Abstract
The genetic relatedness of Clostridium botulinum type E isolates associated with an outbreak of wildlife botulism was studied using random amplification of polymorphic DNA (RAPD). Specimens were collected from November 2000 to December 2008 during a large outbreak of botulism affecting birds and fish living in and around Lake Erie and Lake Ontario. In our present study, a total of 355 wildlife samples were tested for the presence of botulinum toxin and/or organisms. Type E botulinum toxin was detected in 110 samples from birds, 12 samples from fish, and 2 samples from mammals. Sediment samples from Lake Erie were also examined for the presence of C. botulinum. Fifteen of 17 sediment samples were positive for the presence of C. botulinum type E. Eighty-one C. botulinum isolates were obtained from plants, animals, and sediments; of these isolates, 44 C. botulinum isolates produced type E toxin, as determined by mouse bioassay, while the remaining 37 isolates were not toxic for mice. All toxin-producing isolates were typed by RAPD; that analysis showed 12 different RAPD types and multiple subtypes. Our study thus demonstrates that multiple genetically distinct strains of C. botulinum were involved in the present outbreak of wildlife botulism. We found that C. botulinum type E is present in the sediments of Lake Erie and that a large range of bird and fish species is affected.
Botulism is a paralytic disease mediated by a protein toxin produced by the obligate anaerobe Clostridium botulinum. Botulism intoxication can result in respiratory failure and death. This disease in humans, as well as other animals, has been described. In waterfowl, drowning is a common cause of death, because the neck muscles of the affected bird become paralyzed and the bird is no longer able to hold its head above water (32). The natural habitat of C. botulinum is thought to be soil and sediments of both marine and freshwater origins (16, 32, 34).
C. botulinum can be classified according to the antigenic specificity of the toxin produced or according to DNA relatedness. If classified according to toxin type, C. botulinum organisms can be grouped in toxin types A to G (9, 14, 16, 32, 34). If classified according to DNA relatedness, C. botulinum organisms are divided into groups I to IV (16, 32, 34). In the continental United States, C. botulinum type E is primarily found in soils associated with water (33). In Alaska, C. botulinum type E has been found in association with water and salmon (27). Human disease caused by C. botulinum type E has been associated with the consumption of fish and aquatic mammals (9, 16, 32).
Random amplification of polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) are tools that have been used previously to determine genetic relatedness among C. botulinum type E strains (17, 19, 23). All of the previous studies demonstrated that extensive genetic variation exists in C. botulinum type E strains.
Although C. botulinum type E has been associated almost exclusively with aquatic environments, outbreaks of this type of botulism among wild water birds and shore birds are rare. Kaufman et al. reported an outbreak of type E botulism among loons and gulls on Lake Michigan in 1963 (21). Since that time, other researchers have reported outbreaks of type E avian botulism in the Great Lakes region of the United States (5, 6, 15). C. botulinum type E has been reported in the intestinal contents of fish taken from the Great Lakes (3), as well as in dead fish collected from Lake Michigan (6, 28). The presence of C. botulinum type E in the sediments of the Great Lakes (3, 4) and the Gulf of St. Lawrence (22) has been demonstrated.
In 1998, laboratory-confirmed cases of type E botulism from Great Lakes birds were reported in Canada (7). Starting in the year 2000, a large outbreak of avian botulism due to C. botulinum type E has been occurring among birds on the New York shoreline of Lake Erie (30). The first observation of botulism in birds from Lake Ontario occurred in 2002 (1). For the years 2000 to 2008, the potential mortality, calculated by extrapolation, may be as high as 67,793 birds (2). This outbreak is remarkable for its duration, the high-level bird mortality, and the large numbers of bird species involved. While previous outbreaks of avian botulism due to C. botulinum type E have been described, little is known about the biodiversity of this organism in a wildlife botulism outbreak. The present study was designed to determine the genetic relatedness of C. botulinum type E strains isolated from the ongoing outbreak of wildlife botulism.
MATERIALS AND METHODS
Animal samples.
Moribund and dead animals were collected by staff of the New York State Department of Environmental Conservation and shipped to the Wildlife Pathology Unit (WPU). Animals were collected from the New York Lake Erie shoreline, as well as the New York Lake Ontario shoreline. WPU staff identified the species of animal and performed necropsy on the birds. The examination included gross pathology, limited histopathology, toxicology, and stomach content analysis. Stomach content analysis during the years 2000 to 2002 included examination for round goby remains. WPU staff also prepared specimens for bacteriologic examination. The sample matrices from birds and mammals included alimentary canal content, serum, and the liver. The specimen matrix for most fish was either alimentary canal content (for large fish) or the complete animal (for smaller species of fish). Livers, muscles, and hearts from a few individual fish were also tested for the presence of botulinum toxin. Two amphibian livers were individually tested; otherwise, the amphibian and invertebrate specimens were tested as whole animals. A total of 355 animal specimens were submitted to the University of Pennsylvania Botulism Laboratory (Kennett Square, PA), the National Veterinary Services Laboratory (Ames, IA), or the Wadsworth Center, New York State Department of Health (Albany, NY). These samples included 272 birds (31 species), 64 fish (10 species), 2 invertebrates (2 species), 13 amphibians (1 species), and 4 mammals (2 species). Botulinum toxin tests, but not culture for C. botulinum organisms, were performed at the University of Pennsylvania and the National Veterinary Services Laboratory, whereas culture and toxin analysis were performed at the Wadsworth Center. Some of the specimens sent to the Wadsworth Center were of insufficient volume for toxin extraction to be performed; in such cases, only culture was done.
Sediments.
