Abstract
Photorhabdus luminescens lives in a mutualistic association with entomopathogenic nematodes and is pathogenic for insects. Variants of Photorhabdus frequently arise irreversibly and are studied because they have altered phenotypic traits that are potentially important for the host interaction. VAR* is a colonial and phenotypic variant displaying delayed pathogenicity when directly injected into the insect, Spodoptera littoralis. In this study, we evaluated the role of transcriptomic modulation in determining the phenotypic variation and delayed pathogenicity of VAR* with respect to the corresponding wild-type form, TT01α. A P. luminescens microarray identified 148 genes as differentially transcribed between VAR* and TT01α. The net regulator status of VAR* was found to be significantly modified. We also observed in VAR* a decrease in the transcription of genes supporting certain phenotypic traits, such as pigmentation, crystalline inclusion, antibiosis, and protease and lipase activities. Three genes encoding insecticidal toxins (pit and pirB) or putative insecticidal toxins (xnp2) were less transcribed in VAR* than in the TT01α. The overexpression of these genes was not sufficient to restore the virulence of VAR* to the levels of ΤΤ01α, which suggests that the lower virulence of VAR* does not result from impaired toxemia in insects. Three loci involved in oxidative stress responses (sodA, katE, and the hca operon) were found to be downregulated in VAR*. This is consistent with the greater sensitivity of VAR* to H2O2 and may account for the impaired bacteremia in the hemolymph of S. littoralis larvae observed with VAR*. In conclusion, we demonstrate here that some phenotypic traits of VAR* are regulated transcriptionally and highlight the multifactorial nature of pathogenicity in insects.
Photorhabdus luminescens is a member of the Enterobacteriaceae that lives in a mutualistic association with entomopathogenic nematodes and is pathogenic for a wide range of insects. Studies on Photorhabdus pathogenicity have shown this bacterium to be highly virulent, due to the production of an array of insecticidal toxins and the induction of host immune depression (6, 18, 25, 28, 41, 67).
Variants frequently occur within the Photorhabdus genus. Colonial variants form colonies with different morphotypes and are unstable (31, 32, 38, 39, 65, 69). Some of these morphotypes have modified virulence properties and do not support nematode development and reproduction (31, 38). Phenotypic variation is another phenomenon widely described in the Photorhabdus genus. Phenotypic variants generally emerge after prolonged in vitro culture of the wild-type strain collected from the nematode (1, 7). The phenotypic variant is characterized by simultaneous changes in many of the traits of the wild-type form (the production of extracellular enzymes, pigments, antibiotics, and crystalline inclusion bodies and the ability to generate bioluminescence). This phenomenon occurs at a low and unpredictable frequency and is rarely reversible. Generally, both wild-type and variant forms are virulent in insect hosts, but only wild-type forms support nematode growth and development (27, 67). Since the two main types of variation in Photorhabdus affect interactions with invertebrate hosts and/or phenotypes potentially involved in these interactions, several studies have tried to determine the mechanisms underlying such events.
Comparative genomic, proteomic, and transcriptomic approaches have been used to investigate regulation of the phenotypic switch (22, 31, 40, 53, 61). Comparative genomics studies have provided no useful information about this regulation (31). The generation of phenotypic variants in Photorhabdus temperata strain K122 has been shown to be controlled by repressing factors (40, 53), which also play a key role in the regulatory network controlling pathogenicity and mutualism (40). The molecular basis of the phenotypic traits of the wild-type form has also been analyzed. Lipase and protease proteins are present in phenotypic variants, but their activities are inhibited (54, 63). Comparative whole-proteome analysis has been carried out on wild-type and variant forms of P. luminescens TT01 and has identified proteomic variations (22, 61). However, few studies have focused on the molecular basis of phenotypic variation traits at the transcriptomic level.
We have previously described different Photorhabdus variants (31). One of these variants, the wild-type form TT01α, was collected from a laboratory-maintained symbiotic nematode. From a phenotypic point of view, this variant is not distinguishable from the wild-type form TT01, but it differs in having large-scale deletion events in the flexible genome. The VAR* variant is a derivative of the wild-type form TT01α. This variant is of particular interest because it is both a phenotypic and a colonial variant. Moreover, VAR* displays delayed pathogenicity in the insect Spodoptera littoralis. VAR* differs from TT01α principally in the presence of an unusual single-block duplication encompassing 275 kb, corresponding to 4.8% of the TT01 reference genome (31).
The purpose of the present study was to evaluate the role of transcriptomic variation in the phenotypic variation and delayed pathogenicity of the VAR* variant. With this goal in mind, we compared the transcriptomes of TT01α and VAR*. Based on the description of the transcriptomic variation observed, we followed various lines of investigation to account for the observed physiologic variation: phenotypic variation and delayed pathogenicity.
MATERIALS AND METHODS
Bacterial strains and medium.
The strains and plasmids used in the present study and their sources are listed in Table 1. Bacteria were routinely grown in Luria-Bertani (LB) broth, on solid LB medium, on 1.5% nutrient agar (GNO) medium, on NBTA (nutrient agar supplemented with 25 mg of bromothymol blue and 40 mg of triphenyl-2,3,4 tetrazolium chloride per liter) medium, or on TreGNO (nutrient agar with 10 g of trehalose and 25 mg of bromothymol blue per liter) medium (31) at 28°C for P. luminescens and at 37°C for Escherichia coli. When required, the final concentrations of antibiotics used for selection were as follows: 15 μg of erythromycin/ml for P. luminescens, 10 mg (liquid culture) and 18 mg (agar plates) of cefoxitin/liter for P. luminescens and 20 mg of chloramphenicol/liter for E. coli, and 15 mg of chloramphenicol/liter for P. luminescens.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| P. luminescens | ||
| TT01 | Strain isolated from the nematode Heterorhabditis bacteriophora THO1 in Trinidad; wild-type form | 26 |
| TT01α | Genomic variant of TT01 isolated from the laboratory-maintained nematode Heterorhabditis bacteriophora THO1; wild-type form | 31 |
| VAR* | Stabilized TT01α phenotypic and colonial variant | 31 |
| TT01ΔsodA | TT01R sodA::cat | 17 |
| E. coli | ||
| XL1-Blue MRF′ | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac F′ [proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| S17-1 | pro r− n− Tpr Smr RP4-2-Tc::Mu::Tn7 recA thi | 60 |
| Plasmids | ||
| pBBR1-MCS1 | Broad-host-range vector; Cmr, mob | 42 |
| pBB-pit | 0.5-kb PCR fragment obtained with XhoI-L-plu1537 and PstI-R-plu1537 primers and inserted between the XhoI and PstI sites of pBBR1-MCS1 | This study |
| pBB-pirAB | 1.7 kb PCR fragment obtained with XhoI-L-plu4093 and PstI-R-plu4092 primers and inserted between the XhoI and PstI sites of pBBR1-MCS1 | This study |
| pBB-xnp2 | 7.3-kb PCR fragment obtained with XhoI-L-plu2444 and PstI-R-plu2441 primers and inserted between the XhoI and PstI sites of pBBR1-MCS1 | This study |
| pBB-UIDK | pBB-uidA; Kmr | 46 |
| pBB-PlacuidA | PvuII Plac fragment from pUC19 inserted into pBB-UIDK | This study |
Cmr, chloramphenicol resistance; Tpr, trimethoprim resistance; Smr, streptomycin resistance; Kmr, kanamycin resistance.
Phenotypic analysis of Photorhabdus variants.
Wild-type and variant traits were assessed as previously described (5) except for the protease assay, which was monitored by a colorimetric method (14). Agglutination assays were assessed as previously described (50) with Photorhabdus bacterial suspensions (108 cells/ml) prepared from exponential-growth or stationary-phase cultures in LB broth.
