Abstract
Ixodes ricinus ticks collected from 835 birds and from vegetation in the Czech Republic were analyzed. Host-seeking ticks (n = 427) were infected predominantly by Borrelia afzelii (25%). Ticks (n = 1,012) from songbirds (Passeriformes) were infected commonly by Borrelia garinii (12.1%) and Borrelia valaisiana (13.4%). Juveniles of synanthropic birds, Eurasian blackbirds (Turdus merula) and song thrushes (Turdus philomelos), were major reservoir hosts of B. garinii.
In central Europe, including the Czech Republic, the main vector of Lyme disease spirochetes is the Ixodes ricinus tick, feeding on a wide range of vertebrate hosts (7). The role of birds as reservoir hosts of Borrelia spirochetes, mainly B. garinii, has been elucidated in recent years (1, 10). In 2009, based on data acquired during the postbreeding period, we presented a study suggesting a differential role of passerine birds in distribution of Borrelia spirochetes (2). Here we show data acquired from I. ricinus ticks feeding on birds during the spring migration and/or breeding period at the same location. We compare and synthesize our data from both spring and postbreeding periods, together with 2-year data from host-seeking ticks from the same area and with data from 2 years' collection of ticks from birds in a higher-altitude area of the Czech Republic.
Bird infestation by ticks was lower in the spring period than in the postbreeding period.
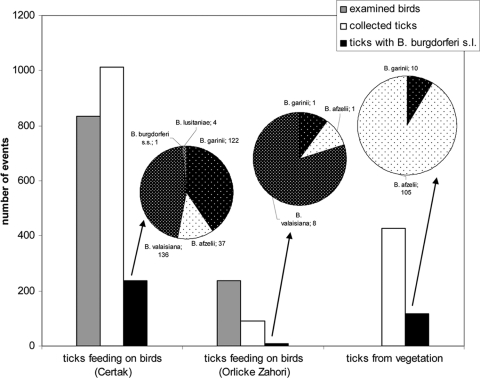
Spring collection at location Certak (370 to 400 m above sea level [ASL], 49°34′N, 17°59′E) (5) was conducted from 31 March to 28 April 2007. Birds were collected with mist nets and then identified, examined, and released after the ticks were removed with tweezers. Host-seeking ticks were collected by blanket dragging. Ticks were placed in 70% ethanol and later in the laboratory were classified according to the species, stage, sex of adults, and blood meal volume as “unfed,” “half fed,” or “fully fed.” All procedures were performed identically to those described in our previous study (2). During spring, the total number of birds captured, including retrapped events, was 835 (Fig. 1; see also Table S1 in the supplemental material). Passerine birds (Passeriformes) represented 99.3% (0.7% belonged to Piciformes). The most common birds were European robins, Erithacus rubecula (39%), and blackcaps, Sylvia atricapilla (15%). The overall infestation of birds with ticks was lower in the spring than in the postbreeding period (2), the average number of ticks per bird was 2.1 in the postbreeding period and 1.2 in the spring, and the mean number of ticks per infested bird was 5.1 in the postbreeding period (2) and 3.7 in birds captured in the spring. The difference in infestation by I. ricinus ticks was not entirely proportional across the bird species. In certain passerines, we observed little or no increase in infestation in the postbreeding period (E. rubecula, 1.3 versus 1.4 [2] ticks/bird; dunnock, Prunella modularis, 5.5 versus 7.5 ticks/bird [2]). On the other hand, certain birds were substantially more infested in the postbreeding period (Eurasian blackbird, Turdus merula, 8.3 versus 18.7 [2]; winter wren, Troglodytes troglodytes, 0.8 versus 4.4 [2]). Generally, ground-foraging species have higher numbers of ticks overall, and the association between foraging behavior and tick infestation was more pronounced in the postbreeding period. There were 1,012 ticks identified as I. ricinus, 1 fed larva of Haemaphysalis concinna, and 3 nymphs of Ixodes arboricola. The proportions of I. ricinus larvae and nymphs were 38% and 61%, respectively, with 2 adult females collected as well, whereas in the postbreeding period, 70% of I. ricinus ticks were represented by larvae (2). The abundance of larvae on birds in the postbreeding period (August/September) suggests infestation by ticks hatched from eggs laid during earlier months. We observed differences in the proportion of subadult ticks on certain bird species; the vast majority of larvae (n = 277) were collected from E. rubecula, representing 68% of ticks on these birds (odds ratio [OR], 10.0; 95% confidence interval [CI], 7.5 to 13.4). The proportion of larvae was substantially lower on the following bird species: song thrush Turdus philomelos, with 12% (OR, 0.2; 95% CI, 0.1 to 0.5), Turdus merula with 19% (OR, 0.3; 95% CI, 0.2 to 0.4), and Dunnock P. modularis with 9% (OR, 0.2; 95% CI, 0.1 to 0.3).
FIG. 1.
Overview of the number of examined birds, their infestation with I. ricinus ticks, and the presence of Borrelia spirochetes in ticks from birds and vegetation, including proportions of Borrelia genospecies. s.s., sensu stricto; s.l., sensu lato.