Sediments from the eastern basin of Lake Erie were collected using a Petite Ponar grab on two occasions. Ten sediment specimens were collected in December 2001, and seven were collected in October 2002. The grab was cleaned in lake water between samples. The sample size was approximately 500 ml. Approximately 30 g of each sample was submitted for analysis. Sediment samples were analyzed as previously described (34). Briefly, 10 g of sediment was inoculated into 100 ml of Trypticase-peptone-glucose-yeast extract broth with 0.01% trypsin (TPGYT) followed by incubation at 28°C for up to 7 days in an anaerobic atmosphere. The broth was examined at 4 days for the presence of C. botulinum by the mouse bioassay and PCR. Broths that were negative at 4 days' incubation were again examined at day 7 for the presence of botulinum toxin and by PCR for the presence of bont/A, bont/B, bont/E, and bont/F genes. Enrichment broths were cultured for C. botulinum by incubation on C. botulinum isolation agar (13) and egg yolk agar supplemented with 100 mg neomycin sulfate/liter.
Plant debris and algae.
Four samples of plant debris/algae (Cladophora spp.) were collected from the Lake Erie shoreline in 2001, and three samples of plant debris/algae were collected from the Lake Erie shoreline in 2002. The specimens collected in 2001 were treated as described below for the animal alimentary tract specimens, and the specimens collected in 2002 were treated as described for the sediment specimens.
Mouse bioassay.
Serum, liver, and alimentary tract specimens were analyzed for C. botulinum toxin by the mouse bioassay test (14). Serum samples were tested without further treatment. On occasion, serum samples had to be pooled in order to yield sufficient volume for performance of toxin tests. Pooling was always done within species. Alimentary tract specimens were tested for toxin by grinding with an equal amount (wt/vol) of gelatin diluent followed by overnight toxin extraction at 5°C. Extracts were centrifuged (12,350 × g at 5°C for 20 min), and the supernatant was tested for the presence of botulinum toxin. For some animals, such as small fish, crayfish, and mudpuppies, as well as liver specimens from animals, the whole bodies were ground with diluent and then tested for the presence of toxin.
Isolates of C. botulinum were tested for toxin production by cultivation of the organism for 2 to 3 days in cooked meat broth at 37°C. The broth was centrifuged (2,000 × g at 5°C for 10 min), and the supernatant was withdrawn. The supernatant was then tested for the presence of botulinum toxin as described below. Mouse bioassay-negative isolates were screened by PCR for the presence of bont/E. Four strains that were originally toxin negative (mouse bioassay) when incubated at 37°C yet PCR positive for the presence of bont/E gene were retested by the mouse bioassay after growth for 2 to 3 days at 20°C in TPGYT.
The mouse bioassay test was performed by a splitting of the sample and neutralization of an aliquot of the specimen with C. botulinum type E antitoxin. Two samples were tested with polyvalent antitoxin (i.e., for types A, B, C, D, and E), and three samples were tested with trivalent antitoxin (i.e., for types A, B, and E). (Antitoxin was provided by the Centers for Disease Control and Prevention, Atlanta, GA.) Female Swiss Webster mice were injected in pairs with either a neutralized or unneutralized extract. Animals that were judged to be moribund were sacrificed. Mice were observed at least daily for 4 to 5 days postinjection for signs of botulism intoxication. Specimens were considered containing botulinum toxin if the mice receiving the neutralized extract survived and the mice that received the unneutralized extract developed signs of botulism intoxication. All procedures with mice were performed in accordance with the Institutional Animal Care and Use Committee at the institution where testing was performed.
Culture of specimens.
Alimentary canal, liver, and ground animal specimens were cultured by inoculation to egg yolk agar supplemented with 100 mg of neomycin sulfate/liter. Most specimens were also cultured with C. botulinum isolation agar (13). Plates were incubated under anaerobic conditions at 37°C for at least 48 h.
C. botulinum organisms were isolated by picking of lipase-positive colonies. Such colonies were identified to the species level by conventional biochemical reactions (14, 18). These tests included fermentation of glucose, maltose, mannitol, lactose, sucrose, xylose, salicin, arabinose, and glycerol. Litmus milk, motility, gelatin liquefaction, nitrate reduction, indole production, esculinase, urease, catalase, growth in bile, anaerobic growth requirement, reaction on egg yolk agar, and H2S production were also used. Generally, only one lipase-positive colony from a specimen was identified to the species level. Three animals, however, were examined for the presence of multiple isolates. Multiple isolates of C. botulinum were picked from the alimentary canal contents of three individual birds. All isolates were identified to the species level with conventional biochemical reactions as described above and were also tested for the presence of bont/A, bont/B, bont/E, and bont/F genes by PCR.
RAPD.
Isolates of C. botulinum were typed using RAPD according to the procedure of Hyytia et al. (19). C. botulinum type E strains were grown on anaerobe blood agar for 48 h. Template DNA was prepared from these strains by making a suspension of each strain in 500 μl of Tris-EDTA (TE) buffer and heating at 115°C for 10 min. Following heat lysis of samples, total nucleic acid purification was performed to ensure high-quality DNA. Briefly, 250 μl of heat-lysed sample was extracted by using the EasyMag system (BioMérieux, Durham, NC) according to the manufacturer's instructions, with a final elution volume of 50 μl. RAPD analysis was performed with Ready-To-Go RAPD analysis beads (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions. The PCR volume was 25 μl, containing 10 μl of water, 25 ng of DNA template, and 25 pmol of RAPD primer 2 (sequence, 5′-GTTTCGCTCC-3′). Amplifications were conducted in an iCycler (Bio-Rad, Hercules, CA) with a 5-min initial denaturation at 95°C followed by a 4°C hold. Amplification products were electrophoresed on a 2% E-Gel (Invitrogen, Carlsbad, CA) for 30 min and were visualized under UV light by using the Gel Doc XR system (Bio-Rad, Hercules, CA). A 100-bp DNA molecular weight marker (Invitrogen, Carlsbad CA) was used as a fragment size marker. All isolates were typed at the same time. A control strain from a 1987 food outbreak of type E botulism was included as a control (35).