RNA preparation.
TT01α and VAR* were grown in LB broth for 6 h (5 × 107 CFU/ml; optical density at 540 nm [OD540] of 0.5; exponential-growth phase) or 45 h (5 × 108 CFU/ml; OD540 of 13 for TT01α and OD540 of 8 for VAR*; stationary-growth phase). Total RNA was extracted with TRIzol reagent, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA) and then purified with a High Pure RNA Isolation kit (Roche, Meylan, France), including a DNase I treatment step. The concentration was determined by spectrophotometric measurement of the OD260. For each RNA preparation, DNA contamination was assessed by carrying out PCR.
Microarray experiments and analysis.
We synthesized cDNA targets from 10 μg of TT01α and VAR* RNA and labeled them as follows. Each RNA preparation was heated at 70°C for 10 min with random hexamer (0.1 μg/μl; Roche) and cooled at room temperature for 10 min. The RNA was then reverse transcribed by incubation at 42°C for 2 h with SuperScript II (10 U/μl; Invitrogen), dithiothreitol (5 mM; Invitrogen), a mixture of deoxynucleoside triphosphates (1 μl; RPK0147; Amersham/GE Healthcare Europe GmbH, Munich, Germany), and dCTP-Cy3 or dCTP-Cy5 (50 μM; Amersham). The remaining RNA was subjected to alkaline lysis by incubation with 0.2 M NaOH for 10 min at 37°C. The labeled cDNAs were neutralized by adding 625 mM HEPES (pH 8) and immediately purified with a QiaQuick PCR purification kit (Qiagen, Courtaboeuf, France), used according to the manufacturer's recommendations, but with two additional washes in PE buffer (provided in the Qiagen kit). Cy5- and Cy3-labeled cDNAs were mixed, dried under a vacuum, and resuspended in hybridization buffer, as previously described (30).
The Photorhabdus DNA microarray (GEO accession number GPL11042) and the prehybridization and hybridization procedures have been described elsewhere (30). Each microarray comparison (TT01α versus VAR* growth in the exponential-growth or stationary phase) included six slides, with three dye-swapping replicates. The intensity of the signal for each microarray spot was determined with ArrayVision software (Amersham).
Microarray data were normalized and statistical analyses were carried out with the Spotfire DecisionSite functional genomic tool (http://spotfire.tibco.com/products/decisionsite-functional-genomics.aspx). The raw intensities were normalized to ensure that the experiments were comparable and to remove sources of systematic variation. For a given hybridization, the median intensity of a given variable is adjusted such that it is equal to the median intensity of the control variable (logR − logG = 0, where R and G are the summed intensities for each variable) without the designation of a baseline. As such, the intensity levels of the two channels are mutually adjusted (dye normalization). The trimmed median method was used to calculate a median value for which the effect of outliers was minimized. The trim cutoff was set at 10%, resulting in the exclusion of the top 5% and the bottom 5% of the values from the calculation. The dye-normalized values were used for a global normalization process in which intensities were rescaled with respect to a baseline intensity control.
After normalization, the fold change (FC) was calculated as follows: FC = R if R > 1 and −1/R if R < 1, where R = (median of normalized intensity for VAR*)/(median of normalized intensity for TT01α). We used a Student t test to identify statistically significant differences between groups. The original hypothesis (i.e., null hypothesis) was that the median expression levels of a gene did not differ between the two groups. The null hypothesis was then either rejected or accepted for each gene considered. The results are expressed in terms of P values, indicating the significance of the differences observed. These values correspond to the probability of a type I error leading to the conclusion that two median expression values for a given gene are different when there is, in fact, no difference. A significant difference is considered to exist if the P value obtained is below 0.01. The microarray data are accessible under the GEO accession number GSE24627.
qRT-PCR.
For the validation of whole-transcriptome data, quantitative reverse transcription-PCR (qRT-PCR) was carried out on the RNA preparations used for Photorhabdus microarray hybridization. cDNAs were synthesized with 1 μg of total RNA using the SuperScript II reverse transcriptase (Invitrogen) and random hexamers (100 ng/μl; Roche Diagnostics). qPCR analyses were performed using a LightCycler FastStart DNA MasterPLUS SYBR green I kit (Roche Diagnostics) with 1 μl of cDNA synthesis mixture and 1 μM concentrations of specific gene primers (Table 2). The enzyme was activated for 10 min at 95°C. The reactions were performed in triplicate at 95°C for 5 s, 61°C for 5 s, and 72°C for 10 s (45 cycles) and monitored in the LightCycler (Roche). Melting curves were analyzed for each reaction, and each curve contained a single peak. The amount of PCR product was calculated from the standard curves obtained from PCR with serially diluted genomic DNA from P. luminescens TT01. All data are presented as a ratio with respect to gyrB, which was used as a control gene. Values are means ± the standard deviation and were compared by using a Student t test.
TABLE 2.
Oligonucleotides used in this study
| Use and oligonucleotide | Sequence (5′-3′)a | Location |
|---|---|---|
| qRT-PCR | ||
| L-0004 | ATACACGAAGAAGAAGGTGTTTCAG | Internal region within plu0004 |
| R-0004 | TACCTGTCTGTTCAGTTTCTCCAAC | Internal region within plu0004 |
| L-0157 | ATGAAGAGCTGATTGTCGATGATG | Internal region within plu0157 |
| R-0157 | CGTAAAAGAGAAAGATAGAGCAGCAC | Internal region within plu0157 |
| L-0183 | AGTGTGGAACGTTGGATAGATAGAG | Internal region within plu0183 |
| R-0183 | AAGTATAGCTGCACCTACAGAACAAC | Internal region within plu0183 |
| L-1537 | ACTTATCCAGGATTCTATTCGTCTG | Internal region within plu1537 |
| R-1537 | ACTATAGATTTCGCCTTTGCTATCC | Internal region within plu1537 |
| L-1752 | CCTACGATATAGGCAATGGTATCAG | Internal region within plu1752 |
| R-1752 | ACAATATCTTGGTAGCCGAAAGAG | Internal region within plu1752 |
| L-1956 | GTACCTGTTTGAACAGACTGAAATG | Internal region within plu1956 |
| R-1956 | TTGTAGTTACTGAATGCTGACTGTG | Internal region within plu1956 |
| L-1991 | GCTAATATTTCCGTAGGCTCTCATC | Internal region within plu1991 |
| R-1991 | GGACGTAATCAAAGTGTAATTCTCG | Internal region within plu1991 |
| L-2442 | AATAGGCAATATGCTGTACGTCTTC | Internal region within plu2442 |
| R-2442 | CAGACCGCAAATACAATAAAGTAGG | Internal region within plu2442 |
| L-3506 | TTAAGGGCGATATTGATGTAGAAGG | Internal region within plu3506 |
| R-3506 | TTGATAGACATACTGCTGCAACGAC | Internal region within plu3506 |
| L-4092 | GACAGTGACCATGAACAGACATAAC | Internal region within plu4092 |
| R-4092 | ATATTGGGTTGCTGGTTTAGAGAG | Internal region within plu4092 |
| L-4546 | CTGCCGATATAATTGCAGCTTTAC | Internal region within plu4546 |
| R-4546 | CCAAATTTCTGAAGGGTGTATTTCC | Internal region within plu4546 |
| Plasmid constructions | ||
| XhoI-L-plu1537 | GCGCCTCGAGTTAAAATCAATCACAAGAGG | Upstream of plu1537 |
| PstI-R-plu1537 | GCGCCTGCAGAATATCTTCTCACAGGAATGG | Downstream of plu1537 |
| XhoI-L-plu4093 | GCGCCTCGAGTTAATGAGGAAAATAAATATGTC | Upstream of plu4093 |
| PstI-R-plu4092 | GCGCCTGCAGCTACGTACATAAAATATACTTGTTAAA | Downstream of plu4092 |
| XhoI-L-plu2444 | GCGCCTCGAGGAGGCCGATAGCTTCCAAC | Upstream of plu2444 |
| PstI-R-plu2441 | GCGCCTGCAGAAAGCGTAGCAATGTTCCTG | Downstream of plu2441 |
Restriction enzyme sites are underlined.
