Abstract
An uncharacterized gene from Thermus thermophilus, thought to encode a mannose-6-phosphate isomerase, was cloned and expressed in Escherichia coli. The maximal activity of the recombinant enzyme for l-ribulose isomerization was observed at pH 7.0 and 75°C in the presence of 0.5 mM Cu2+. Among all of the pentoses and hexoses evaluated, the enzyme exhibited the highest activity for the conversion of l-ribulose to l-ribose, a potential starting material for many l-nucleoside-based pharmaceutical compounds. The active-site residues, predicted according to a homology-based model, were separately replaced with Ala. The residue at position 142 was correlated with an increase in l-ribulose isomerization activity. The R142N mutant showed the highest activity among mutants modified with Ala, Glu, Tyr, Lys, Asn, or Gln. The specific activity and catalytic efficiency (kcat/Km) for l-ribulose using the R142N mutant were 1.4- and 1.6-fold higher than those of the wild-type enzyme, respectively. The kcat/Km of the R142N mutant was 3.8-fold higher than that of Geobacillus thermodenitrificans mannose-6-phosphate isomerase, which exhibited the highest activity to date for the previously reported kcat/Km. The R142N mutant enzyme produced 213 g/liter l-ribose from 300 g/liter l-ribulose for 2 h, with a volumetric productivity of 107 g liter−1 h−1, which was 1.5-fold higher than that of the wild-type enzyme.
Optically pure carbohydrates are important precursors for pharmaceutical, food, and agrochemical products (22). Among carbohydrates, l-enantiomers have been widely used as antiviral nucleoside analogue drugs in the treatment of severe viral diseases due to their potent biological activities and lower toxicity than the corresponding d-nucleosides (3). l-Ribose, a pentose sugar, can be used as a precursor for the synthesis of antiviral drugs, such as l-nucleoside derivatives (2, 5, 14). l-Ribose can be synthesized by chemical methods from l-arabinose (1, 6, 9), l-xylose (13), d-glucose (15), d-galactose (19), d-ribose (26), or d-mannono-1,4-lactone (20). However, chemical synthesis has several disadvantages, including multiple steps, by-product formation, and chemical waste production.
Recently, the enzymatic production of l-ribose has been investigated using l-arabinose (7) or l-ribulose (25). l-Ribose has been produced primarily from the cheap sugar l-arabinose because l-ribulose is an expensive sugar. An l-arabinose isomerase mutant of Escherichia coli (4) and a d-xylose isomerase mutant of Actinoplanes missouriensis (17) converted l-arabinose to l-ribose by a two-step isomerization reaction with low productivity. A recombinant E. coli strain containing l-arabinose isomerase and l-ribose isomerase (7) and purified l-arabinose isomerase and mannose-6-phosphate isomerase from Geobacillus thermodenitrificans (24) were used to produce l-ribose from l-arabinose via l-ribulose with high productivity. However, a rate-limiting step in the two enzyme systems is the conversion of l-ribulose to l-ribose using l-ribose isomerase (7, 12) or mannose-6-phosphate isomerase (23-25). Thus, biotechnological production of l-ribose has been focused on these enzymes.
Mannose-6-phosphate isomerase from G. thermodenitrificans exhibits the highest activity to date for l-ribose production. Greater efficiency can be attained only through the discovery or synthesis of l-ribose-producing enzymes with higher kcat/Km. Increases in the kcat/Km ratio can be realized by genetic improvements via directed evolution and by structural modification of the determinant residues at or near the active site, based on homology models or the determined structure of the enzymes.
In this study, the activities of a recombinant mannose-6-phosphate isomerase from Thermus thermophilus with different metal ions, pHs, and temperatures for l-ribulose isomerization and its substrate specificities for various aldoses and ketoses were characterized. Mutational analyses were performed with predicted active-site residues obtained from homology studies; the R142N mutant was selected as an effective l-ribose producer. The specific activity, kcat/Km, and conversion for l-ribulose using the R142N mutant were determined.
MATERIALS AND METHODS
Materials.