RAPD analysis.
Comparisons of DNA patterns obtained by RAPD were analyzed using BioNumerics version 4.0 software (Applied Maths, Belgium). The measure of similarity of DNA fragment patterns was estimated utilizing the Dice coefficient correlation. Visual interpretation of the fragment patterns generated by RAPD was performed. Different types of C. botulinum were distinguished from each other by the numbers of bands detected, as well as the molecular weights of the bands. Patterns with 3 or more different bands were classified as different RAPD types, while RAPD subtypes were defined as having 1 or 2 banding pattern differences within each RAPD type.
PCR.
Selected samples were examined for the botulinum toxin gene (bont) by either conventional PCR (25) or an in-house-developed real-time PCR procedure (12). Initial specimens (alimentary canal contents, isolates, and sediments) were analyzed by using a conventional protocol, while subsequent specimens (alimentary canal contents and isolates) were analyzed using the real-time procedure. Specimens were heat treated at 95°C for 30 min and then centrifuged for 10 s, and the supernatant was used to obtain DNA. Extraction was performed with a MasterPure Complete DNA and RNA purification kit (Epicentre Biotechnologies, Madison, WI), with the modification of a 30-min lysis step being used instead of a 15-min lysis step, followed by filtering in a spin module column (BIO 101, Vista, CA) to remove unlysed spores. Primers and probes were manufactured by Integrated DNA Technologies, Coralville, IA. Primers for the conventional PCR were designed according to the procedure of Lindstrom et al. (25). Primers for the real-time PCR procedure were designed at the Wadsworth Center. Conventional PCR was performed with a 100-μl reaction mixture containing 10 μl of template, 2.5 mM MgCl2, 200 mM each deoxynucleotide substrate, 1 mM each primer, and 1 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). Bovine serum albumin was added to a final concentration of 0.05%. PCR conditions were 9 min at 95°C, followed by 40 cycles of 0.3 min at 94°C and 1 min at 60°C, and finally 1.25 min at 72°C. PCR products were visualized on a 2% agarose gel run at 150 V for 50 min in 0.5% Tris-borate-EDTA (TBE) with 10 mg/ml ethidium bromide or on E-Gels (Invitrogen, Carlsbad, CA) run at 70 V for 30 min. The proper size of the amplicon was determined through comparison with a 1-kb DNA ladder (1 μg/μl; Life Technologies, Inc., Gaithersburg, MD). Real-time PCR conditions differed from those of the conventional PCR method in that a LightCycler hybridization probe kit (Roche, Indianapolis, IN) was used. Real-time PCR conditions were 1 cycle at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and 60°C for 1 min. Samples were run in either a TaqMan 7000 or TaqMan 7500 system (Applied Biosystems, Foster City, CA).
RESULTS
Animals tested for C. botulinum.
A total of 272 birds collected from November 2000 to December 2008 were tested for the presence of C. botulinum toxin and/or organisms; 31 species of birds were included. Twenty-one of the species yielded samples that were positive for C. botulinum type E toxin (Table 1). The gross pathology of the birds was characterized by lack of lesions. Toxicology tests for chlorinated hydrocarbons, pesticides, and polychlorinated biphenyls were also negative. Some birds had elevated, but nonlethal, levels of lead and mercury. Necropsy of loon carcasses indicated that drowning was the cause of death. Seven of eight serum samples (88%) examined had C. botulinum type E toxin according to the mouse bioassay. Twenty-three of 53 (43%) avian livers and 71 of 160 (44%) avian alimentary tract samples were positive for the presence of type E botulinum toxin by the mouse bioassay. Two hundred thirteen avian specimens (alimentary tracts and livers) were cultured for C. botulinum. Forty-two toxin-producing isolates of C. botulinum were isolated from birds; 38 of the C. botulinum strains produced toxin when grown in cooked meat broth at 37°C, whereas 4 strains did not produce toxin under those conditions, although they did produce toxin when grown at 20°C in TPGYT. Stomach content analysis revealed that many of the birds examined had fed on round gobies (Neogobius melanostomus) (Table 2). Among a total of 53 fish examined for the presence of C. botulinum type E toxin, 13 (25%) of the fish (9 freshwater drum [Aplodinotus grunniens], 3 lake sturgeons [Acipenser fulvescens], and 1 channel catfish [Ictalurus punctatus]) had C. botulinum type E toxin, as determined by the mouse bioassay (Table 3). Eleven fish samples could not be analyzed for toxin due to small sample size. Two of 41 (5%) fish analyzed, one round goby and one freshwater drum, were culture positive for toxin-producing C. botulinum type E.
TABLE 1.
Presence of C. botulinum toxin type E in birds
| Species | No. of birds positive for type E toxin/no. testeda |
|---|---|
| Common loon (Gavia immer) | 32/83 |
| Ring-billed gull (Larus delawarensis) | 18/35 |
| Red-breasted merganser (Mergus serrator) | 12/21 |
| Long-tailed duck (Clangula hyemalis) | 11/30 |
| Caspian tern (Sterna caspia) | 6/6 |
| Herring gull (Larus argentatus) | 4/7 |
| White-winged scoter (Melanitta deglandi) | 4/4 |
| Great black-backed gull (Larus marinus) | 3/8 |
| Red-necked grebe (Podiceps grisegena) | 3/4 |
| Bald eagle (Haliaeetus leucocephalus) | 2/2 |
| Semipalmated sandpiper (Calidris pusilla) | 2/2 |
| Common goldeneye (Bucephala clangula) | 2/3 |
| Sanderling (Calidris alba) | 2/6 |
| Red-throated loon (Gavia stellata) | 1/1 |
| Surf scoter (Melanitta perspicillata) | 1/1 |
| Double-crested cormorant (Phalacrocorax auritus) | 1/1 |
| Lesser black-backed gull (Larus fuscus) | 1/1 |
| Great blue heron (Ardea herodias) | 1/2 |
| Greater scaup (Aythya marila) | 1/2 |
| Horned grebe (Podiceps auritus) | 1/4 |
| Mallard duck (Anas platyrhynchos) | 1/4 |
| Bonaparte's gull (Larus philadelphia) | 0/1 |
| Black duck (Anas rubripes) | 0/1 |
| Wood duck (Aix sponsa) | 0/1 |
| Tundra swan (Cygnus columbianus) | 0/1 |
The total group of specimens includes 6 specimens for which the toxin could not be typed (i.e., polyvalent positive).