Sequence analysis.
The sequence annotations of the TT01 genome were from or updated with PhotoScope, the Photorhabdus database of the annotation and comparative analysis platform, MaGe (https://www.genoscope.cns.fr/agc/mage/wwwpkgdb/Login/log.php?pid=13).
Assays of sensitivity to antimicrobial peptides, antibiotics, and toxic compounds.
We assessed the susceptibility of the Photorhabdus variants to antimicrobial peptides (AMPs) or antibiotics, by determining the MICs of colistin, polymyxin B, cecropin A, and chloramphenicol. Stock solutions of colistin methanesulfonate (Sigma) and polymyxin B (Sigma) were prepared in sterile water, to obtain concentrations of 20 and 0.5 mg/ml, respectively. Stock solutions of cecropin A and B (Sigma synthetic molecules C6830 and C1796, respectively) were prepared in 1% acetic acid to obtain a concentration of 0.25 mg/ml. A stock solution of chloramphenicol (Sigma) was prepared in absolute ethanol to obtain a concentration of 20 mg/ml. The antimicrobial molecules were then added directly into the 96-well microtiter plates in 2-fold serial dilutions. Bacterial cells were grown at 28°C overnight in LB broth and were then washed and resuspended in Mueller-Hinton (MH) broth (Biokar, Marnes-la-Coquette, France). A final inoculum equivalent to 103 CFU was dispensed into the wells of 96-well microtiter plates. The MICs were determined after incubation at 28°C for 48 h. The microtiter plates were read by eye. β-Lactam susceptibility assays were carried out by the agar diffusion method in accordance with the instructions of the Antimicrobial Committee of the French Society for Microbiology. The susceptibilities of these cultures to other toxic compounds were assessed on solid media as follows: an inoculum of Photorhabdus variants equivalent to 103 CFU was evenly spread onto solidified MH medium containing 1.5% agar (BD Difco, Franklin Lakes, NJ) and allowed to dry at room temperature for 20 min. The various toxic compounds (1.5 mM ZnSO4, 30% H2O2, crystal violet [4 mg/ml], 20% sodium dodecyl sulfate, 25 mM deoxycholate) were diluted in sterile water and sterilized by passage through a filter with 0.2-μm-pore-size pores. Filter paper discs (bioMérieux S.A, Marcy l'Etoile, France) were impregnated with 5 μl of a solution of the toxic compound to be tested and placed in the center of a MH agar plate. Bacterial growth inhibition was evaluated by measuring the diameter of the zone in which no bacterial growth was observed.
DNA manipulation.
Standard DNA manipulations in Escherichia coli were carried out as described by Ausubel et al. (2). We evaluated the functional complementation of VAR* with genes encoding insecticidal toxins (plu1537, pit; plu4092, pirB; and plu2442, xnp2) by constructing low-copy-number plasmids as follows. pit, pirA-pirB, and the operon containing xnp2 (plu2444-plu2443-plu2442-plu2441) were amplified by PCR using the primer pairs XhoI-L-plu1537/PstI-R-plu1537, XhoI-L-plu4093/PstI-R-plu4092, and XhoI-L-plu2444/PstI-R-plu2441, respectively (see Table 2). PCR fragments were ligated into pBBR1-MCS hydrolyzed with XhoI and PstI (Roche) to ensure that these fragments were inserted downstream from the Plac promoter. The resulting plasmids were named pBB-pit, pBB-pirAB, and pBB-xnp2, respectively. All constructs were checked by sequencing (Millegen, Labège, France). Plasmids were introduced into VAR* by mating experiments, as previously described (11). We checked that the Plac promoter was functional in the VAR* variant by constructing the pBB-Plac uidA plasmid as follows. The Plac fragment from pUC19 was inserted upstream from the promoter-less uidA gene in pBB-UIDK (46). The uidA gene encodes the β-glucuronidase enzyme, the specific activity of which was measured as previously described (46).
Pathogenicity assays.
Pathogenicity experiments were performed as previously described (12). Statistical analysis (Wilcoxon test) was performed as previously described (11, 33) using SPSS version 14.0 (SPSS, Inc., Chicago, IL) to compare the mortality patterns.
Quantification of P. luminescens colonization of the S. littoralis hemolymph.
The bacteria recoverable from the hemolymph were counted as previously described (59) with the following modifications. Insects were injected with 5 × 104 bacteria. Hemolymph was collected by making a lesion in a proleg 0, 3, 6, 9, 24, 30, and 48 h after injection (four larvae per time point). We then plated serial dilution of hemolymph in LB broth on NBTA medium and on TreGNO medium, both of which were supplemented with erythromycin (final concentration, 15 μg ml−1) to prevent the growth of insect-associated bacteria other than P. luminescens. The NBTA and TreGNO plates were incubated at 28°C for 48 to 72 h at 28°C, and the CFU were counted. The results are expressed as the number of CFU in the total hemolymph (equivalent to 500 μl of hemolymph).
RESULTS AND DISCUSSION
Comparative analysis of the transcription profiling of TT01α and VAR* by microarray hybridization.
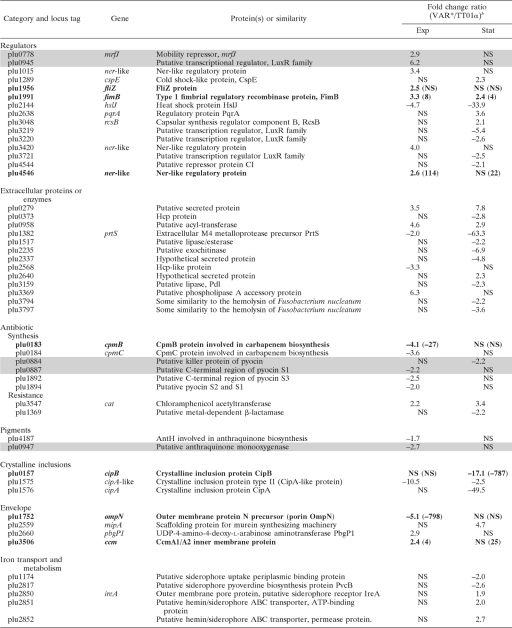
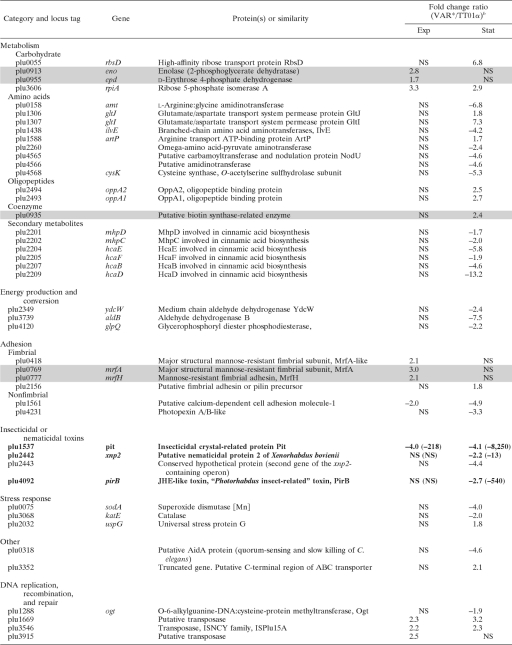
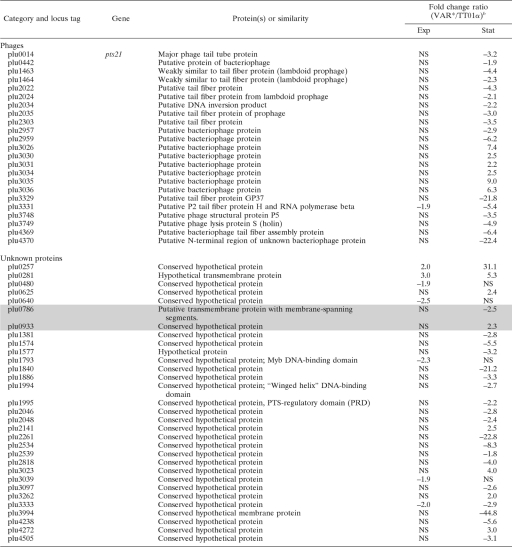
For comparison of the transcription profiles of TT01α and VAR* growing in LB broth, we first checked that the two forms had similar growth curves in this medium (data not shown). We then extracted RNA from cells in the exponential growth phase (6-h-old culture, 5 × 107 CFU/ml) and stationary phase (45-h-old culture, 5 × 108 CFU/ml). For each of these growth phases, two independent biological replicates were performed. Genes displaying a significant difference in transcription between TT01α and VAR* in the two biological replicates (P < 0.01) and with a fold change in signal intensity (VAR*/TT01α) greater than 1.5 or smaller than −1.5 are listed in Table 3. We found that 148 genes displayed differential patterns of transcription between the two variants: 104 during stationary phase, 28 genes during the exponential growth phase, and 16 in both phases, with the pattern of transcription varying similarly in the two phases.