The monosaccharide standards d- and l-lyxose, d- and l-xylose, d-arabinose, d- and l-ribose, d-xylulose, d-galactose, d-mannose, d-glucose, d-altrose, d-allose, d-fructose, d-psicose, d-tagatose, and d-sorbose were purchased from Sigma (St. Louis, MO), and l-arabinose, d- and l-ribulose, l-xylulose, d- and l-talose, l-galactose, d- and l-idose, d- and l-gulose, l-mannose, l-glucose, l-altrose, l-allose, l-fructose, l-psicose, l-tagatose, and l-sorbose were purchased from Carbosynth (Newbury, Berkshire, United Kingdom).
Microorganisms, plasmid, medium, and culture conditions.
T. thermophilus KCCM 40879, E. coli ER2566, and plasmid pET-28a (Novagen, Darmstadt, Germany) were used as the sources of genomic DNA of mannose-6-phosphate isomerase, as host cells, and as the expression vector, respectively. The recombinant E. coli cells for the expression of the enzyme were cultivated as described previously (23).
Gene cloning and site-directed mutagenesis of mannose-6-phosphate isomerase.
The gene (765 bp) encoding putative mannose-6-phosphate isomerase was obtained using the genomic DNA isolated from T. thermophilus KCCM 40879. The sequences of the primers used for gene cloning were based on the DNA sequence of the mannose-6-phosphate isomerase from T. thermophilus HB8 (GenBank accession number AP008226). Forward (5-TTTCATATGAGGCGGTTGGAGCCCAA) and reverse (5-TTTGAATTCACTCACGCCCCCTCCTT) primers were designed to introduce the underlined NdeI and EcoRI restriction sites, respectively. The amplified DNA fragment was purified using a PCR purification kit (Promega) and was ligated into the NdeI and EcoRI sites of pET-28a(+). The resulting plasmid was used to transform the E. coli ER2566 strain. Site-directed mutagenesis was performed using the Quick-Change kit and protocol (Stratagene, Beverly, MA).
Purification and molecular mass determination of mannose-6-phosphate isomerase.
The purification and the determination of subunit and total molecular masses of mannose-6-phosphate isomerase were performed as described previously (23).
Determination of specific activities and kinetic parameters.
One unit of mannose-6-phosphate isomerase activity was defined as the amount of enzyme required to produce 1 nmol of l-ribose per min at 75°C and pH 7.0. To measure the specific activity for monosaccharide, the reaction was performed in 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 7.0) containing 10 mM monosaccharide and 1 to 100 U/ml of enzyme in the presence of 0.5 mM Cu2+ at 75°C for 5 min. The specific activity was defined as the increased amount of l-ribose as a product per enzyme amount per reaction time.
Various amounts of substrates (10 to 1,000 mM) were incubated in 50 mM PIPES buffer (pH 7.0) containing enzyme at 75°C for 5 min. The reaction was stopped by adding HCl to the reaction mixture at a final concentration of 200 mM. The Km and kcat were calculated using the enzyme concentration and a Lineweaver-Burk plot of the Michaelis-Menten equation.
Effects of metal ions, pH, and temperature on enzyme activity.
The metal ion-treated enzyme was prepared by adding 0.5 mM metal ion, such as Co2+, Mn2+, Mg2+, Ca2+, Zn2+, Cu2+, Fe2+, or Ni2+, to the purified enzyme after treatment with 10 mM EDTA, followed by dialysis at 4°C for 16 h against distilled water. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 10 U/ml of enzyme and 0.5 mM metal ion at 75°C for 5 min. The effect of the concentration of Cu2+ was investigated in the range of 0 to 2 mM.
To examine the effects of pH and temperature on the activity of T. thermophilus mannose-6-phosphate isomerase, the pH was varied from 6.5 to 8.5 using 50 mM PIPES buffer (pH 6.5 to 7.5) and 50 mM N-(2-hyroxyethyl)piperazine-N′-(3-propanesulfonic acid) (EPPS) buffer (pH 7.5 to 8.5), and the temperature was varied from 70 to 90°C. The influence of the temperature on enzyme stability was monitored at temperatures from 65 to 85°C at pH 7.0. The experimental data for thermal deactivation of enzymes were fitted to a first-order curve, and the half-life of the enzyme was calculated using SigmaPlot 10.0 software (Systat Software, San Jose, CA).
Comparative homology modeling.