TABLE 2.
Incidence of round goby remains in stomach contents of avian predators
| Species | % (no.) of examined birds with round goby remains in stomach content by yr |
||
|---|---|---|---|
| 2000 | 2001 | 2002 | |
| Common loon (Gavia immer) | 56 (62) | 59 (192) | 61 (131) |
| Horned grebe (Podiceps auritus) | 0 | 0 | 54 (26) |
| Long-tailed duck (Clangula hyemalis) | 0 | 0 | 60 (169) |
| Red-breasted merganser (Mergus serrator) | 84 (97) | 0 | 78 (124) |
| Herring gull (Larus argentatus) | 24 (44) | 8 (52) | 21 (29) |
| Ring-billed gull (Larus delawarensis) | 70 (104) | 22 (110) | 8 (117) |
TABLE 3.
Presence of C. botulinum toxin type E in fish species
| Species | No. of fish positive for type E toxin/no. tested |
|---|---|
| Round goby (Neogobius melanostomus) | 0/13 |
| Freshwater drum (Aplodinotus grunniens) | 9/19 |
| Lake sturgeon (Acipenser fulvescens) | 3/7 |
| Channel catfish (Ictalurus punctatus) | 1/1 |
| Lake trout (Salvelinus namaycush) | 0/1 |
| Smallmouth bass (Micropterus dolomieu) | 0/9 |
| Muskellunge (Esox masquinongy) | 0/1 |
| Rainbow trout (Oncorhynchus mykiss) | 0/1 |
| Common carp (Cyprinus carpio) | 0/1 |
Since the stomach content analysis indicated that many birds had fed on round gobies, a total of 14 round gobies were examined individually by culture and toxin extraction. One of the round gobies was culture positive for a C. botulinum type E toxin-producing organism, six were culture positive for non-toxin-producing C. botulinum organisms, and the remaining seven fish were culture negative. Thirteen of the round gobies were negative for the presence of botulinum toxin by mouse bioassay, and one fish was not tested for toxin due to insufficient sample size. A subset of six round gobies was also tested by PCR for the presence of the bont/E gene. PCR analysis showed the presence of the bont/E gene in four individual fish and gave negative results for the other two fish.
The amphibian (13 mudpuppies [Necturus maculosus]) and invertebrate (1 zebra mussel [Dreissena polymorpha] and 1 crayfish [species undetermined]) specimens were all negative for the presence of botulinum toxin, as determined by mouse bioassay and culture. Both of the invertebrate specimens and 10 of 13 mudpuppy specimens were cultured for the presence of C. botulinum. All were culture negative.
The four mammals tested for botulinum toxin comprised two opossums and two raccoons. Serum from one opossum was negative for toxin, while the intestinal content from the other opossum was positive for C. botulinum type E toxin. Both raccoons were positive for C. botulinum type E toxin in stomach contents and in livers. Mammals were not cultured for C. botulinum.
Sediments tested for C. botulinum.
The sediment analysis showed that sediments from Lake Erie contained toxin-producing C. botulinum type E organisms. Among samples taken in the year 2001, 9 of 10 were positive for C. botulinum toxin type E by mouse bioassay. PCR for the presence of the bont/E gene was also positive on these specimens. The remaining specimen was negative by PCR and mouse bioassay. None of the sediment samples was culture positive for toxin-producing C. botulinum type E. Among samples taken in the year 2002, six of seven were positive for C. botulinum type E toxin by mouse bioassay. Four of the sediment samples had non-toxin-producing C. botulinum organisms present. Five of 10 sediment samples were also positive for bont/E. C. botulinum type B was not detected in the culture fluid, but one sediment specimen was culture positive for C. botulinum type B.
Plant debris and algae tested for C. botulinum.
For algae and plant debris specimens from Lake Erie collected in 2002 (three specimens), we enriched them in TPGYT prior to testing for the presence of toxin. These specimens were also tested for the presence of the bont/E gene by PCR. Only one of the specimens was positive for type E botulinum toxin, by mouse bioassay, as well as for the bont/E gene. One of the specimens was culture positive, but the isolate failed to produce toxin, and the remaining specimen was culture negative and toxin negative. The specimens collected in 2001 (four specimens) were not enriched prior to testing for the presence of toxin, and all four specimens were culture negative and toxin negative.
Non-toxin-producing organisms.
One unexpected finding was that 22 specimens from birds (8%), 10 samples from fish (16%), 1 alga sample (25%), and 4 sediment specimens (24%) contained non-toxin-producing organisms that resembled C. botulinum type E organisms. Interestingly, 9 of 22 (41%) bird alimentary canal content samples that were culture positive for the non-toxin-producing organisms had detectable levels of type E botulinum toxin by mouse bioassay. Three of the sediment strains, as well as the isolate from the alga, also came from a TPGYT enrichment culture that had demonstrable levels of type E botulinum toxin in the culture fluid. A total of 15 specimens (bird, fish, sediment enrichment, and alga enrichment) that had demonstrable levels of botulinum toxin by mouse bioassay were culture positive for non-toxin-producing organisms.