TABLE 3.
Genes differentially transcribed between TT01α and VAR* in exponentially growing or stationary-phase culturesa
Genes were differentially transcribed between TT01α and VAR* in exponentially growing (exp) or stationary phase (stat) cultures (fold change VAR*/TT01α >1.5 or <−1.5; P < 0.01). Shaded rows indicate genes located in the previously described 275-kb single-block duplication (31). Rows in boldface indicate genes that were chosen for qRT-PCR validation.
b Exp, exponentially growing culture; Stat, stationary-phase culture. Values in parentheses indicate the qRT-PCR fold change (VAR*/TT01α) when the validation was undergone. NS, not significant.
The transcription data collected by microarray hybridization were checked on the two biological replicates by qRT-PCR on a sample of 10 genes (Table 3). These genes were chosen because they were potentially related to the phenotypic variation and the pathogenicity. As frequently reported, the qRT-PCR fold changes for the expression ratios were greater than the fold changes obtained with microarray data. Nevertheless, 17 on 20 comparisons between TT01α and VAR* gave similar results in microarray hybridization and qRT-PCR (Table 3). We also compared our results with the results of a proteomic approach comparing the TT01 wild-type form and its phenotypic variant (61). As in our study, the difference in expression pattern between the wild-type form and the phenotypic variant was found to be greater in the stationary phase than in the exponential growth phase. These findings validate our microarray analysis.
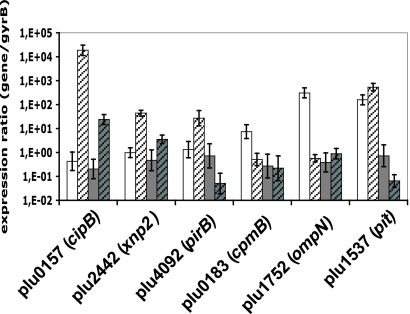
A high proportion of genes (93/148) displayed higher levels of transcription in TT01α than in VAR*. In order to determine what kind of regulation alteration occurs in VAR*, we compared the qRT-PCR data of six of these genes (Fig. 1). One gene (pit) displayed a growth-independent pattern of transcription in both strains, which suggests a repression phenomenon during whole growth in VAR*. Five genes displayed a growth-dependent pattern in TT01α. cipB, xnp2, and pirB transcription was specifically induced in the stationary phase of TT01α, and this induction was diminished or absent in the stationary phase of VAR*. cpmB and ompN displayed a specific transcriptional induction in the exponential growth phase of TT01α that disappeared in VAR*. Therefore, both a transcriptional repression and an absence of transcriptional induction occurs in VAR*, and the second phenomenon seems more important.
FIG. 1.
qRT-PCR data on six genes displaying lower transcription in TT01α than in VAR*. qRT-PCR was carried out with total RNA from TT01α (white bars) and VAR* (gray bars) variants in the growth exponential phase (plain bars) and the stationary phase (hatched bars). The specific internal primers for each gene are listed in Table 2. The data are presented as a ratio, with gyrB used as the control gene. Values are means of six assays (three technical replicates on each biological replicates) and were compared using a Student t test (P > 0.05). The confidence limits are shown.
The regions affected by the transcriptional modifications in VAR* were not colocalized. A 275-kb single-block duplication has previously been identified in the VAR* genome but was not observed in the TT01α genome (31). Gene amplification may confer physiological adaptation through the overproduction of transcripts and proteins, including those conferring antibiotic resistance, for example (57). Nevertheless, only 12 of the 148 differentially transcribed genes were located in the duplicated region. Furthermore, four of these genes were less strongly expressed in VAR* than in TT01α. This duplication therefore has no massive effect on transcription as a whole in the VAR* variant. This result confirms that phenotypic variant status is independent of global genomic architecture, as previously suggested (31).
A modified regulator network in VAR*.
The transcription of 15 regulators or potential regulators (see regulators in Table 3, column 1) was found to be modified. For 10 of them, the transcription levels were higher in VAR* than in TT01α: the MrfJ repressor, a putative transcriptional regulator from the LuxR family, three of the 13 Ner-like regulatory proteins encoded in the P. luminescens TT01 genome, the CspE cold-shock protein, the FliZ regulator, the FimB recombinase, and the PqrA and RcsB regulators. Higher levels of FimB and MrfJ proteins may have affects on fimbrial production. This is validated from a genetic perspective, since four genes encoding fimbrial components—a mrfA-like gene (plu0418), mrfA (plu0769), mrfH (plu0777), and a putative adhesin-encoding gene (plu2156)—are more transcribed in VAR* than in TT01α. Moreover, sheep erythrocytes agglutination was only detected with VAR* bacterial cells (Table 4).
TABLE 4.
Phenotypes of the TT01α and VAR* variantsa
| Phenotype | TT01α | VAR* |
|---|---|---|
| Colony morphology | Convex, mucoid | Flat, nonmucoid |
| Pigmentation | + (orange) | − |
| Coloration on TreGNO mediumb | Green | Yellow |
| Bioluminescence | + | − |
| Crystal | + | − |
| Antibiotic productionc | + | − |
| Sheep blood hemolysisd | T | − |
| Lipase activity on: | ||
| Tween 20-60e | ++ | + |
| Tween 80-85 | ++ | +v |
| Protease activity | ++ | w |
| Motility | + | + |
| Hemagglutination titerf | ||
| Rabbit hemagglutination titer | <1 | <1 |
| Sheep hemagglutination titer | <1 | 8 |
Unless noted otherwise, the results are scored as follows: +, positive; -, negative; v, variable; and w, weak.
On TreGNO medium, phenotypic variant colonies undergoing trehalose fermentation acidify the medium, turning the bromothymol blue yellow, whereas wild-type colonies do not undergo trehalose fermentation and remain green (31).
+, Zone of growth inhibition for Micrococcus luteus (laboratory collection); -, no growth inhibition.
T, total hemolysis; -, no hemolysis.
Halo of precipitation (diameter): ++, >20 mm; +, >10 mm.
Agglutination assays were assessed on Photorhabdus bacterial suspensions (108 cells/ml) prepared from exponential growth or stationary-phase cultures in LB broth.