Homology modeling of the T. thermophilus mannose-6-phosphate isomerase was performed using Discovery Studio 2.5 (Accelrys, San Diego, CA) based on the X-ray structure of mannose-6-phosphate isomerase from Bacillus subtilis (Protein Data Bank [PDB] entry 1QWR). The homologous search and sequence alignment were conducted using sequence analysis and multiple-sequence alignment modules, respectively. Based on the optimized alignment, 5 comparative models of the target sequence were conducted with MODELLER (11) by applying the default model-building routine “model” with fast refinement. This procedure has an advantage in that one can select the best model from several candidates. Furthermore, the variability among the models can be used to evaluate modeling reliability. Energy minimization was performed using the consistent valence force field and the Discover program with the steepest descent and conjugated gradient algorithms. The qualities of these models were analyzed with PROCHECK (10). l-Ribulose was docked in the model of T. thermophilus mannose-6-phosphate isomerase using the Surflex docking program (Tripos, St. Louis, MO) (8). The active site was defined as a collection of amino acid residues enclosed within a sphere of 4.5-Å radius centered on the substrate binding site. Each docking run consisted of 100 independent docks with 1,000 iteration cycles. A random start was used to generate the substrate position within the docking box. The substrate orientation giving the lowest interaction energy was chosen for additional rounds of docking.
Analytical methods.
The concentrations of monosaccharides were determined with a Bio-LC system (ICS-3000; Dionex, Sunnyvale, CA) with an electrochemical detector using a CarboPac PA1 column. The column was eluted at 30°C with 200 mM sodium hydroxide at a flow rate of 1 ml/min.
RESULTS AND DISCUSSION
Amino acid sequence alignment, purification, and molecular mass determination of mannose-6-phosphate isomerase from T. thermophilus.
A BLAST search of mannose-6-phosphate isomerase from T. thermophilus revealed relatively low amino acid sequence identities of 34, 27, 15, 13, and 15% relative to the characterized mannose-6-phosphate isomerases from B. subtilis (23), G. thermodenitrificans (25), Salmonella enterica serovar Typhimurium (16), Candida albicans (21), and Homo sapiens (18), respectively.
The mannose-6-phosphate isomerase from T. thermophilus was purified, with a final purification of 30-fold, a yield of 30%, and a specific activity of 25 U/mg. The subunit molecular mass of the purified enzyme was approximately 29 kDa, as determined by SDS-PAGE (see Fig. S1 in the supplemental material), which is in good agreement with the value of 29,054 Da calculated with the Compute pI/Mw software. Based on the reference protein masses, the native enzyme existed as a monomer with a molecular mass of 29 kDa, as determined by gel filtration chromatography, using a Sephacryl S-300 HR 16/60 column (see Fig. S3 in the supplemental material). The molecular mass of mannose-6-phosphate isomerase from T. thermophilus differed significantly from those from G. thermodenitrificans (25), B. subtilis (23), Salmonella Typhimurium (16), C. albicans (21), and H. sapiens (18), which are 36,452, 36,444, 42,591, 48,867, and 46,656 Da, respectively.
Effects of metal ions, pH, and temperature on the activity of mannose-6-phosphate isomerase from T. thermophilus.
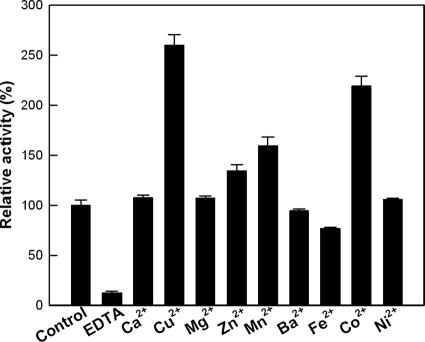
Mannose-6-phosphate isomerases are metalloenzymes that require a divalent ion metal cofactor, such as a cobalt ion, for sugar isomerization (23, 25). After the removal of metal ions by treatment with EDTA, the relative activity was 12% of that prior to EDTA treatment (Fig. 1). The addition of Cu2+, Co2+, Mn2+, or Zn2+ ions enhanced the activity of l-ribulose isomerization by T. thermophilus mannose-6-phosphate isomerase, whereas the addition of Fe2+ ion inhibited the activity. Among the metal ions tested, Cu2+ was the most effective, with an optimal concentration of 0.5 mM (see Fig. S4 in the supplemental material).
FIG. 1.