Birds from higher altitudes are less infested by Ixodes ticks.
In 2 to 4 June 2006 and from 27 May to 2 June 2007, field investigation was performed at another location in the northern part of the Czech Republic, Orlicke Zahori (680 m ASL, 50°30′N, 16°44′E). The total number of birds captured was 236, of which 43 birds were infested by a total of 92 ticks (Fig. 1; see also Table S2 in the supplemental material); 91 I. ricinus ticks (62% larvae and 38% nymphs) and 1 I. arboricola subadult tick. The most common birds were blackcaps, S. atricapilla, and common whitethroats, Sylvia communis, which accounted for 18% and 17%, respectively. The mean number of ticks per bird was 0.4, which is significantly less than findings from a lower-altitude (Certak) location and corresponds to the altitude-dependent tick density (9).
The presence of Borrelia spirochetes is lower in bird-feeding ticks from higher altitudes.
DNA was extracted from ticks by alkaline hydrolysis, and borrelial DNA was detected by the nested PCR targeted ffr (5S)-rrl (23S) intergenic spacer, followed by a reverse line blotting assay for genospecies identification. In analyzing the spring tick collection from Certak, Borrelia spirochetes were found in 238 (23.4%) I. ricinus ticks, specifically 122 cases of B. garinii, 37 of B. afzelii, 136 of Borrelia valaisiana, 1 of B. burgdorferi sensu stricto, and 4 Borrelia lusitaniae infections (Fig. 1), thus resulting in 1.26 infection events per infected tick caused by frequent coinfections; e.g., 5.8% of examined ticks were infected by both B. garinii and B. valaisiana. Adult females of I. ricinus and other tick species found on birds were not infected. Borrelia spirochetes were more frequent in nymphs (32.6%) than in larvae (9.2%). In 92 ticks collected at Orlicke Zahori, B. garinii was found in 1 nymph from the Eurasian blackbird, T. merula, B. afzelii was found in 1 nymph from the gray wagtail, Motacilla cinerea, and B. valaisiana was found in 8 ticks feeding on 2 Eurasian blackbirds (T. merula; 4 ticks), 1 fieldfare, Turdus pilaris (2), and 2 S. atricapilla robins (2) (see Table S2 in the supplemental material).
The overall level of B. garinii infections in bird-feeding ticks is lower in the spring than in the postbreeding period.
To estimate the ability to transmit Borrelia spirochetes to ticks feeding on a bird host that might be infected, we did the following: (i) compared the proportions of infected fed and unfed larvae, assuming that a higher proportion of fed infected larvae reflects the host's ability to transmit Borrelia to ticks, (ii) compared the proportion of infected nymphs and infected larvae, assuming that a low or no difference in the infection rate between subadult stages reflects the ability of the current host to transmit Borrelia to ticks rather than infection from a former stage, and (iii) calculated the noninfectivity coefficient (n-I) by determining the proportion of noninfected larvae among larvae feeding on a bird carrying ≥1 infected tick. Proportions were evaluated using odds ratio (OR) procedures with 95% confidence intervals (95% CI) (2). B. garinii (see Table S3 in the supplemental material) was detected in 12.1% of I. ricinus ticks, more frequently in nymphs than in larvae (OR, 5.7; 95% CI, 3.2 to 10.0), suggesting that a substantial number of infected nymphs were the result of infection during a former stage. B. garinii infection was associated with ticks feeding on T. merula (29.3%), T. philomelos (26.0%), and to a lesser extent the great tit, Parus major (12.5%). This was accompanied by decreased noninfectivity indices (n-I) in these bird species. Comparing the proportions of B. garinii infection in fed and unfed larvae (OR, 5.1; 95% CI, 0.7 to 40), a significant association was not found between blood volume and the presence of B. garinii during the spring period. The nymph-to-larva infection rate was substantially higher in the spring (OR, 5.7; see Table S3) than in the postbreeding period (OR, 1.5) (2), suggesting also an increased risk of B. garinii acquisition by ticks from spirochetemic birds in the postbreeding period. We observed a decreased infection rate in ticks feeding on adult birds in late summer compared to that in the spring. This suggests that the statistically increased proportion of B. garinii infections during the postbreeding period (22.2%) (2) reflects mainly the activity of juvenile Turdus merula and T. philomelos after breeding season.
The presence of other Borrelia species in ticks from birds does not differ between the spring and the postbreeding period.
B. valaisiana (see Table S4 in the supplemental material) infection was detected in 13.4% of I. ricinus ticks and was more frequent in nymphs than in larvae (OR, 2.6; 95% CI, 1.7 to 4.0). The presence of B. valaisiana in ticks was associated with T. merula (37.2%) and T. philomelos (30.0%), with low n-I coefficients. We did not observe overall or bird species-related association between blood volume and the presence of B. valaisiana in the spring collection. B. afzelii (see Table S5) was found only in nymphs with a n-I equal to 1.0, and the infection in nymphs (5.0%) did not differ from that in the postbreeding period (6.0% [2]). A negative association between blood volume and the presence of B. afzelii (OR, 10.9; 95% CI, 1.3 to 9397) supports the theory of an inhibiting effect of bird blood, presumably complement based, on the viability of B. afzelii (6). B. burgdorferi sensu stricto was found in one half-fed nymph feeding on P. major. B. lusitaniae was detected in 4 nymphs: 1 unfed on E. rubecula, 1 full on T. merula, and 1 half-fed and 1 full on the same T. merula individual retrapped 5 days later; thus, we cannot rule out the capability of T. merula to transmit B. lusitaniae.