For five molecules, lower levels of transcription were observed in VAR* than in TT01α: the HslJ heat shock protein, three putative transcriptional regulators from the LuxR family, and a putative CI repressor. This large number of regulators with modified transcription patterns suggests that the generation of phenotypic variants in P. luminescens TT01 depends on transcription switching mediated by a network of regulators rather than a single regulator.
Previous regulation analyses have deciphered the role of particular regulatory genes in the generation of Photorhabdus phenotypic variants (22, 40, 53). The AstR-AstS two-component system controls the timing of the phenotypic switch between the wild-type form and phenotypic variants in P. luminescens TT01 (22). Activation of the two-component systems is not dependent on the level of transcription of the genes encoding the proteins involved. Instead, it depends on the phosphorylation status of the cytoplasmic receptor (3). Our approach therefore did not allow the detection of RNA variation for the AstR-AstS or any other two-component system. The gene encoding the HexA/LrhA repressor, a LysR-type transcriptional regulator, is not transcribed in wild-type forms of P. temperata K122 (40). However, our data show no difference in the transcription patterns of the genes encoding HexA/LrhA (Plu3090) or any other LysR-type transcriptional regulator between TT01α and VAR*. Finally, the Ner-like repressor has a more ambiguous role (53). Similar RNA levels for this repressor are found in the wild-type form and phenotypic variants of P. temperata K122. Repression of the wild-type form is observed when the ner gene is harbored on a plasmid and overexpressed in a phenotypic variant. Our data show that three of the 13 ner-like genes present in the P. luminescens TT01 genome are more strongly transcribed in VAR* than in TT01α. Therefore, as in P. temperata K122, ner overexpression is observed in a phenotypic variant of P. luminescens TT01. This overexpression may be mediated by both the redundancy of ner-like genes in the TT01 genome and the higher level of transcription observed in the VAR* variant. The reason for this higher level of transcription is unknown, but it seems likely to be controlled by an upstream global regulator.
Transcriptomic variations of genes supporting phenotypic variations.
The wild-type strain and phenotypic variants of P. luminescens can be distinguished by a set of simple bacteriological tests, such as pigmentation on agar medium, trehalose fermentation, luminescence, the production of intracellular protein inclusions, and antimicrobial and extracellular activities (7, 31). TT01α and VAR* harbor the typical wild-type and phenotypic variant traits, respectively (Table 4). We explored the possibility that some phenotypic variant trait-related genes are regulated at the transcriptional level.
(i) Pigmentation.
On agar media, wild-type forms generate convex, mucoid, orange colonies, whereas phenotypic variants generate flat, nonmucoid colonies without pigmentation (7, 31). The antH gene (plu4187) and plu0947, which are involved in the anthraquinone biosynthesis pathway (10), are less strongly transcribed in VAR* than in TT01α, probably accounting for the loss of pigmentation of VAR*.
(ii) Crystalline inclusion.
One of the characteristic phenotypes of the wild-type bacterium is the presence of intracellular protein inclusions, known as crystals, which are absent from phenotypic variants (4). Crystalline inclusion proteins, CipA and CipB, are regulated transcriptionally in VAR*, since the cipA gene (plu1576), the cipB gene (plu0157), and a cipA-like gene (plu1575) displaying some similarity to cipA (identity, 36%; E-value, 8.4e−06) are less strongly transcribed in VAR* than in TT01α. The role of the crystalline inclusions remains unclear. They may be involved in nutrient storage, including sulfur storage in particular, due to the high methionine content of CipA (4). Moreover, the inactivation of either cipA or cipB through insertion results in mutants displaying variation in phenotypic traits and alteration in interactions with nematodes (4). For these reasons, Bintrim and Ensign speculated that Cip proteins might play a role in the global regulation of phenotypic variation. Since VAR* displayed changes to phenotypic traits but not to interactions with nematodes (D. Clarke, unpublished data), these two sets of phenotypes are probably regulated differently. For example, a small number of cip transcripts may be sufficient for correct interaction with nematodes but not for intracellular crystal formation and the generation of wild-type form traits.
(iii) Antibiotic synthesis.
The phenotypic variant form had lower levels of antimicrobial activity (5). Because classical antibiosis tests are carried out in a Gram-positive bacterium, Micrococcus luteus (Table 4), we extended the antibacterial activity test to other genera of bacteria. The antibacterial activity against the four Gram-positive bacteria observed with TT01α was not observed with VAR* (Table 5). Stilben is the only antimicrobial molecule produced by P. luminescens that has been shown to be active against Gram-positive bacteria (68). None of the genes involved in stilben biosynthesis was transcribed to different levels in VAR* and TT01α. The defect in antibiotic production by VAR* is therefore probably due to posttranscriptional modulation of the stilben biosynthesis pathway or antimicrobial molecules that have yet to be identified. Three kinds of antibiosis patterns were observed against Gram-negative bacteria: (i) no antibacterial activity for either form, (ii) antibacterial activity with TT01α but not with VAR* on both closed and more distantly related bacterial species, and (iii) antibacterial activity with VAR* but not with TT01α on Proteus vulgaris (Table 5). The pyocin-like bacteriocins of P. luminescens can kill related Gram-negative bacteria such as other Photorhabdus strains and E. coli (58). Two integral and two truncated genes encoding pyocin-like bacteriocins (bacteriocins Plu0884 and Plu1894 and bacteriocins Plu0887 and Plu1892, respectively) are less strongly transcribed in VAR* than in TT01α in the exponential growth or in the stationary phase. P. luminescens produces carbapenems of TT01, a class of β-lactam antibiotics with broad-spectrum activity mostly targeting Gram-negative bacteria (21). The cpmB and cpmC genes (plu0183 and plu0184), involved in the biosynthesis of carbapenems are less strongly transcribed in VAR* than in TT01α. The downregulation of both pyocin-like bacteriocins and carbapenems may therefore account for the lower levels of antimicrobial activity against the related E. coli, S. enterica, and Y. enterocolitica strains observed for VAR*, whereas the downregulation of carbapenems may only be sufficient to account for the lower levels of activity against the more distantly related O. anthropi and Pseudomonas sp. strains. For P. vulgaris, against which VAR* displayed a higher level of antibiosis than TT01α, other antimicrobial molecules are probably involved.
TABLE 5.
Antibacterial activities of the TT01α and VAR* variants against a panel of microorganisms
| Strain | Inhibition zone diam (mm)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria |
Gram-negative bacteria |
||||||||||||
| M. luteus | C. xerosis | S. epidermidis | S. aureus | E. coli | P. vulgaris | P. aeruginosa | O. intermedium | O. anthropi | E. amylovora | Pseudomonas sp. | S. enterica | Y. enterocolitica | |
| TT01α | 20 | 20 | 20 | 20 | 10 | 1 | 0 | 0 | 14 | 0 | 10 | 2 | 2 |
| VAR* | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Antibacterial activities are expressed as the diameter of inhibition zones observed in petri plate bioassays. The full strain designations were as follows: Micrococcus luteus CIP6821, Corynebacterium xerosis, Staphylococcus epidermidis CIP6821, Staphylococcus aureus CIP7625, Escherichia coli CIP7624, Proteus vulgaris CIP5860, Pseudomonas aeruginosa CIP76.110, Ochrobactrum intermedium LMG3301T, Ochrobactrum anthropi ATCC 49188T, Erwinia amylovora CFBP1430, Pseudomonas sp. strain BW11M, Salmonella enterica 14028s, and Yersinia enterocolitica serotype O8.
(iv) Extracellular protein activities.