Effects of metal ions on the activity of T. thermophilus mannose-6-phosphate isomerase. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 10 mM l-ribulose, 10 U/ml of enzyme, and 0.5 mM each metal ion at 75°C for 5 min. The control was the purified enzyme without treatment with EDTA. The data represent the means of three experiments, and the error bars represent standard deviations.
The effects of pH and temperature on enzyme activity were also investigated, and maximal activity for l-ribulose was observed at pH 7.0 and 75°C (see Fig. S5 and S6 in the supplemental material). The enzyme displayed first-order kinetics of thermal inactivation, with half-lives of 22, 10, 5.5, 2.1, and 0.3 h at 65, 70, 75, 80, and 85°C, respectively (Fig. 2).
FIG. 2.
Thermal inactivation of the activity of mannose-6-phosphate isomerase from T. thermophilus at temperatures of 65 (•), 70 (▪), 75 (▴), 80 (○), and 85°C (□). A sample was withdrawn at each time interval, and the relative activity was determined in 50 mM PIPES buffer (pH 7.0) containing 10 mM l-ribulose, 10 U/ml of enzyme, and 0.5 mM Cu2+ at 75°C for 5 min. The data represent the means of three experiments, and the error bars represent standard deviations.
Substrate specificity of mannose-6-phosphate isomerase from T. thermophilus.
The specific activity of the enzyme was investigated with all pentoses and hexoses. Among the aldose substrates, the specific activity was highest for d-talose, followed by d-mannose, l-allose, l-ribose, d-lyxose, d-allose, l-talose, d-ribose, l-lyxose, and l-mannose (Table 1). The products formed from different substrates were analyzed with a Bio-LC system using a CarboPac PA1 column and showed the same retention times as the monosaccharide standards. Among the ketose substrates, the highest specific activity was observed with l-ribulose, followed by d-fructose, d-xylulose, d-ribulose, d-tagatose, l-fructose, l-psicose, l-xylulose, l-tagatose, and d-psicose. The specific activity of mannose-6-phosphate isomerase from T. thermophilus for l-ribulose (1,493 U/mg) was 16-fold higher than that from B. subtilis (92 U/mg) (23). These results suggested that the T. thermophilus mannose-6-phosphate isomerase was a potential l-ribose producer.
TABLE 1.
Specific activities of mannose-6-phosphate isomerases from T. thermophilus for monosaccharides
| Substrate | Product | Sp acta (U/mg) |
|---|---|---|
| Aldose | ||
| d-Talose | d-Tagatose | 1,619 ± 21 |
| l-Talose | l-Tagatose | 63 ± 2 |
| d-Allose | d-Psicose | 165 ± 2 |
| l-Allose | l-Psicose | 742 ± 5 |
| d-Mannose | d-Fructose | 842 ± 32 |
| l-Mannose | l-Fructose | 19 ± 0.5 |
| d-Ribose | d-Ribulose | 58 ± 2 |
| l-Ribose | l-Ribulose | 425 ± 24 |
| d-Lyxose | d-Xylulose | 333 ± 14 |
| l-Lyxose | l-Xylulose | 23 ± 0.2 |
| Ketose | ||
| d-Ribulose | d-Ribose | 126 ± 2 |
| l-Ribulose | l-Ribose | 1,493 ± 57 |
| d-Xylulose | d-Lyxose | 128 ± 7 |
| l-Xylulose | l-Lyxose | 22 ± 0.2 |
| d-Tagatose | d-Talose | 85 ± 0.6 |
| l-Tagatose | l-Talose | 16 ± 0.1 |
| d-Fructose | d-Mannose | 167 ± 11 |
| l-Fructose | l-Mannose | 57 ± 3 |
| d-Psicose | d-Allose | 3 ± 0.1 |
| l-Psicose | l-Allose | 42 ± 0.9 |
The data represent the means and standard deviations from three separate experiments. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 10 mM monosaccharide and 0.5 mM Cu2+ at 75°C for 5 min by adjusting the enzyme amount (1 to 100 U/ml).
Mannose-6-phosphate isomerases from T. thermophilus, B. subtilis (23), and G. thermodenitrificans (25) prefer aldose substrates with C-2 and C-3 hydroxyl groups in the left-hand configuration (Fischer projections), such as d-talose, d-mannose, l-allose, l-ribose, and d-lyxose, relative to aldoses with right-hand hydroxyl groups, such as d-allose, l-talose, d-ribose, l-lyxose, and l-mannose. Additionally, mannose-6-phosphate isomerase from T. thermophilus preferred aldohexoses, such as d-talose, d-mannose, and l-allose, whereas mannose-6-phosphate isomerases from B. subtilis (23) and G. thermodenitrificans (25) preferred aldopentoses, such as l-ribose and d-lyxose.
