
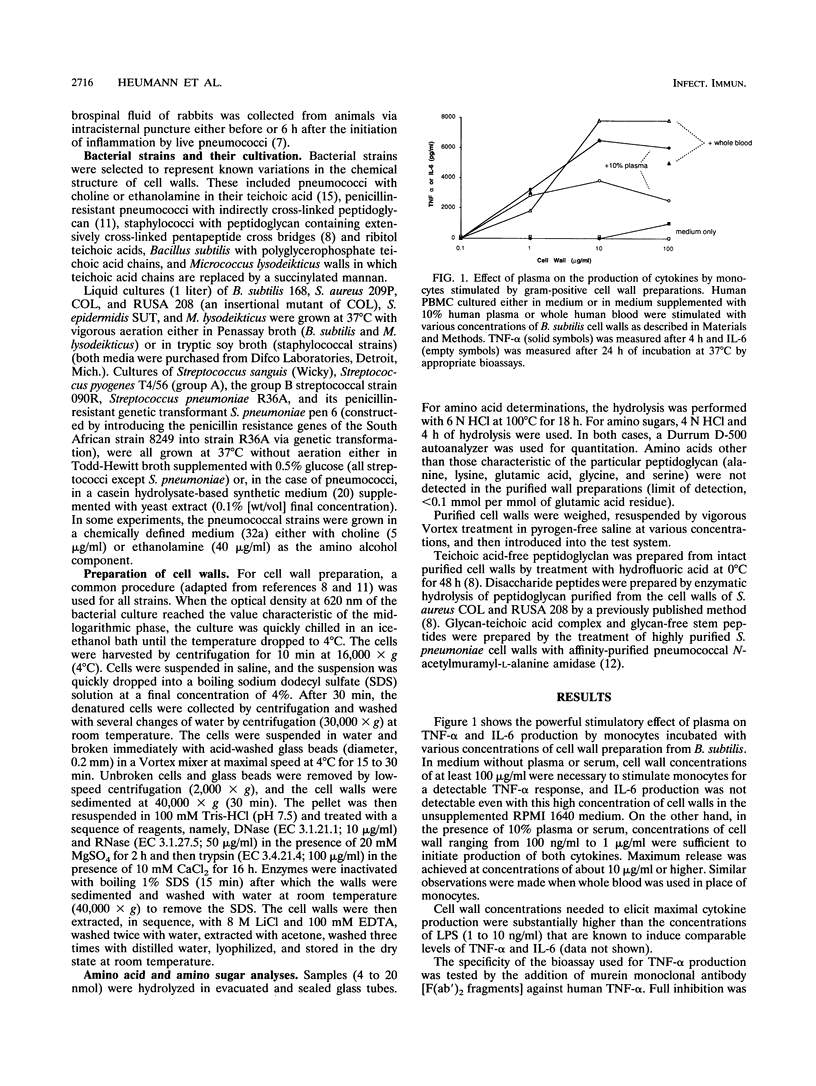
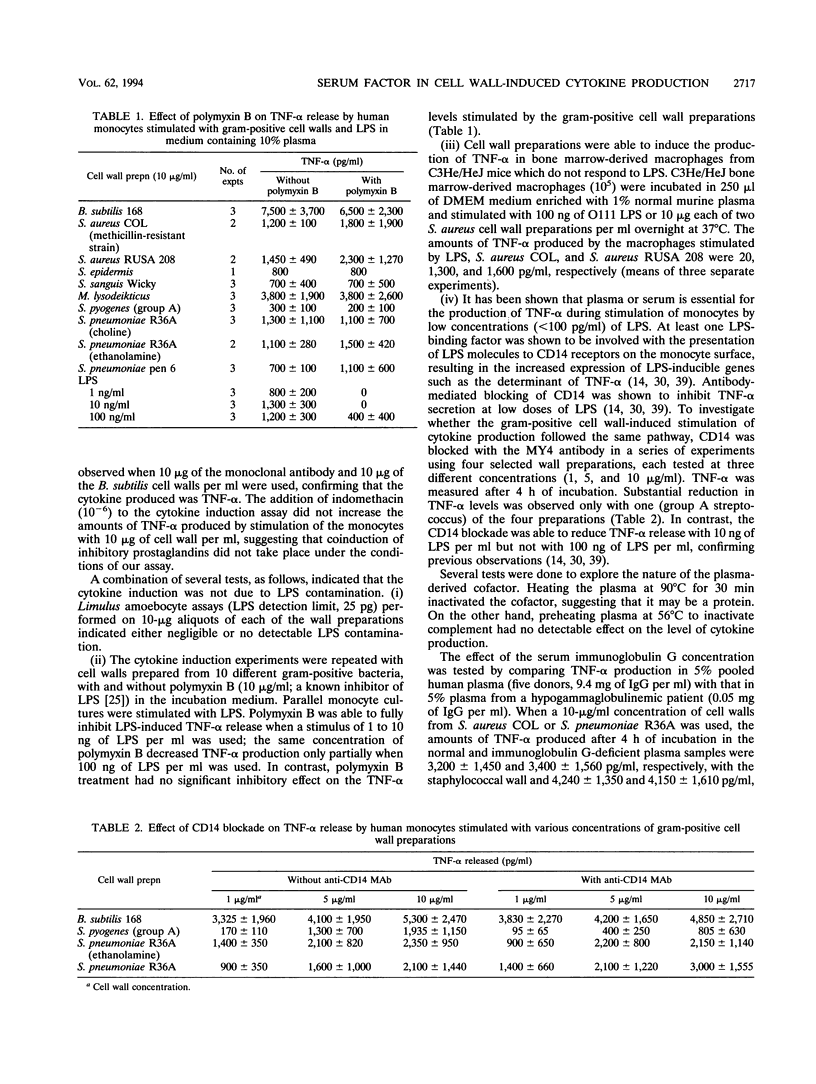
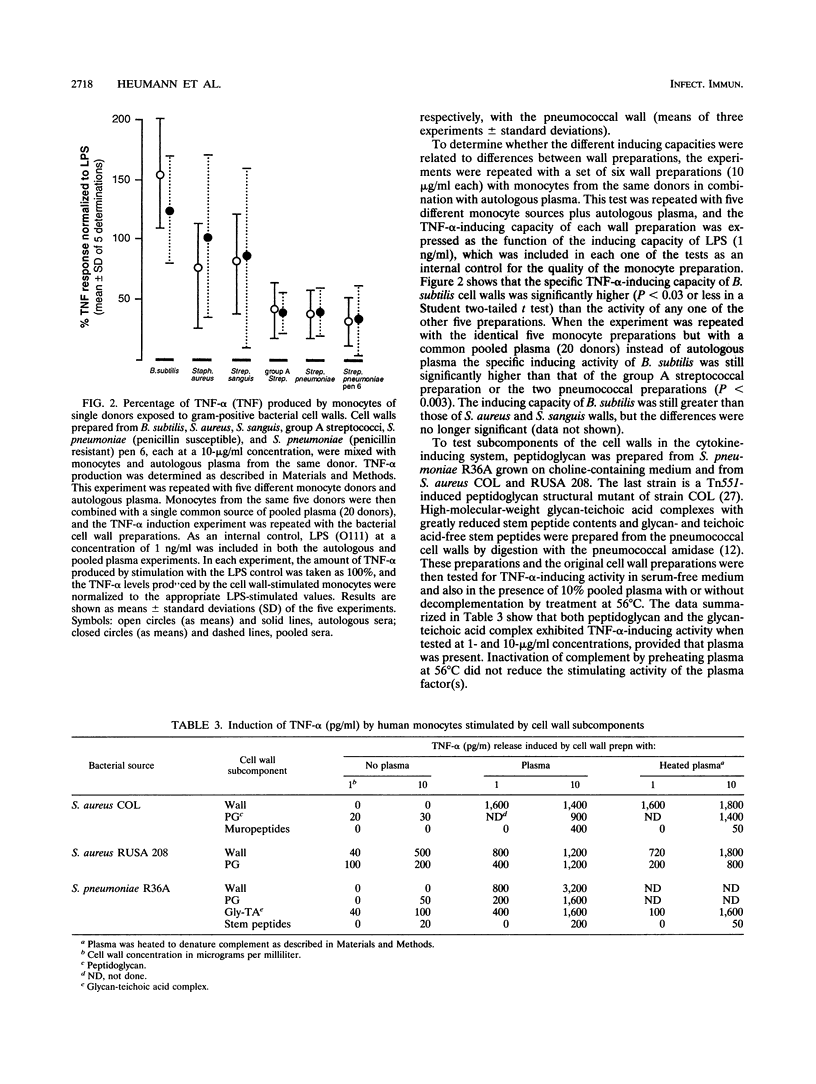
Purified cell walls representing a wide variety in teichoic acid and peptidoglycan structure prepared from eight different gram-positive bacterial species induced the production of tumor necrosis factor alpha (TNF-alpha) and interleukin-6 from human monocytes in the presence of 10% plasma or serum. Significant amounts of cytokines began to be produced at concentrations above 100 ng to 1 microgram of cell walls per ml, with maximal production requiring 10 to 100 micrograms of cell wall material per ml. In the absence of plasma, the cytokine-inducing capacity of cell wall preparations was lower by at least an order of magnitude. The serum-derived cofactor was inactivated by heating at 90 degrees C for 30 min, suggesting that the activity is associated with a protein. On the other hand, replacement of normal with hypogammaglobulinemic plasma, inactivation of complement (at 56 degrees C), and blockade by the monoclonal antibody MY4 of the CD14 receptors on monocytes did not inhibit the production of TNF-alpha induced by whole cell walls. Cell walls also stimulated production of TNF-alpha induced by whole cell walls. Cell walls also stimulated production of TNF-alpha in the presence of polymyxin B, and macrophages derived from the lipopolysaccharide-insensitive cell line of C3He/HeJ mice also produced this cytokine when stimulated by cell walls. Both peptidoglycan and the soluble glycan-teichoic acid component prepared by an enzymatic method from the same wall preparation exhibited a serum-dependent induction of TNF-alpha from monocytes, while stem peptides and disacharride peptides had only poor, if any, activity. Cell walls may contribute to the septic shock induced by gram-positive bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Klonisch T., Nuber P., Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991 Dec;59(12):4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard K. W., Minor L. B., Slotman G. J., Gann D. S. Staphylococcus epidermidis sepsis in surgical patients. Arch Surg. 1984 Jan;119(1):96–100. doi: 10.1001/archsurg.1984.01390130078014. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J., Gallay P., Heumann D., Ulevitch R. J., Tobias P. S. Enhancement of murine macrophage binding of and response to bacterial lipopolysaccharide (LPS) by LPS-binding protein. J Leukoc Biol. 1992 Oct;52(4):363–368. doi: 10.1002/jlb.52.4.363. [DOI] [PubMed] [Google Scholar]
- Crass B. A., Bergdoll M. S. Toxin involvement in toxic shock syndrome. J Infect Dis. 1986 May;153(5):918–926. doi: 10.1093/infdis/153.5.918. [DOI] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Chait B. T., Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987 Nov 15;262(32):15400–15405. [PubMed] [Google Scholar]
- Garcia-Bustos J., Tomasz A. A biological price of antibiotic resistance: major changes in the peptidoglycan structure of penicillin-resistant pneumococci. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5415–5419. doi: 10.1073/pnas.87.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Fischer W., Keist R., Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992 Sep;60(9):3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Gustafson J. E., Keist R. The macrophage response to bacteria. Modulation of macrophage functional activity by peptidoglycan from Moraxella (Branhamella) catarrhalis. Clin Exp Immunol. 1992 Sep;89(3):384–389. doi: 10.1111/j.1365-2249.1992.tb06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Pfaller M. A., Wenzel R. P. Coagulase-negative staphylococcal bacteremia. Mortality and hospital stay. Ann Intern Med. 1989 Jan 1;110(1):9–16. doi: 10.7326/0003-4819-110-1-9. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Tobias P. S., Wolfson E., Ulevitch R. J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992 Jul 1;149(1):200–206. [PubMed] [Google Scholar]
- Mattsson E., Verhage L., Rollof J., Fleer A., Verhoef J., van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993 Oct;7(3):281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Miethke T., Wahl C., Heeg K., Echtenacher B., Krammer P. H., Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992 Jan 