Finally, extracellular activities, such as hemolysis and lipase and protease activities, were found to be weaker in the phenotypic variants (5). The activities of two metalloproteases, PrtA and PrtS, were attenuated in the supernatant of the P. temperata K122 phenotypic variant (9, 49). Our data suggest that the transcription of prtA (plu0655) is not modified but that the prtS gene (plu1382) and its downstream gene (plu1381), which probably belongs to the same transcription unit, are less strongly transcribed in VAR* than in TT01α. The regulation of lip-1 (plu3510), encoding a lipase responsible for Tween 80 degradation, is posttranslational in the K122 phenotypic variant of P. temperata (63). Our data confirm this result in P. luminescens TT01α, since no change in transcription was detected for the lip-1 gene. Nevertheless, the uncharacterized lipase genes, plu1517 and plu3159, were less strongly transcribed in VAR*. The low rates of transcription of these two genes may account for poor Tween 20-60 degradation by VAR* (Table 4).
It is widely accepted that posttranscriptional and posttranslational regulation processes are responsible for the differential phenotypes expressed by the two forms of Photorhabdus (22, 29, 54, 61, 63, 64). In the present study, we show that genes involved in pigmentation, crystalline inclusion, antibiosis, and protease and lipase activities are controlled at the transcriptional level. Interestingly, for the lipase and protease activities, downregulation by both transcriptional and posttranscriptional mechanisms is probably involved in phenotypic variation.
Toxin overproduction in the VAR* variant does not restore virulence.
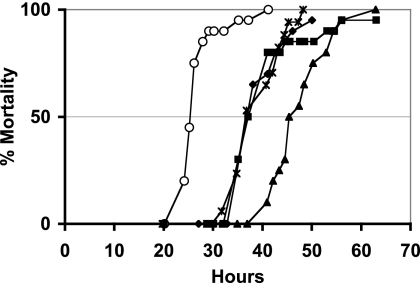
VAR* is less virulent than ΤΤ01α when injected into Spodoptera littoralis larvae: 50% mortality (LT50) was reached 25 h after injection with ΤΤ01α, but not until 37 h after injection for VAR*, although a larva mortality rate of 100% was nonetheless reached 3 days after infection (Fig. 2).
FIG. 2.
Mortality in S. littoralis. Shown is the mortality in S. littoralis infected with the TT01α P. luminescens wild-type form (○), the VAR* phenotypic variant (▪), and VAR* overexpressing toxins or potential toxin genes: the pirAB locus (✳), the pit gene (⧫), and the xnp2 gene (▴). Bacteria obtained at the end of the exponential growth phase were injected into fourth-instar larvae. Mortality values are based on data obtained after the injection of 20 larvae. Note that the virulence of the VAR* variant harboring the pBB-xnp2 plasmid is more attenuated than the VAR* virulence; this is probably due to the energy cost necessary to replicate a plasmid with a large insert (7.3 kb) compared to the 0.5- and 1.7-kb inserts of the pBB-pit and pBB-pirAB plasmids, respectively.
P. luminescens can kill insects by toxemia. The P. luminescens TT01 genome contains a large number of genes thought to encode insecticidal toxins—i.e., the Tc, Mcf, Pir, Txp40, and Pit toxins—and a gene predicted to encode a putative nematicidal protein, the Xnp2 toxin (8, 19, 24, 47, 66). Our transcriptional analyses (microarray hybridization and qRT-PCR) showed that pit (plu1537), pirB (plu4092), and xnp2 (plu2442) were less strongly transcribed in VAR* than in TT01α (Table 3 and Fig. 1). Interestingly, plu2443, the second gene of the operon containing xnp2, is also less strongly transcribed in VAR* than in TT01α.
We investigated the possible involvement of a defect in toxin gene transcription in the attenuation of virulence in VAR* by constructing strains overproducing the toxins. This was achieved by placing the pit gene, the pirAB genes and the four genes of the xnp2-containing operon under the control of the Plac promoter of the pBBR1-MCS1 plasmid and transferring these constructs into VAR*. We first checked that the Plac promoter was functional in VAR*, by measuring the β-glucuronidase activity of the pBB-PlacuidA plasmid in VAR* (data not shown). We then carried out pathological assays, by injecting VAR*/pBB-pit, VAR*/pBB-pirAB, and VAR*/pBB-xnp2 into S. littoralis larvae. The overproduction of these three proteins did not restore virulence in VAR* (Fig. 2).
Delayed growth of VAR* in S. littoralis hemolymph.
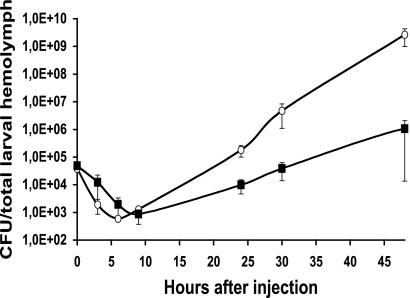
P. luminescens can also kill insects by bacteremia. We evaluated the role of bacteremia in insect death by assessing the growth of VAR* and TT01α bacteria in S. littoralis hemolymph by injecting each variant into the hemocoel of larvae (5 × 104 CFU/larva) and counting the CFU produced. As for the closely related bacterium Xenorhabdus nematophila, but at a later time point after bacterial injection (59), a partial clearance of circulating bacteria was observed for both TT01α and VAR* (between 6 and 9 h after injection) (Fig. 3). Bacterial growth then increased for both TT01α and VAR*. Nevertheless, in contrast to the results obtained for LB broth, in which TT01α and VAR* grew similarly, TT01α grew more rapidly than VAR* in hemolymph (Fig. 3). The delayed virulence of VAR* may therefore be due to impaired VAR* bacteremia.
FIG. 3.
Bacterial growth after the injection of TT01α (○) and VAR* (▪) into S. littoralis. The graphs show the mean numbers of CFU recovered from the total hemolymph of single larvae (four larvae per time point). Larvae were each injected with 5 × 104 bacteria at time zero. Errors bars indicate the standard errors of the means.
Downregulation of genes involved in drug or stress resistance.
Our transcription profiling study showed that the expression of genes associated with resistance to antimicrobial compounds or the detoxification of toxic compounds was affected in VAR* (Table 3). Five genes were more strongly transcribed in VAR* than in TT01α: mipA, which encodes a protein involved in peptidoglycan synthesis and controlling the growth of the stress-bearing sacculus of Escherichia coli (62); pbgP1, the first gene of an operon encoding the components of an enzymatic pathway involved in arabinose incorporation in the lipid A moiety of lipopolysaccharide and responsible for polymyxin resistance in Salmonella enterica (23, 34, 35); cat, which encodes a chloramphenicol acetyltransferase (13); ccm, which encodes an internal protein, CcmA, influencing cell shape in Proteus mirabilis (37); and uspG, which encodes a universal stress protein that may, in some cases, be linked to resistance to DNA-damaging agents and to respiratory uncouplers (45). Eight genes were found to be less strongly transcribed in VAR* than in TT01α: plu1369, which encodes a putative β-lactamase (15); ompN, encoding a protein of the porin family responsible for the permeability of Gram-negative bacteria to small, polar molecules (55); sodA and katE (plu3068), which encode the manganese-dependent superoxide dismutase SodA and the catalase KatE, respectively, both of which are involved in the detoxification of reactive oxygen species in Escherichia coli (48); and the hcaE, hcaF, hcaB, and hcaD genes, which belong to an operon encoding the components of a pathway involved in the degradation of 3-phenylpropionate and cinnamic acid and participate in oxidative stress resistance in Photorhabdus (16, 17).