The kcat/Km of the enzyme for mannose-6-phosphate was 6,685 mM−1 s−1 and was 18-fold higher than that for l-ribulose (374 mM−1 s−1), indicating that the enzyme is a true mannose-6-phosphate isomerase and that mannose-6-phosphate is the authentic substrate of the enzyme.
Selection of residues related to increased activity of l-ribulose isomerization by molecular modeling and Ala substitution.
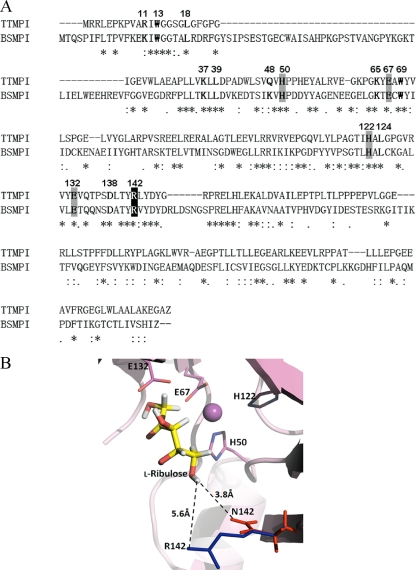
The homology model for the active site for mannose-6-phosphate isomerase from T. thermophilus was generated based on sequence alignment with the known structure of B. subtilis mannose-6-phosphate isomerase (PDB entry 1QWR). Although the level of sequence identity between mannose-6-phosphate isomerases from T. thermophilus and B. subtilis was relatively low (34% identity and 58% similarity), the suggested metal-binding sites (H50, E67, H122, and E132 of T. thermophilus mannose-6-phosphate isomerase) of two enzymes were absolutely conserved, as shown in Fig. 3 A. The Ala substitution of the metal-binding site of T. thermophilus mannose-6-phosphate isomerase exhibited no activity for l-ribulose (Table 2), suggesting that the metal-binding site was involved in the active site. Moreover, the active-site residues around the substrate, such as R11, W13, L18, K37, L39, Q48, H50, K65, E67, W69, H122, L124, E132, D138, and R142, which were identified within a sphere of 4.5-Å radius centered on the substrate binding pocket by the homology model (Fig. 3B), were completely conserved, except R11 and Q48. Thus, the active-site structure of the homology model, including the metal-binding site and position 142, was used in docking studies, and these 15 residues were selected as candidate residues related to the increase in l-ribulose isomerization activity.
FIG. 3.
(A) Alignment of amino acid sequences of mannose-6-phosphate isomerases from T. thermophilus (TTMPI) and B. subtilis (BSMPI). The GenBank accession numbers of the mannose-6-phosphate isomerases of T. thermophilus and B. subtilis are AP008226 and BAA08088, respectively. The putative residues are in boldface, the conserved metal-binding residues are highlighted with a gray background, and the residue at position 142 is highlighted with a black background. The positions of the15 active-site residues within a sphere of 4.5-Å radius centered on the substrate binding pocket are numbered on the amino acid sequences. Dashes, asterisks, colons, and periods indicate deletions, identities, conserved substitutions, and semiconserved substitutions, respectively. (B) Docking of l-ribulose into the active site of the wild-type and mutant enzymes. The yellow, blue, and red sticks represent l-ribulose as a substrate and arginine and asparagine residues at position 142, respectively. The dashed line is the distance between residue and substrate.
TABLE 2.
Specific activities of the wild-type and alanine-substituted mutant mannose-6-phosphate isomerases from T. thermophilus for l-ribulose
| Enzyme | Sp act (U/mg)a |
|---|---|
| Wild type | 1,493 ± 25 |
| R11A | 1,482 ± 45 |
| W13A | 838 ± 30 |
| L18A | 1,194 ± 42 |
| K37A | 474 ± 12 |
| L39A | 1,065 ± 60 |
| Q48A | 1,016 ± 90 |
| H50A | ND |
| K65A | 1,011 ± 60 |
| E67A | ND |
| W69A | 1,046 ± 30 |
| H122A | ND |
| L124A | 1,076 ± 45 |
| E132A | ND |
| D138A | 972 ± 45 |
| R142A | 1,791 ± 13 |
The data represent the means and standard deviations from three separate experiments. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 10 mM l-ribulose and 0.5 mM Cu2+ at 75°C for 5 min by adjusting the enzyme amount (1 to 100 U/ml). ND, specific activity was not detected by the analytical methods in this study.