PCR analysis of the non-toxin-producing strains was negative for the presence of the bont/E gene. Sequence analysis of the 16S rRNA gene was performed on a bird and a fish isolate (26). A search of the Microseq (ABI, Foster City, CA) and GenBank (http://www.ncbi.nlm.nih.gov/genbank/) databases yielded a strong (>99%) homology with C. botulinum group II; this group includes C. botulinum type E.
In order to determine whether an alimentary canal specimen from a single bird carcass could harbor both toxin-producing and non-toxin-producing organisms, we subsequently isolated multiple strains of C. botulinum from three individual birds. All isolates were tested by PCR for the presence of the bont/A, bont/B, bont/E, and bont/F genes. Seven of 10 isolates from a herring gull (Larus argentatus) were positive for the bont/E gene, and 2 of 9 isolates from a common loon (Gavia immer) were positive for bont/E. The seven isolates from a white-winged scoter (Melanitta deglandi) were all negative for the bont/E gene. Toxin production was confirmed in a single bont/E-positive strain from both the gull and the loon. Lack of toxin production was also confirmed in a bont/E-negative strain from each of the three birds. The alimentary canal contents from the herring gull and the common loon had detectable levels of type E botulinum toxin by the mouse bioassay, and the alimentary canal content of the white-winged scoter was negative for botulinum toxin.
RAPD of C. botulinum type E isolates from birds and fish.
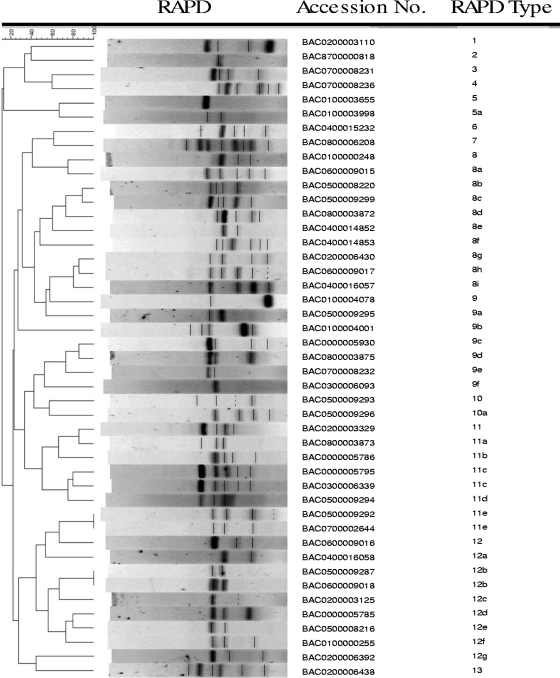
Forty-four C. botulinum type E toxin-producing isolates from the current outbreak were typed by RAPD (Fig. 1). This total comprised 42 isolates from birds and 2 from fish (Table 4). A strain from a 1987 food outbreak (35) was included as a control. The isolates from the ongoing outbreak clustered into 12 distinct types of C. botulinum, including 29 subtypes. All types and subtypes were distinct from the 1987 control strain.
FIG. 1.
Dendrogram of 44 C. botulinum type E isolates from wildlife and 1 C. botulinum type E control by using RAPD primer 2 for RAPD patterns. The measure of similarity of DNA fragment patterns was estimated utilizing the Dice coefficient correlation. The similarity among strains was determined using visual comparison of bands produced. The RAPD types determined by such comparison are listed. Accession numbers are unique identifiers assigned to samples when received.
TABLE 4.
Clostridium botulinum type E strains used in this study
| Yr/strain | Source | Species | County (lake)a | RAPD type |
|---|---|---|---|---|
| 1987/818 | Stool | Human | NA | 2 |
| 2000 | ||||
| 5785 | Alimentary canal | Red-breasted merganser | Erie (Erie) | 12d |
| 5786 | Alimentary canal | Red-breasted merganser | Erie (Erie) | 11b |
| 5795 | Alimentary canal | Ring-billed gull | Erie (Erie) | 11c |
| 5930 | Alimentary canal | Common loon | Erie (Erie) | 9c |
| 2001 | ||||
| 248 | Alimentary canal | Red-breasted merganser | Chautauqua (Erie) | 8 |
| 255 | Alimentary canal | Red-breasted merganser | Chautauqua (Erie) | 12f |
| 3655 | Whole fish | Freshwater drum | Chautauqua (Erie) | 5 |
| 3998 | Alimentary canal | Bald eagle | Chautauqua (Erie) | 5a |
| 4001 | Alimentary canal | American crow | Chautauqua (Erie) | 9b |
| 4078 | Alimentary canal | Sanderling | Chautauqua (Erie) | 9 |
| 2002 | ||||
| 3110 | Alimentary canal | Ring-billed gull | Oswego (Ontario) | 1 |
| 3125 | Alimentary canal | Ring-billed gull | Erie (Erie) | 12c |
| 3329 | Alimentary canal | Ring-billed gull | Cayuga (Ontario) | 11 |
| 6392 | Alimentary canal | Long-tailed duck | Erie (Erie) | 12g |
| 6430 | Whole fish | Round goby | Chautauqua (Erie) | 8g |
| 6438 | Alimentary canal | Common loon | Erie (Erie) | 13 |
| 2003 | ||||
| 6093 | Alimentary canal | Herring gull | Chautauqua (Erie) | 9f |
| 6339 | Alimentary canal | Common loon | Chautauqua (Erie) | 11c |
| 2004 | ||||
| 14852 | Alimentary canal | Common loon | Erie (Erie) | 8e |
| 14853 | Liver | Long-tailed duck | Erie (Erie) | 8f |
| 15232 | Alimentary canal | Long-tailed duck | Erie (Erie) | 6 |
| 16057 | Alimentary canal | Common loon | Erie (Erie) | 8i |
| 16058 | Alimentary canal | Common loon | Erie (Erie) | 12a |
| 2005 | ||||
| 8216 | Alimentary canal | White-winged scoter | Monroe (Ontario) | 12e |
| 8220 | Alimentary canal | Common loon | Erie (Erie) | 8b |
| 9287 | Alimentary canal | Common loon | Jefferson (Ontario) | 12b |
| 9292 | Alimentary canal | Common loon | Chautauqua (Erie) | 11e |
| 9293 | Alimentary canal | Common loon | Chautauqua (Erie) | 10 |
| 9294 | Alimentary canal | Common loon | Chautauqua (Erie) | 11d |
| 9295 | Alimentary canal | Common loon | Chautauqua (Erie) | 9a |
| 9296 | Alimentary canal | Common loon | Chautauqua (Erie) | 10a |
| 9299 | Alimentary canal | Common loon | Chautauqua (Erie) | 8c |
| 2006 | ||||
| 9015 | Liver | Common loon | Jefferson (Ontario) | 8a |
| 9016 | Liver | Common loon | Jefferson (Ontario) | 12 |
| 9017 | Liver | Common loon | Erie (Erie) | 8h |
| 9018 | Liver | Common loon | Erie (Erie) | 12b |
| 2007 | ||||
| 2644 | Liver | Common loon | Jefferson (Ontario) | 11e |
| 8231 | Liver | Long-tailed duck | Jefferson (Ontario) | 3 |