We investigated the sensitivity of TT01α and VAR* to various antimicrobial molecules, such as antimicrobial peptides (colistin, cecropin A, and polymyxinB) and antibiotics (ampicillin and chloramphenicol), but both forms were found to be equally resistant to the five molecules (data not shown). We then investigated the sensitivity of the variant to toxic compounds. A significant difference in growth between the variant and the wild type was detected only with hydrogen peroxide (H2O2), to which VAR* was more susceptible than TT01α. The lower levels of transcription of the sodA gene, the katE gene and the hca operon are probably responsible for this sensitivity. Interestingly, the difference in inhibition diameters between VAR* and TT01α was similar to that between a P. luminescens TT01 ΔsodA mutant and the corresponding wild-type strain (data not shown). We further investigated the arsenal of genes providing resistance to oxidative stress in the P. luminescens TT01 genome. A careful search of the genome showed that P. luminescens TT01 does not have the other canonical enzymes involved in resistance to oxidative stress, i.e., those encoding SodB, SodC, and KatG (48). Under oxygen-rich conditions resulting from the Fenton reaction, iron is also a source of dangerous radicals (36), and some iron-transporters protect bacterial cells against lethal doses of hydrogen peroxide (20, 56). Interestingly, the transcription patterns of five genes involved in iron uptake were found to be modified in VAR* (Table 3). Dealing with oxidative stress is a relevant challenge for insect pathogens. Indeed, insect hemocytes engaged in phagocytosis generate reactive oxygen intermediates (51). For instance, during encapsulation of the eggs of the wasp parasitoid Leptopilina boulardi, H2O2 is one of the principal generated oxidants (52). Therefore, transcriptional alteration of genic arsenal involved in resistance to oxidative stress may explain the altered pathogenicity of VAR*.
Conclusion.
In the present study, we evaluated the extent and impact of transcriptomic modulation in the phenotypes of the VAR* variant, focusing on its phenotypic variant status and lower virulence than the wild-type form TT01α.
In terms of the phenotypic variant status of VAR*, we described the transcriptional regulation of both regulators and genes supporting some of the traits of the wild-type form. Mixed regulation (transcriptional and posttranscriptional) probably occurs for some traits, suggesting that phenotypic variation is a highly complex regulation phenomenon.
Our investigation of the attenuated virulence of VAR* showed in VAR* lower levels of transcription of genes involved in insecticidal toxin production and in the oxidative stress response than in the wild type. Similar effects have already been reported for isogenic mutants displaying impaired production of the regulatory proteins HcaR, SodA, UvrY, and LuxS (17, 43, 44). Like VAR*, none of these four mutants displayed a complete lack of virulence. This is probably due to the high degree of redundancy of toxins and oxidative stress responses in P. luminescens. These studies also suggest that there is a close relationship between toxin production, oxidative stress resistance, and virulence, probably mediated by a complex array of regulators. Overall, our transcriptomic data for the VAR* variant displaying delayed pathogenicity for insects, but no defect for nematode reproduction in contrast to other Photorhabdus variants, show that different forms and mechanisms of phenotypic variation coexist in the Photorhabdus genus. Moreover, our data are consistent with the view that bacterial virulence is both multifactorial and combinatorial.
Acknowledgments
We thank Nadège Ginibre for assistance with bacterial growth experiments in the S. littoralis hemolymph.
This study was supported by INRA (grant SPE 2004-1133-2) and by the Ministère de l'Industrie (AAV ASG no. 30; contract A01307).
Footnotes
Published ahead of print on 3 December 2010.
REFERENCES
- 1.Akhurst, R. J. 1980. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 121:303-309. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., et al. 1993. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 3.Beier, D., and R. Gross. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 4.Bintrim, S. B., and J. C. Ensign. 1998. Insertional inactivation of genes encoding the crystalline inclusion proteins of Photorhabdus luminescens results in mutants with pleiotropic phenotypes. J. Bacteriol. 180:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boemare, N., J.-O. Thaler, and A. Lanois. 1997. Simple bacteriological tests for phenotypic characterization of Xenorhabdus and Photorhabdus phase variants. Symbiosis 22:167-175. [Google Scholar]
- 6.Boemare, N. E. 2002. Biology, taxonomy, and systematics of Photorhabdus and Xenorhabdus, p. 35-56. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 7.Boemare, N. E., and R. J. Akhurst. 1988. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. ( Enterobacteriaceae). J. Gen. Microbiol. 134:751-761. [Google Scholar]
- 8.Bowen, D., et al. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129-2132. [DOI] [PubMed] [Google Scholar]
- 9.Bowen, D. J., et al. 2003. Genetic and biochemical characterization of PrtA, an RTX-like metalloprotease from Photorhabdus. Microbiology 149:1581-1591. [DOI] [PubMed] [Google Scholar]
- 10.Brachmann, A. O., et al. 2007. A type II polyketide synthase is responsible for anthraquinone biosynthesis in Photorhabdus luminescens. Chembiochem 8:1721-1728. [DOI] [PubMed] [Google Scholar]
- 11.Brillard, J., E. Duchaud, N. Boemare, F. Kunst, and A. Givaudan. 2002. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. J. Bacteriol. 184:3871-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugirard-Ricaud, K., et al. 2005. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell Microbiol. 7:363-371. [DOI] [PubMed] [Google Scholar]
- 13.Bunny, K. L., R. M. Hall, and H. W. Stokes. 1995. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob. Agents Chemother. 39:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabral, C. M., A. Cherqui, A. Pereira, and N. Simoes. 2004. Purification and characterization of two distinct metalloproteases secreted by the entomopathogenic bacterium Photorhabdus sp. strain Az29. Appl. Environ. Microbiol. 70:3831-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carfi, A., et al. 1995. The 3-D structure of a zinc metallo-beta-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 14:4914-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalabaev, S., et al. 2008. Cinnamic acid, an autoinducer of its own biosynthesis, is processed via Hca enzymes in Photorhabdus luminescens. Appl. Environ. Microbiol. 74:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalabaev, S., et al. 2007. The HcaR regulatory protein of Photorhabdus luminescens affects the production of proteins involved in oxidative stress and toxemia. Proteomics 7:4499-4510. [DOI] [PubMed] [Google Scholar]
- 18.Clarke, D. J. 2008. Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell Microbiol. 10:2159-2167. [DOI] [PubMed] [Google Scholar]
- 19.Daborn, P. J., et al. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. U. S. A. 99:10742-10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellagi, A., M. N. Brisset, J. P. Paulin, and D. Expert. 1998. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol. Plant-Microbe Interact. 11:734-742. [DOI] [PubMed] [Google Scholar]
- 21.Derzelle, S., E. Duchaud, F. Kunst, A. Danchin, and P. Bertin. 2002. Identification, characterization, and regulation of a cluster of genes involved in carbapenem biosynthesis in Photorhabdus luminescens. Appl. Environ. Microbiol. 68:3780-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derzelle, S., et al. 2004. AstR-AstS, a new two-component signal transduction system, mediates swarming, adaptation to stationary phase and phenotypic variation in Photorhabdus luminescens. Microbiology 150:897-910. [DOI] [PubMed] [Google Scholar]
- 23.Derzelle, S., et al. 2004. The PhoP-PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J. Bacteriol. 186:1270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchaud, E., et al. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. [DOI] [PubMed] [Google Scholar]
- 25.ffrench-Constant, R., et al. 2003. Photorhabdus: toward a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 26:433-456. [DOI] [PubMed] [Google Scholar]
- 26.Fischer-Le Saux, M., V. Viallard, B. Brunel, P. Normand, and N. E. Boemare. 1999. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov., and P. asymbiotica sp. nov. Int. J. Syst. Bacteriol. 49 Pt. 4:1645-1656. [DOI] [PubMed] [Google Scholar]
- 27.Forst, S., and D. Clarke. 2002. Bacteria-nematode symbiosis, p. 57-77. In R. Gaugler (ed.), Entomopathogenic nematology. CAB International, Wallingford, Oxfordshire, United Kingdom.