The 15 proposed residues were each replaced separately with Ala. The wild-type and all mutant enzymes were expressed and purified by His trap chromatography as a single band with a molecular mass of approximately 29 kDa (data not shown). The specific activities of the wild-type and mutant enzymes for l-ribulose are shown in Table 2. The mutant enzymes of the suggested metal-binding site (H50A, E67A, H122A, and E132A) have no activity for l-ribulose. The activities of the other mutant enzymes for l-ribulose decreased, with the exception of the R142A mutant, which exhibited an activity 1.2-fold higher than that of the wild-type enzyme. These results suggested that the amino acid at position 142 was related to the observed increase in l-ribulose isomerization activity.
Kinetic analysis of the wild-type and R142N mutant mannose-6-phosphate isomerases from T. thermophilus.
The Arg residue at position 142 of the mannose-6-phosphate isomerase from T. thermophilus was replaced separately with the nonpolar aliphatic residue Ala, the polar uncharged residues Asn and Gln, the aromatic residue Tyr, the negatively charged residue Glu, and the positively charged residue Lys. Expression of the wild-type and mutant enzymes was purified by His trap chromatography and confirmed by SDS-PAGE (see Fig. S2 in the supplemental material). The specific activities and kinetic parameters of l-ribulose isomerization with the wild-type and mutant enzymes at position 142 are presented in Table 3. The highest specific activity and kcat/Km was observed with the R142N mutant, suggesting that the R142N mutant was the most efficient producer of l-ribose.
TABLE 3.
Kinetic parameters and specific activities of the wild-type and mutant mannose-6-phosphate isomerase enzymes from T. thermophilus at position 142 for l-ribulosea
| Enzyme | Sp act (U/mg) | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| Wild type | 1,493 ± 25 | 136 ± 4 | 50,644 ± 709 | 374 ± 11 |
| R142A | 1,791 ± 13 | 184 ± 5 | 64,873 ± 908 | 353 ± 11 |
| R142N | 2,152 ± 37 | 140 ± 4 | 81,063 ± 929 | 579 ± 18 |
| R142Q | 1,270 ± 12 | 150 ± 2 | 48,300 ± 523 | 322 ± 6 |
| R142K | 1,214 ± 18 | 228 ± 6 | 68,877 ± 964 | 302 ± 9 |
| R142E | 1,045 ± 11 | 151 ± 5 | 32,666 ± 536 | 216 ± 8 |
| R142Y | 1,092 ± 10 | 308 ± 6 | 56,178 ± 865 | 182 ± 5 |
The data represent the means and standard deviations from three separate experiments. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing various amounts of l-ribulose (10 to 1,000 mM) and 0.5 mM Cu2+ at 75°C for 5 min.
In the molecular model, position 142 is located near the 5-OH of l-ribulose when the substrate is docked in the active-site pocket (Fig. 3). The distance from the 5-OH of l-ribulose to the side chain of the Arg residue (5.6 Å) in the wild-type enzyme was longer than the distance to the side chain of the Asn residue in the R142N mutant (3.8 Å). This shorter distance may explain the enhanced kcat/Km for l-ribulose over the wild-type enzyme.
The kcat/Km of the R142N mutant (579 mM−1 s−1) of mannose-6-phosphate isomerase from T. thermophilus for l-ribulose was 1.5-, 13.3-, and 3.8-fold higher than those of mannose-6-phosphate isomerases from T. thermophilus (375 mM−1 s−1), B. subtilis (43.5 mM−1 s−1), and G. thermodenitrificans (152 mM−1 s−1), respectively (23, 25). The R142N mutant exhibited the highest catalytic efficiency for l-ribulose among all of the l-ribose-converting enzymes.
l-Ribose production from l-ribulose by mannose-6-phosphate isomerase from T. thermophilus.