Host-seeking ticks are infected mainly by B. afzelii.
Host-seeking ticks were collected from vegetation by blanket dragging at Certak at the time of bird netting. All 427 collected ticks were identified as I. ricinus: 3 larvae, 366 nymphs, 22 females, and 36 males. We detected presence of B. garinii in 2.3% of host-seeking ticks and B. afzelii in 24.6%, respectively (Fig. 1; see also Table S6 in the supplemental material).
Our summary data show that B. garinii and B. valaisiana are associated with I. ricinus ticks feeding on synanthropic passerines, such as Eurasian blackbirds (T. merula), song thrushes (T. philomelos), and great tits (P. major) and that the B. garinii occurrence is attenuated at higher altitudes. Although the tick infestation rate reflects foraging behavior, it is not the key to the Borrelia infection rate. One determinant may be the tick's ability to feed on certain bird species. For example, despite a high infestation of P. modularis, the low infection rate is in accordance with low blood meal volume in ticks feeding on P. modularis. This can be related to the differences in the host T-cell-mediated immune response, presumably to tick salivary proteins, as is described for tick-sensitive and tick-resistant animal hosts (3, 8). Bird immune response to Borrelia pathogens may be another biological determinant of a competent reservoir host. Birds are likely naturally resistant to B. afzelii, and rodents are the main reservoir hosts of B. afzelii in Europe (4). Some individuals of Turdidae and P. major (Paridae) are competent reservoir hosts for B. garinii and B. valaisiana depending on acquired resistance. This is implicated by a decreasing B. garinii infection rate in ticks feeding on T. merula according to age consecution: juvenile bird, 72%; breeding adult bird, 29%; adult bird during postbreeding, 6% (see Table S3 in the supplemental material) (2). Comparing data from host-seeking and bird-feeding ticks, we conclude that the occurrence of B. garinii and B. valaisiana in ticks is associated with current-host (bird) feeding. This suggests that transstadial transmission of B. garinii and B. valaisiana may be less efficient, although possible, based on an increased infection in nymphs compared to larvae and the presence of B. garinii in adult ticks. Thus, we conclude that major competent reservoir hosts of the bird-associated Lyme disease spirochete B. garinii are juvenile Eurasian blackbirds and song thrushes, with epidemiological relevance due to their synanthropic behavior and distribution potential. The biological determinants of competent bird reservoirs of B. garinii need to be elucidated.
Supplementary Material
Acknowledgments
We thank all field coworkers and Eva Suchanova for excellent technical cooperation in the laboratory.
This work was supported by the Czech Science Foundation (grant 524-08-P139), by the Czech Ministry of Education, Youth and Sports (grant MSM 6215712402), and by the Scientific Grant Agency of Ministry of Education and Slovak Academy of Sciences (grant 2/0161).
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Comstedt, P., et al. 2006. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg. Infect. Dis. 12:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubska, L., I. Literak, E. Kocianova, V. Taragelova, and O. Sychra. 2009. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl. Environ. Microbiol. 75:596-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira, B. R., M. J. Szabo, K. A. Cavassani, G. H. Bechara, and J. S. Silva. 2003. Antigens from Rhipicephalus sanguineus ticks elicit potent cell-mediated immune responses in resistant but not in susceptible animals. Vet. Parasitol. 115:35-48. [DOI] [PubMed] [Google Scholar]
- 4.Hanincova, K., et al. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126:11-20. [DOI] [PubMed] [Google Scholar]
- 5.Kulich, P., et al. 2008. Avipoxvirus in blackcaps (Sylvia atricapilla). Avian Pathol. 37:101-107. [DOI] [PubMed] [Google Scholar]
- 6.Kurtenbach, K., et al. 2002. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immun. 70:5893-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piesman, J., and L. Gern. 2004. Lyme borreliosis in Europe and North America. Parasitology 129:S191-S220. [DOI] [PubMed] [Google Scholar]
- 8.Piper, E. K., et al. 2009. Immunological profiles of Bos taurus and Bos indicus cattle infested with the cattle tick, Rhipicephalus (Boophilus) microplus. Clin. Vaccine Immunol. 16:1074-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suss, J., C. Klaus, F. W. Gerstengarbe, and P. C. Werner. 2008. What makes ticks tick? Climate change, ticks, and tick-borne diseases. J. Travel Med. 15:39-45. [DOI] [PubMed] [Google Scholar]
- 10.Taragel'ova, V., et al. 2008. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl. Environ. Microbiol. 74:1289-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.