- 28.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 29.Frackman, S., M. Anhalt, and K. H. Nealson. 1990. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J. Bacteriol. 172:5767-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudriault, S., et al. 2006. Whole-genome comparison between Photorhabdus strains to identify genomic regions involved in the specificity of nematode interaction. J. Bacteriol. 188:809-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudriault, S., et al. 2008. Plastic architecture of bacterial genome revealed by comparative genomics of Photorhabdus variants. Genome Biol. 9:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerritsen, L. J., G. de Raay, and P. H. Smits. 1992. Characterization of form variants of Xenorhabdus luminescens. Appl. Environ. Microbiol. 58:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Givaudan, A., and A. Lanois. 2000. flhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J. Bacteriol. 182:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn, J. S., et al. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 35.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 37.Hay, N. A., D. J. Tipper, D. Gygi, and C. Hughes. 1999. A novel membrane protein influencing cell shape and multicellular swarming of Proteus mirabilis. J. Bacteriol. 181:2008-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu, K., and J. M. Webster. 1998. In vitro and in vivo characterization of a small-colony variant of the primary form of Photorhabdus luminescens MD (Enterobacteriaceae). Appl. Environ. Microbiol. 64:3214-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurlbert, R. E., J. Xu, and C. L. Small. 1989. Colonial and cellular polymorphism in Xenorhabdus luminescens. Appl. Environ. Microbiol. 55:1136-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joyce, S. A., and D. J. Clarke. 2003. A hexA homologue from Photorhabdus regulates pathogenicity, symbiosis, and phenotypic variation. Mol. Microbiol. 47:1445-1457. [DOI] [PubMed] [Google Scholar]
- 41.Joyce, S. A., R. J. Watson, and D. J. Clarke. 2006. The regulation of pathogenicity and mutualism in Photorhabdus. Curr. Opin. Microbiol. 9:127-132. [DOI] [PubMed] [Google Scholar]
- 42.Kovach, M. E., et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 66:175-176. [DOI] [PubMed] [Google Scholar]
- 43.Krin, E., et al. 2006. Pleiotropic role of quorum-sensing autoinducer 2 in Photorhabdus luminescens. Appl. Environ. Microbiol. 72:6439-6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krin, E., et al. 2008. Regulatory role of UvrY in adaptation of Photorhabdus luminescens growth inside the insect. Environ. Microbiol. 10:1118-1134. [DOI] [PubMed] [Google Scholar]
- 45.Kvint, K., L. Nachin, A. Diez, and T. Nyström. 2003. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 6:140-145. [DOI] [PubMed] [Google Scholar]
- 46.Lanois, A., G. Jubelin, and A. Givaudan. 2008. FliZ, a flagellar regulator, is at the crossroads between motility, haemolysin expression and virulence in the insect pathogenic bacterium Xenorhabdus. Mol. Microbiol. 68:516-533. [DOI] [PubMed] [Google Scholar]
- 47.Li, M., et al. 2008. Expression and activity of a probable toxin from Photorhabdus luminescens. Mol. Biol. Rep. 36:785-790. [DOI] [PubMed] [Google Scholar]
- 48.Lynch, A. S., and E. C. C. Lin. 1996. Responses to molecular oxygen, p. 1526-1535. In F. C. Neidhart et al. (ed.), Escherichia coli and Salmonella, vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 49.Marokhazi, J., et al. 2004. Comparison of proteolytic activities produced by entomopathogenic Photorhabdus bacteria: strain- and phase-dependent heterogeneity in composition and activity of four enzymes. Appl. Environ. Microbiol. 70:7311-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moureaux, N., T. Karjalainen, A. Givaudan, P. Bourlioux, and N. Boemare. 1995. Biochemical characterization and agglutinating properties of Xenorhabdus nematophilus F1 fimbriae. Appl. Environ. Microbiol. 61:2707-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nappi, A. J., and E. Ottaviani. 2000. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays 22:469-480. [DOI] [PubMed] [Google Scholar]
- 52.Nappi, A. J., and E. Vass. 1998. Hydrogen peroxide production in immune-reactive Drosophila melanogaster. J. Parasitol. 84:1150-1157. [PubMed] [Google Scholar]
- 53.O'Neill, K. H., D. M. Roche, D. J. Clarke, and B. C. Dowds. 2002. The ner gene of Photorhabdus: effects on primary-form-specific phenotypes and outer membrane protein composition. J. Bacteriol. 184:3096-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ong, K. L., and F. N. Chang. 1997. Analysis of proteins from different phase variants of the entomopathogenic bacteria Photorhabdus luminescens by two-dimensional zymography. Electrophoresis 18:834-839. [DOI] [PubMed] [Google Scholar]
- 55.Prilipov, A., P. S. Phale, R. Koebnik, C. Widmer, and J. P. Rosenbusch. 1998. Identification and characterization of two quiescent porin genes, nmpC and ompN, in Escherichia coli BE. J. Bacteriol. 180:3388-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabri, M., S. Leveille, and C. M. Dozois. 2006. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 152:745-758. [DOI] [PubMed] [Google Scholar]
- 57.Sandegren, L., and D. I. Andersson. 2009. Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat. Rev. Microbiol. 7:578-588. [DOI] [PubMed] [Google Scholar]
- 58.Sharma, S., et al. 2002. The lumicins: novel bacteriocins from Photorhabdus luminescens with similarity to the uropathogenic-specific protein (USP) from uropathogenic Escherichia coli. FEMS Microbiol. Lett. 214:241-249. [DOI] [PubMed] [Google Scholar]
- 59.Sicard, M., et al. 2004. Stages of infection during the tripartite interaction between Xenorhabdus nematophila, its nematode vector, and insect hosts. Appl. Environ. Microbiol. 70:6473-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon, R., U. Priefer, and A. Pülher. 1983. A broad hostrange mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology (NY) 1:784-791. [Google Scholar]
- 61.Turlin, E., et al. 2006. Proteome analysis of the phenotypic variation process in Photorhabdus luminescens. Proteomics 6:2705-2725. [DOI] [PubMed] [Google Scholar]
- 62.Vollmer, W., M. von Rechenberg, and J. V. Holtje. 1999. Demonstration of molecular interactions between the murein polymerase PBP1B, the lytic transglycosylase MltA, and the scaffolding protein MipA of Escherichia coli. J. Biol. Chem. 274:6726-6734. [DOI] [PubMed] [Google Scholar]
- 63.Wang, H., and B. C. Dowds. 1993. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J. Bacteriol. 175:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, H., and B. C. A. Dowds. 1991. Molecular cloning and characterization of the lux genes from the secondary form of Xenorhabdus luminescens K122. Biochem. Soc. Trans. 20:68S. [DOI] [PubMed] [Google Scholar]
- 65.Wang, Y., A. L. Bilgrami, D. Shapiro-Ilan, and R. Gaugler. 2007. Stability of entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens, during in vitro culture. J. Ind. Microbiol. Biotechnol. 34:73-81. [DOI] [PubMed] [Google Scholar]
- 66.Waterfield, N., S. G. Kamita, B. D. Hammock, and R. ffrench-Constant. 2005. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol. Lett. 245:47-52. [DOI] [PubMed] [Google Scholar]
- 67.Waterfield, N. R., T. Ciche, and D. Clarke. 2009. Photorhabdus and a host of hosts. Annu. Rev. Microbiol. 63:557-574. [DOI] [PubMed] [Google Scholar]
- 68.Williams, J. S., M. Thomas, and D. J. Clarke. 2005. The gene stlA encodes a phenylalanine ammonia-lyase that is involved in the production of a stilbene antibiotic in Photorhabdus luminescens TT01. Microbiology 151:2543-2550. [DOI] [PubMed] [Google Scholar]
- 69.Wouts, W. M. 1990. The primary form of Xenorhabdus species (Enterobacteriaceae, Eubacteriales) may consist of more than one bacterial species. Nematologica 36:313-318. [Google Scholar]