| 8232 | Alimentary canal | Common loon | Chautauqua (Erie) | 9e |
| 8236 | Alimentary canal | Red-necked grebe | Oswego (Ontario) | 4 |
| 2008 | ||||
| 3872 | Alimentary canal | Ring-billed gull | Cayuga (Ontario) | 8d |
| 3873 | Alimentary canal | Ring-billed gull | Cayuga (Ontario) | 11a |
| 3875 | Alimentary canal | Ring-billed gull | Cayuga (Ontario) | 9d |
| 6208 | Alimentary canal | Red-breasted merganser | Oswego (Ontario) | 7 |
NA, not applicable.
Reproducibility of RAPD banding patterns was tested using a subset of 15 isolates. Reproducibility after two runs was determined to be 73%; reproducibility after three runs fell to 27%. The lack of reproducibility was primarily due to differences in faint bands, although occasionally intense bands were not reproducible. Duplicate RAPD banding patterns normally differed by 1 or 2 bands.
We investigated the use of pulsed-field gel electrophoresis (PFGE) to type the isolates from this outbreak. The first 18 strains obtained were typed by PFGE. This technique was abandoned when 4 of the 18 strains (22%) failed to yield a discernible pattern, due to DNA degradation (data not shown).
DISCUSSION
While the biodiversity of strains of Clostridium botulinum type E has been studied at trout farms, for fish and fishery products, and in the Canadian Arctic (17, 19, 23), little is known about the genetic diversity of Clostridium botulinum type E in a wildlife outbreak setting. One question posed by the ongoing botulism outbreak in Lake Erie and Lake Ontario was whether a single strain of C. botulinum was responsible for the majority of deaths seen among the bird population. RAPD typing of the toxin-producing isolates from birds and fish associated with the outbreak revealed 12 distinct types as well as numerous subtypes. Previous molecular studies of C. botulinum type E reported by researchers in Finland and Canada also revealed a great deal of diversity among C. botulinum type E isolates (17, 19, 23).
Hielm et al. (17) examined the biodiversity of C. botulinum type E in trout farms. Their study included 20 farms in Finland and 1 farm in Sweden. A total of 30 strains of C. botulinum type E were isolated from fish, and 18 strains were isolated from fish farm sediments. This study also included 12 archived strains of North American and North Atlantic origins. PFGE of the 60 isolates revealed 28 pulsotypes; 6 strains were untypeable by PFGE. Hyytia et al. (19) obtained similar results when they analyzed the biodiversity of C. botulinum type E toxin in fish and fishery products by using RAPD and PFGE methodology on a total of 92 strains. The isolates comprised 67 isolates from fish farmed or caught in Finland, 15 isolates from German farmed fish, and 10 archived specimens of North Atlantic and North American origins. A total of 62 different subtypes were detected in that sample group.
Leclair et al. (23) utilized RAPD and PFGE methodology to investigate the diversity of group II C. botulinum in the Canadian Arctic. Their isolates were 39 strains from human, environmental, and food sources. Their strains included organisms isolated from four food-borne outbreaks. That study revealed 28 RAPD types. PFGE of the isolates also indicated a great deal of diversity. Depending on the choice of enzyme used by the researchers, either 18 or 23 pulsotypes were detected.
In our study, 44 toxin-producing strains of C. botulinum type E from fish and birds isolated over a 9-year period were examined by RAPD methodology. Twelve major types and numerous subtypes were detected in our analysis, and this indicates a wide genetic diversity of C. botulinum type E organisms in a wildlife outbreak setting. In our study, only three types had more than one strain represented in a single group. Our types 11c, 11e, and 12b had two strains each, while all of the other types and subtypes were represented by only a single strain. Like the previous studies on environmental isolates of C. botulinum type E, the present study has demonstrated that C. botulinum type E isolates from a wildlife outbreak exhibit a great deal of biodiversity.
While the reproducibility of RAPD typing methodology has been problematic, the extent of reproducibility of the RAPD analysis for consecutive runs reported here is comparable to the results from a previous study (23). In the previous study, depending on the choice of primer, 65.4 to 78.5% of C. botulinum type E isolates had RAPD patterns that were reproducible. In our study, patterns for 73% of our isolates were reproducible, and the remaining 27% differed by only 1 or 2 bands. Such a small variation is not likely to change the epidemiologic type of the strain.
Our results demonstrated the presence of bont/E in sediments and plants in Lake Erie. bont/E was detected in 1 of 7 (14%) plant samples and 15 of 17 (88%) sediment samples. An obvious limitation of this finding is that the apparatus used to collect the sediment samples was washed in lake water after each sample was taken, and cross-contamination of samples could have occurred. While our results show that C. botulinum type E is present in the environment of Lake Erie, the true distribution remains unknown.