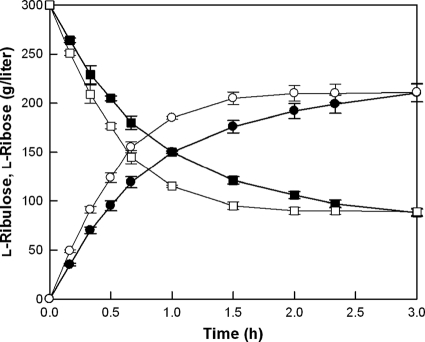
For practical biotechnological applications, time-course reactions of l-ribose production by the purified wild-type and R142N mutant enzymes (see Fig. S7 in the supplemental material) were performed with the high concentration of l-ribulose with 0.5 mM Cu2+ at 70°C and pH 7.0 for 3 h (Fig. 4). The concentrations of substrate and enzyme in the reaction mixtures were optimized at 300 g/liter l-ribulose and 2 mg/ml, respectively (see Fig. S8 and S9 in the supplemental material). As a result, 213 g/liter l-ribose was obtained from 300 g/liter l-ribulose using the wild-type enzyme after 3 h, with a conversion yield of 70% and a volumetric productivity of 71 g liter−1 h−1, and the same concentration of l-ribose was obtained from 300 g/liter l-ribulose using the R142N mutant enzyme after 120 min, with a volumetric productivity of 107 g liter−1 h−1, which was 1.5-fold higher than that using the wild-type enzyme.
FIG. 4.
Time courses of l-ribose production from l-ribulose by the wild-type and R142N mutant mannose-6-phosphate isomerases from T. thermophilus. l-Ribose of the wild type (•) and R142N mutant (○) and l-ribulose of the wild type (▪) and R142N mutant (□) are shown. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 300 g/liter l-ribulose, 2 mg/ml of enzyme, and 0.5 mM Cu2+ at 70°C for 3 h. The data represent the means of three experiments, and the error bars represent standard deviations.
In summary, a previously uncharacterized gene from T. thermophilus, thought to encode a mannose-6-phosphate isomerase, was cloned and expressed in E. coli. The R142N mutant, which exhibited an enhanced activity of l-ribulose isomerization over the wild-type enzyme, was obtained using molecular modeling and site-directed mutagenesis. The kcat/Km of the R142N mutant was 1.6-fold higher than that of the wild-type enzyme and was 3.8-fold higher than that of G. thermodenitrificans mannose-6-phosphate isomerase, which had previously exhibited the highest reported kcat/Km. These results suggest that the R142N mutant of T. thermophilus mannose-6-phosphate may be useful for the industrial production of l-ribose.
Supplementary Material
Acknowledgments
This study was supported by a grant (R0A-2007-000-20015-0) from the National Research Laboratory Program of the Ministry of Education, Science and Technology and by a grant (20090054) from the Agricultural R&D Promotion Center.
Footnotes
Published ahead of print on 29 November 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Akagi, M., et al. 2002. A practical synthesis of L-ribose. Chem. Pharm. Bull. (Tokyo) 50:866-868. [DOI] [PubMed] [Google Scholar]
- 2.Casey, J., et al. December 2003. Method of treating hepatitis delta virus infection. U.S. patent 6,670,342.
- 3.Cho, B. H., J. H. Kim, H. B. Jeon, and K. S. Kim. 2005. A new efficient and practical synthesis of 2-deoxy-L-ribose. Tetrahedron 61:4340-4341. [Google Scholar]
- 4.De Muynck, C., et al. 2007. Development of a selection system for the detection of L-ribose isomerase expressing mutants of Escherichia coli. Appl. Microbiol. Biotechnol. 76:1051-1057. [DOI] [PubMed] [Google Scholar]
- 5.Doong, S. L., C. H. Tsai, R. F. Schinazi, D. C. Liotta, and Y. C. Cheng. 1991. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc. Natl. Acad. Sci. U. S. A. 88:8495-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, J., et al. 1999. A practical synthesis of L-FMAU from L-arabinose. Nucleosides Nucleotides 18:187-195. [DOI] [PubMed] [Google Scholar]
- 7.Helanto, M., K. Kiviharju, T. Granstrom, M. Leisola, and A. Nyyssola. 2009. Biotechnological production of L-ribose from L-arabinose. Appl. Microbiol. Biotechnol. 83:77-83. [DOI] [PubMed] [Google Scholar]
- 8.Jain, A. N. 2003. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 46:499-511. [DOI] [PubMed] [Google Scholar]
- 9.Jumppanen, J., J. Nurmi, and O. Pastinen. October 2000. Process for the continuous production of high purity of L-ribose. U.S. patent 6,140,498.