1;175(1):91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Natanson C., Danner R. L., Elin R. J., Hosseini J. M., Peart K. W., Banks S. M., MacVittie T. J., Walker R. I., Parrillo J. E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest. 1989 Jan;83(1):243–251. doi: 10.1172/JCI113866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas-Soares A., de Lencastre H., de Jonge B., Gage D., Chang Y. S., Tomasz A. The peptidoglycan composition of a Staphylococcus aureus mutant selected for reduced methicillin resistance. J Biol Chem. 1993 Dec 15;268(35):26268–26272. [PubMed] [Google Scholar]
- Parsonnet J. Mediators in the pathogenesis of toxic shock syndrome: overview. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S263–S269. doi: 10.1093/clinids/11.supplement_1.s263. [DOI] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- See R. H., Chow A. W. Staphylococcal toxic shock syndrome toxin 1-induced tumor necrosis factor alpha and interleukin-1 beta secretion by human peripheral blood monocytes and T lymphocytes is differentially suppressed by protein kinase inhibitors. Infect Immun. 1992 Aug;60(8):3456–3459. doi: 10.1128/iai.60.8.3456-3459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F. The role of cytokine mediators in septic shock. Adv Surg. 1990;23:21–56. [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Jung W. K., Connolly R. J., Burke J. F., Dinarello C. A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Invest. 1991 Jun;87(6):1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle N. Bacteraemic and endotoxic shock. Br J Hosp Med. 1979 Jul;22(1):104–104. [PubMed] [Google Scholar]
- Wiles J. B., Cerra F. B., Siegel J. H., Border J. R. The systemic septic response: does the organism matter? Crit Care Med. 1980 Feb;8(2):55–60. doi: 10.1097/00003246-198002000-00001. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Patel M., Miller D. S. Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992 Sep 1;176(3):719–727. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- de Jonge B. L., Chang Y. S., Gage D., Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem. 1992 Jun 5;267(16):11248–11254. [PubMed] [Google Scholar]