One unexpected finding of our study was that a total of 37 isolates identified by traditional biochemical reactions as C. botulinum failed to produce toxin. All of these isolates were also negative for bont genes. Interestingly, 41% (15/37) of non-toxin-producing organisms were isolated from specimens that had detectable levels of botulinum toxin by mouse bioassay. These 15 specimens, at least, could have contained a mixture of toxin-producing and non-toxin-producing strains. When multiple isolates of C. botulinum from two birds with demonstrable levels of botulinum toxin by mouse bioassay were examined, a mixture of toxin-producing and non-toxin-producing strains could be found in the same sample. This finding suggests that a good strategy by which to screen wildlife samples for the presence of toxin-producing C. botulinum is to use PCR on the primary sample as an initial test before performing the mouse bioassay. Nontoxigenic isolates of C. botulinum have been described previously (24, 36). The significance of the non-toxin-producing isolates in the present outbreak is unknown, but it is interesting to note that 41% of our isolates failed to produce botulinum toxin.
The demonstration of the presence of type E botulinum toxin, as well as the isolation of C. botulinum type E toxin-producing organisms, suggests that botulism was the cause of death for thousands of birds during the years 2000 to 2008. It has been estimated that a total of 67,793 bird deaths were attributed to botulism in New York State alone (2). That total included 15,672 from Lake Ontario and 52,121 from Lake Erie. It is important to mention that the Canadian province of Ontario has reported type E avian botulism during the same time period (29). The bird mortality reported for the present outbreak is greater than the mortality reported in previous outbreaks of type E avian botulism (6, 15).
One investigator has postulated a possible link between the round goby and the current outbreak of avian botulism (31). The round goby is a recently introduced species; it was first detected in the Great Lakes Basin in 1990 and is thought to have arrived via ballast water discharge from a foreign freighter (20). This species was first detected in Lake Erie in 1993 and had become widespread in New York waters by 2000. The dramatic increase in the population of this fish also coincided with the start of the current outbreak (11). The round goby is a benthic fish that feeds on benthic organisms, including filter-feeding bivalves (8, 10, 21), and would therefore be exposed to bacteria, such as C. botulinum type E, that are found in lake sediments. One study (37) on round gobies that were experimentally intoxicated with type E botulinum toxin found that the fish exhibited pigmentation changes and displayed erratic swimming behavior. A reasonable hypothesis is that such changes make intoxicated fish easier prey for a piscivorous bird.
Many of the necropsies performed for the current study demonstrated the presence of round goby remains in the digestive tracts of affected birds. One other investigator (7) has previously demonstrated that birds affected by botulinum toxin had remains of round goby in their alimentary canals. Our toxin tests on round gobies failed to produce a positive toxin result in the mouse bioassay, although four of six round gobies tested were PCR positive for bont/E, and one of the fish tested yielded a type E toxin-producing C. botulinum organism. While the potential round goby-avian botulism link is intriguing, we found that another fish, the freshwater drum, was also culture positive for C. botulinum toxin-producing organisms. Freshwater drum, as well as lake sturgeons, had C. botulinum type E toxin in their alimentary canals, while a channel catfish had botulinum toxin in its liver. Thus, while the round goby may be a vector involved in the current outbreak, it appears not to be the only source of botulism.
In conclusion, our study demonstrates that many different bird species have been affected by the ongoing outbreak of wildlife botulism and that many distinct strains of toxin-producing C. botulinum type E are present in the Great Lakes ecosystem. We have also shown that non-toxin-producing strains of C. botulinum are common in birds and fish taken from Lake Erie and Lake Ontario and that both toxin-producing strains of C. botulinum and non-toxin-producing strains can be isolated from a single animal. Our data indicate that while the round goby could be a vector in this outbreak, other fish species are also carriers of C. botulinum. More work on the ecology of C. botulinum in the Great Lakes ecosystem is needed to elucidate the source of botulism intoxication in birds as well as the impact of botulism on the wild bird population.
Acknowledgments
We thank Kimberlee Musser for advice, Adriana Verschoor for critical review of the manuscript, Dianna Schoonmaker-Bopp for her assistance with the RAPD analysis, and Susan McAdams at the Botulism Laboratory, University of Pennsylvania, and Beverly Schmitt of the National Veterinary Services Laboratory for providing toxin tests.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Adams, D., K. Roblee, and W. Stone. 2004. Botulism caused fish & waterbird mortality in New York waters of Lakes Erie & Ontario, p. 17-22. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc04.pdf.
- 2.Adams, D., K. Roblee, and W. Stone. 2009. NYS waterbird mortality as a result of type E botulism and nonnative invasive species, poster B. Abstr. Odum Conf. 2009. New York Invasive Species Research Institute, Cornell University, Ithaca, NY. http://www.nyisri.org/odumposterB.aspx.
- 3.Bott, T. L., J. S. Deffner, E. McCoy, and E. M. Foster. 1966. Clostridium botulinum type E in fish from the Great Lakes. J. Bacteriol. 91:919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bott, T. L., J. Johnson, Jr., E. M. Foster, and H. Sugiyama. 1968. Possible origin of the high incidence of Clostridium botulinum type E in an inland bay (Green Bay of Lake Michigan). J. Bacteriol. 95:1542-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand, C. J., R. M. Duncan, S. P. Garrow, D. Olson, and L. E. Schumann. 1983. Water bird mortality from botulism type E in Lake Michigan: an update. Wilson Bull. 95:269-275. [Google Scholar]
- 6.Brand, C. J., S. M. Schmitt, R. M. Duncan, and T. M. Cooley. 1988. An outbreak of type E botulism among common loons (Gavia immer) in Michigan's upper peninsula. J. Wildl. Dis. 24:471-476. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, D. 2002. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc02.pdf.