- 10.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 11.Martí-Renom, M. A., et al. 2000. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29:291-325. [DOI] [PubMed] [Google Scholar]
- 12.Mizanur, R. M., G. Takata, and K. Izumori. 2001. Cloning and characterization of a novel gene encoding L-ribose isomerase from Acinetobacter sp. strain DL-28 in Escherichia coli. Biochim. Biophys. Acta 1521:141-145. [DOI] [PubMed] [Google Scholar]
- 13.Moyroud, E., and P. Strazewski. 1999. L-ribonucleosides from L-xylose. Tetrahedron 55:1277-1284. [Google Scholar]
- 14.Okano, K. 2009. Synthesis and pharmaceutical application of L-ribose. Tetrahedron 65:1937-1949. [Google Scholar]
- 15.Pitsch, S. 1997. An efficient synthesis of enantiomeric ribonucleic acids from D-glucose. Helv. Chim. Acta 80:2286-2314. [Google Scholar]
- 16.Sagurthi, S. R., G. Gowda, H. S. Savithri, and M. R. Murthy. 2009. Structures of mannose-6-phosphate isomerase from Salmonella typhimurium bound to metal atoms and substrate: implications for catalytic mechanism. Acta Crystallogr. D Biol. Crystallogr. 65:724-732. [DOI] [PubMed] [Google Scholar]
- 17.Santa, H., et al. 2005. Stochastic boundary molecular dynamics simulation of L-ribose in the active site of Actinoplanes missouriensis xylose isomerase and its Val135Asn mutant with improved reaction rate. Biochim. Biophys. Acta 1749:65-73. [DOI] [PubMed] [Google Scholar]
- 18.Schollen, E., et al. 2000. Genomic organization of the human phosphomannose isomerase (MPI) gene and mutation analysis in patients with congenital disorders of glycosylation type Ib (CDG-Ib). Hum. Mutat. 16:247-252. [DOI] [PubMed] [Google Scholar]
- 19.Shi, Z. D., B. H. Yang, and Y. L. Wu. 2002. A stereospecific synthesis of L-deoxyribose, L-ribose and L-ribosides. Tetrahedron 58:3287-3296. [Google Scholar]
- 20.Takahashi, H., Y. Iwai, Y. Hitomi, and S. Ikegami. 2002. Novel synthesis of L-ribose from D-mannono-1,4-lactone. Org. Lett. 4:2401-2403. [DOI] [PubMed] [Google Scholar]
- 21.Wells, T. N., P. Scully, and E. Magnenat. 1994. Arginine 304 is an active site residue in phosphomannose isomerase from Candida albicans. Biochemistry 33:5777-5782. [DOI] [PubMed] [Google Scholar]
- 22.Woodyer, R. D., N. J. Wymer, F. M. Racine, S. N. Khan, and B. C. Saha. 2008. Efficient production of L-ribose with a recombinant Escherichia coli biocatalyst. Appl. Environ. Microbiol. 74:2967-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeom, S. J., J. H. Ji, N. H. Kim, C. S. Park, and D. K. Oh. 2009. Substrate specificity of a mannose-6-phosphate isomerase from Bacillus subtilis and its application in the production of l-ribose. Appl. Environ. Microbiol. 75:4705-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeom, S.-J., N.-H. Kim, C.-S. Park, and D.-K. Oh. 2009. l-Ribose production from l-arabinose by using purified l-arabinose isomerase and mannose-6-phosphate isomerase from Geobacillus thermodenitrificans. Appl. Environ. Microbiol. 75:6941-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeom, S. J., et al. 2009. Characterization of a mannose-6-phosphate isomerase from Geobacillus thermodenitrificans that converts monosaccharides. Biotechnol. Lett. 31:1273-1278. [DOI] [PubMed] [Google Scholar]
- 26.Yun, M., et al. 2005. A highly efficient synthesis of unnatural L-sugars from D-ribose. Tetrahedron Lett. 46:5903-5905. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.