- 8.Charlebois, P. M., L. D. Corkum, D. J. Jude, and C. Knight. 2001. The round goby (Neogobius melanostomus) invasion: current research and future needs. J. Great Lakes Res. 27:263-266. [Google Scholar]
- 9.Collins, M. D., and A. K. East. 1998. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J. Appl. Microbiol. 84:5-17. [DOI] [PubMed] [Google Scholar]
- 10.Corkum, L. D., M. R. Sapota, and K. E. Skora. 2004. The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biol. Invasions 6:173-181. [Google Scholar]
- 11.Culligan, B. 2002. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc02.pdf.
- 12.Davis, S. W., C. Eagan, and N. Cirino. 2006. Abstr. 106th Am. Soc. Microbiol. Gen. Meet., abstr. Y-014.
- 13.Dezfulian, M., L. M. McCroskey, C. L. Hatheway, and V. R. Dowell, Jr. 1981. Selective medium for isolation of Clostridium botulinum from human feces. J. Clin. Microbiol. 13:526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell, V. R. 1974. Laboratory methods in anaerobic bacteriology. Centers for Disease Control, Atlanta, GA.
- 15.Fay, L. D. 1966. Type E botulism in Great Lakes water birds. Trans. 31st North Am. Wildl. Nat. Resour. Conf. Wildlife Management Institute, Washington, DC.
- 16.Hauschild, A. H. W., and K. L. Dodds. 1993. Clostridium botulinum ecology and control in foods. Marcel Dekker Inc., New York, NY.
- 17.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1998. Prevalence of Clostridium botulinum in Finnish trout farms: pulsed-field gel electrophoresis typing reveals extensive genetic diversity among type E isolates. Appl. Environ. Microbiol. 64:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holdeman, L. V., E. P. Cato, and W. E. C. Moore. 1977. Anaerobe laboratory manual. Virginia Polytechnic Institute and State University, Blacksburg, VA.
- 19.Hyytiä, E., S. Hielm, J. Bjorkroth, and H. Korkeala. 1999. Biodiversity of Clostridium botulinum type E strains isolated from fish and fishery products. Appl. Environ. Microbiol. 65:2057-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jude, D. J., R. H. Reider, and G. R. Smith. 1992. Establishment of Gobiidae in the Great Lakes Basin. Can. J. Fish. Aquat. Sci. 49:416-421. [Google Scholar]
- 21.Kaufman, O. W., and L. D. Fay. 1964. Clostridium botulinum type E toxin in tissues of dead loons and gulls. Q. Bull. Michigan State Univ. Agric. Exp. Stn. 47:236-242. [Google Scholar]
- 22.Laycock, R. A., and D. H. Loring. 1972. Distribution of Clostridium botulinum type E in the Gulf of St. Lawrence in relation to the physical environment. Can. J. Microbiol. 18:763-773. [DOI] [PubMed] [Google Scholar]
- 23.Leclair, D., F. Pagotto, J. M. Farber, B. Cadieux, and J. W. Austin. 2006. Comparison of DNA fingerprinting methods for use in investigation of type E botulism outbreaks in the Canadian Arctic. J. Clin. Microbiol. 44:1635-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, W. H., and H. Riemann. 1970. Correlation of toxic and non-toxic strains of Clostridium botulinum by DNA composition and homology. J. Gen. Microbiol. 60:117-123. [DOI] [PubMed] [Google Scholar]
- 25.Lindström, M., et al. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67:5694-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiwald, M. 2004. Broad-range PCR for detection and identification of bacteria, p. 379-390. In D. H. Persing, et al. (ed.), Molecular microbiology: diagnostic principles and practice. ASM Press, Washington, DC.
- 27.Miller, L. G. 1975. Observations on the distribution and ecology of Clostridium botulinum type E in Alaska. Can. J. Microbiol. 21:920-926. [DOI] [PubMed] [Google Scholar]
- 28.Monheimer, R. H. 1968. The relationship of Lake Michigan waterbird mortalities to naturally occurring Clostridium botulinum type E toxin. Bull. Wildl. Dis. Assoc. 4:81-85. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, J. 2002. Fish and bird die-offs on Canadian side of Lake Erie—2001, p. 28-34. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc02.pdf.
- 30.Roblee, K. 2002. Botulism caused waterbird mortality in New York waters of Lake Erie—2001, p. 20-27. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc02.pdf.
- 31.Ruffing, R. 2004. The round goby botulism connection, p. 23-29. In Botulism in Lake Erie: Workshop Proceedings. Sea Grant, Stony Brook, NY. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc04.pdf.
- 32.Smith, L. D. S. 1977. Botulism: the organism, its toxins, the disease. Charles C. Thomas, Springfield, IL.
- 33.Smith, L. D. S. 1978. The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Lab Science 15:74-80. [PubMed] [Google Scholar]
- 34.Smith, L. D. S., and H. Sugiyama. 1988. Botulism. The organism, its toxins, the disease. Charles C. Thomas, Springfield, IL.
- 35.Telzak, E. E., et al. 1990. An international outbreak of type E botulism due to uneviscerated fish. J. Infect. Dis. 161:340-342. http://www.seagrant.sunysb.edu/botulism/pdfs/Botulism-Proc04.pdf. [DOI] [PubMed] [Google Scholar]
- 36.Yamakawa, K., et al. 1997. Emergence of Clostridium botulinum type B-like nontoxigenic organisms in a patient with type B infant botulism. J. Clin. Microbiol. 35:2163-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yule, A. M., I. K. Barker, J. W. Austin, and R. D. Democcia. 2006. Toxicity of Clostridium botulinum type E neurotoxin to Great Lakes fish: implications for avian botulism. J. Wildl. Dis. 42:479-493. [DOI] [PubMed] [Google Scholar]